Abstract
Surgical wound infection is a serious and potentially catastrophic complication after joint arthroplasty. Urinary tract infection is a common infection that creates a potential reservoir of resistant pathogens and increases patient morbidity. We asked whether treated preoperative and postoperative urinary tract infections are risk factors for deep joint infection. We examined the medical records of 19,735 patients. The minimum had joint infections develop. Of these, three had preoperative and four had postoperative urinary tract infections. The majority of bacteria were not enteric. The bacteria in the two types of infections were not identical. Control subjects were randomly selected from a list of patients matched with patients having infections. Of these, eight had preoperative and one had postoperative urinary tract infections. We found no association between the preoperative urinary tract infection (odds ratio, 0.341; 95% confidence interval, 0.086–1.357) or postoperative urinary tract infection (odds ratio, 4.222; 95% confidence interval, 0.457–38.9) and wound infection. Only one of the 58 patients with wound infections had a urinary tract infection with the same bacteria in both infections. Given the infection rate was very low (0.29%), the power of the study was only 25%. Although limited, the data suggest patients with urinary tract infections had no more likelihood of postoperative infection. We believe treated urinary tract infection should not be a reason to delay or postpone surgery.
Level of Evidence: Level III, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Infection is one of the most serious complications after joint arthroplasty. Revision of joint arthroplasty because of infection is associated with long hospitalization, many operations, and with a current mortality rate of 1% to 2.7% [6, 18, 27, 33, 49]. Although its reported incidence now is less than 1%, its treatment is among the most expensive of orthopaedic procedures with costs often greater than $50,000 [4, 40]. The importance of prevention is critical because of the devastating clinical and economic consequences.
Numerous authors have proposed theories [8, 12, 14, 17, 28, 35, 46, 48] and risk factors [3, 12, 30, 35] (Table 1) regarding the pathogenesis of infection after joint arthroplasty to prevent and control this condition. However, identification of risk factors often is difficult [3, 18, 27, 30].
Table 1.
| Risk factor |
| Immune system impairment |
| Systemic disease (rheumatoid arthritis, lupus, diabetes, malignancy) |
| Advanced age (> 80 years) |
| Malnutrition (< 15% body mass index) |
| Obesity (> 20% over ideal weight) |
| Urinary tract infection |
| Remote site infection |
| Previous joint infection |
| Closed suction drain |
| High postoperative international normalized ratio |
| Long duration of surgery (> 120 minutes) |
| Preoperative stay in hospital (> 4 days) |
Urinary tract infection (UTI) is a common nosocomial infection creating potential bacteria [5, 45]. The presence of a urinary catheter is the main risk factor for UTI [44, 45, 50] and can precipitate bacteremia [32, 44, 45]. In orthopaedic surgery, UTI as a source for joint infection is the subject of controversy [9, 36, 41]. Some authors suggest UTI should be treated before the joint arthroplasty [9] whereas others have stated there are no well-documented data to support the association between UTI and joint infection [15, 36, 41]. Some authors report an association between postoperative bladder catheterization and subsequent joint infection [7, 28, 30]. However, these data are from case reports or case series [7, 28, 30] and their validity is questionable [10].
We therefore asked whether a treated preoperative or postoperative UTI or asymptomatic bacteriuria increases the risk of joint infection and whether the organisms are the same when UTI and joint infection occur in the same patient.
Materials and Methods
In our hospital, more than 5000 joint arthroplasties are performed annually. We performed a retrospective chart review and case-control analysis on all 19,735 joint arthroplasties from our institution performed between 2000 and 2004 with followup of at least 1 year. Four hundred thirty-five patients were lost to followup. These patients had only 3 or 6 months followup and some might have had subsequent late wound infections. We found 58 patients (0.29%) with joint infections and obtained a control group without joint infections matched to this group. For both groups of patients, we searched their medical records for the presence or absence of preoperative or postoperative UTIs, determined the types of bacteria in the UTIs and wound infections, and determined whether there was an association between UTI and joint infection. The project was reviewed and approved by the Institutional Review Board committee (IRB #25050).
Given the measured rates of UTI in our sample, our study had a Type II error of 75% (ie, power of 25%) to detect a major association between UTI and wound infection for a Type I error of 0.05. To obtain a Type II error of 20% (ie, power of 80%), one would have to include in the analyses a population of 260 patients with wound infections and 260 control subjects. Given our hospital’s rates, this number could have been obtained from a database approximately four times larger than the current one (that would correspond to approximately 80,000 subjects).
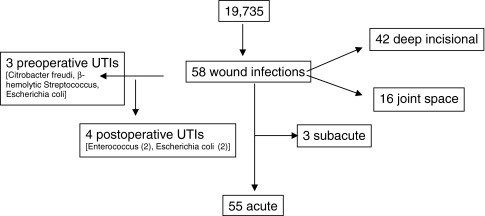
We obtained a list of all patients undergoing THAs and TKAs at our hospital from January 2000 to December 2004 from the hospital database. The hospital infection control group prospectively collects data on all infections in the hospital including data on perioperative UTI and joint infection. All cases were identified from these data. In addition, all patients who underwent a surgical procedure for exploration of a wound, débridement of a wound, or removal of an infected prosthesis from January 2000 until December 2005 were identified. We registered all the patients who had débridement in the operating room owing to wound infection, all who had implant extraction resulting from deep infection, and all who had revision attributable to joint infection during the study period. At the same time, we identified the same cases in the microbiology department to register the bacteria. Moreover, we followed all the cultures from joint arthroplasty cases in the microbiology department to identify positive cultures from superficial drainage in which débridement was not performed and antibiotic treatment was adequate. We did not register cases with negative cultures and cases in which additional therapy was not used. However, if additional therapy was used, we should have identified them. From these data, we identified 58 patients who had postoperative wound infections (Fig. 1; Table 2). There were 32 primary TKAs, 16 primary THAs, five hip revisions, three knee revisions, one bilateral THA, and one bilateral TKA. There were 55 acute and three subacute postoperative wound infections of which 42 were deep incisional and 16 were joint space infections (Fig. 1). The minimum followup of the 58 patients was 1 year (mean, 3 years; range, 1–16 years).
Fig. 1.
A flowchart illustrates the distribution of patients with postoperative wound infections after joint arthroplasty according to type of wound infection and the presence of preoperative or postoperative UTI.
Table 2.
Demographics of patients with wound infections and control subjects
| Demographic characteristics | Patients with infection (n = 58) | Control subjects (n = 58) |
|---|---|---|
| Gender | ||
| Female | 38 | 38 |
| Male | 20 | 20 |
| Age (years)* | 66.7 ± 12.2 (27–90) | 65.4 ± 12.2 (32–88) |
| Catheterization time (hours)* | 43.4 ± 16.6 (8–87) | 44 ± 15.5 (12–85) |
| Followup (years)* | 3.09 ± 0.756 (2–4) | 3.02 ± 0.69 (2–4) |
| Joints | ||
| Knee | 36 | 36 |
| Hip | 22 | 22 |
| Type of wound infection | ||
| Deep incisional | 42 | |
| Joint space | 16 | |
| Acute | 55 | |
| Subacute | 3 | |
| Bacteria (urine cultures) | ||
| Preoperative UTI | 3 [Citrobacter freudi, β-hemolytic Streptococcus, Escherichia coli] | 8 [Enterococcus, Escherichia coli (4), β-hemolytic Streptococcus, bacteriuria + symptoms (no culture), Citrobacter freudi] |
| Postoperative UTI | 4 [Enterococcus (2), Escherichia coli (2), Staphylococcus epidermis, Staphylococcus aureus, Enterococcus-Escherichia coli, β-hemolytic Streptococcus] | 1 [Escherichia coli] |
| Bacteria (wound cultures) | ||
| Staphylococcus aureus | 38 | |
| Staphylococcus epidermidis | 7 | |
| Morganella morgani | 6 | |
| Klebsiella pneumonia | 1 | |
| Proteus mirabilis | 1 | |
| Citrobacter freudi | 1 | |
| β-Hemolytic Streptococcus | 2 | |
| Pseudomonas | 1 | |
* Values are expressed as mean ± standard deviation, with range in parentheses; UTI = urinary tract infection.
Control subjects were randomly selected from a list of patients without infections matched 1:1 with the patients with infections by age (± 2 years), gender, surgeon, joint (hip or knee), and year of surgery (Table 2). Control subjects also were matched with patients with infections by equal length of followup. There were no changes in clinical practices during the study period.
A week before surgery, all patients were examined by an internist and any preoperative UTI was treated with antibiotics as determined by susceptibility test results. Our policy for preoperative UTI or asymptomatic bacteriuria during the study period was antibiotic therapy for 5 to 8 days and thereafter the performance of joint arthroplasty, which was approximately 1 week after the diagnosis of UTI.
All patients undergoing joint arthroplasty were admitted to the hospital on the day of surgery. All patients received epidural or combined spinal epidural anesthesia, and urinary catheters were placed routinely, sometimes in the operating room but usually immediately after surgery in the postanesthesia care unit. Catheters were placed aseptically by trained personnel using closed systems. Institutional policy was to administer intravenous antibiotics within 1 hour of the surgical incision. Antibiotic-impregnated cement was used for patients at high-risk or for those with prior infection, although this was not used routinely in patients with asymptomatic UTI. Prophylactic intravenous antibiotics were administered 24 hours postoperatively for primary cases and until routine operative cultures were negative and final for revision cases. The catheters were discontinued when patients could stand or when the epidural analgesia was discontinued.
We defined wound infections, in accordance with the 2004 Centers for Disease Control and Prevention criteria for surgical site infections (SSIs), as superficial incisional, deep incisional, or joint space infection [19]. A superficial incisional SSI met the following criteria [19]: (1) infection within 30 days of the procedure involving only skin and subcutaneous tissue and (2) the patient has at least one of the following: purulent wound drainage, organisms obtained from aseptically obtained culture of fluid or tissue from a superficial incision, at least one sign or symptom of infection (pain, tenderness, localized swelling, redness, or heat and superficial incision is opened by a surgeon unless the incision is culture-negative), or a diagnosis of superficial incisional SSI by the surgeon. A deep incisional SSI met the following criteria [19]: (1) infection within 30 days of the procedure if no implant is left in place or within 1 year if an implant is in place and involves deep soft tissues (fascial and muscle layers) of the incision and (2) the patient has at least one of the following: purulent drainage from the deep incision but not from the joint space, deep incision spontaneously dehisces or is deliberately opened by a surgeon when the patient has at least one of the following signs or symptoms (fever greater than 38°C, pain, tenderness unless the incision is culture-negative), evidence of an abscess or other evidence of infection is found on examination, during reoperation, or by radiographic or histopathologic examination, or a diagnosis of deep incisional SSI by the surgeon. A joint space SSI met the following criteria [19]: (1) infection within 30 days of the procedure if no implant is left in place or within 1 year if an implant is in place and involves any part of the body, excluding the skin incision, fascia, and muscle layers, that is opened or manipulated during the operation and (2) the patient has at least one of the following: purulent drainage from a drain that is placed through a stab wound into the joint space, organisms aseptically cultured from joint fluid or synovial biopsy, evidence of an abscess or other evidence of infection involving the joint space is found on examination, during reoperation, or by radiographic or histopathologic examination, or a diagnosis of joint space SSI by the surgeon.
We defined UTI, in accordance with the 2004 Centers for Disease Control and Prevention criteria, as an infection that meets at least one of the following criteria [19]: (1) at least one sign or symptom (fever greater than 38°C, urgency, frequency, dysuria, or suprapubic tenderness) with no other recognized cause and a positive urine culture with 105/cm3 or more microorganisms of urine with no more than two species of microorganisms and (2) at least two signs or symptoms (fever greater than 38°C, urgency, frequency, dysuria, or suprapubic tenderness) with no other recognized cause and at least one of the following: positive leukocyte esterase or nitrite, pyuria (urine specimen with 10 leukocytes/mm3 or more), organisms on Gram stain of unspun urine, two positive urine cultures with the same uropathogen with 102 colonies/mL or more in nonvoided specimens, 105 colonies/mL or less of one uropathogen in a patient being treated for a UTI, physician diagnosis of a UTI, or the physician starts therapy for a UTI.
The isolated bacteria in UTIs and in wound infections were determined for both groups. We had complete bacteriologic data on all patients with UTIs and all patients with joint infections. Comorbidities and the American Society of Anesthesiologists risk classification also were reviewed.
We considered the UTI to be the most likely bacterial source if there was a common pathogen in the urine and the infected joint [19, 28]. UTI and joint infection were considered dichotomous variables (presence versus absence). We used relative risk calculations (odds ratio) to assess the association between UTI and joint infection in the two groups. Statistical significance was set at a level of p = 0.05. All analyses were performed using SPSS® 12 for Windows® (SPSS Inc, Chicago, IL).
Results
We found no association (odds ratio, 0.747; 95% confidence interval, 0.258–2.163; p = 0.394) between the total rate of UTI and the rate of wound infection in the case and control groups (Table 3).
Table 3.
Patients with or without joint infections and with or without UTIs*
| UTI | Joint infection | No joint infection |
|---|---|---|
| Yes | 7 | 9 |
| No | 51 | 49 |
* Odds ratio for joint infection with UTI was 0.747 (95% confidence interval, 0.258–2.163; p = 0.394); UTI = urinary tract infection.
We found no association (odds ratio, 0.341; 95% confidence interval, 0.086–1.357; p = 0.127) between preoperative UTI and wound infection. Eight of the control subjects had preoperative UTIs, whereas three of the 58 patients with infections had preoperative UTIs and received antimicrobial therapy for 8 days before the surgery. These three patients had deep incisional infections develop within 1 month after the surgery and were admitted for débridement. The isolated bacteria from wound cultures were Staphylococcus aureus in two patients and Enterococcus in the third patient. The isolated bacteria from urine cultures were Citrobacter, β-hemolytic Streptococcus, and Escherichia coli (Table 2).
We also observed no association (odds ratio, 4.222; 95% confidence interval, 0.457–38.9; p = 0.204) between postoperative UTI and wound infection. In the control group, one patient had a postoperative UTI. Of the 58 patients with acute wound infections, four contracted UTIs after surgery (three deep incisional and one deep joint infection) (Table 2). The isolated bacteria from urine cultures in these four patients with acute wound infections were E. coli in two cases and Enterococcus faecalis in the other two. In one patient, the bacteria cultured from the wound and urine were the same (E. faecalis).
The patient who had the same bacteria cultured in the urine and the wound was an 80-year-old woman who had an uncomplicated primary THA. She was readmitted from a rehabilitation center on postoperative Day 20 with wound drainage. A clean-catch urinalysis had 15 to 20 leukocytes/high-power field and urine culture was positive for two organisms (E. faecalis and E. coli, > 100,000/mL). The next day, her joint fluid aspirate had 1150 leukocytes/mL and the culture was negative. On postoperative Day 30, irrigation and débridement of suprafascial tissues and local flap closure of her wound were performed. The deep fascia was intact, so the infection appeared to be isolated to suprafascial tissues. E. faecalis grew on two postoperative cultures with a similar sensitivity pattern as the urine isolate.
Discussion
Surgical wound infection is a serious and potentially catastrophic complication after joint arthroplasty [23, 26, 31]. Although prosthetic joint infection has decreased during the past few decades, it remains one of the major complications that may lead to prosthesis removal [10, 18]. One-third of infections arise as a result of hematogenous seeding of the joint during the first 2 years after the procedure [10, 16, 42]. UTI is a common hospital-acquired infection that creates a potential reservoir of resistant pathogens and increases patient morbidity [43, 45, 47, 50, 51]. We conducted this study to investigate the risk of perioperatively treated UTI as the remote source of wound infection in patients undergoing joint arthroplasty.
The major limitation of this study is the likelihood that some patients with wound infections were not identified owing to loss of followup at 1 year, which could have resulted in a wound infection rate greater than 0.29%. It is possible that patients with acute infections may have been treated at other hospitals and not identified in our study. Nevertheless, we believe we most likely identified most cases because patients are routinely followed by their surgeons, and because this is a tertiary care facility specializing in joint arthroplasty, and patients return here for care of complications. We also searched for cases of infection using four methods: (1) searching the hospital epidemiology files; (2) checking the database of all operative procedures likely to include infection (evacuation of hematoma, débridement of a wound, removal of an infected prosthesis, etc); (3) using the surveillance methods incorporated in at least a 1-year followup; and (4) reviewing all wound cultures from postoperative patients to be sure all patients were captured. We cannot comment on symptomatic UTIs that may have led to a delay in surgery. However, none of our patients with infections (or control subjects) had cancellation of surgery because of symptomatic UTI. The rate of postoperative wound infection was identified from readmission to the hospital. Finally, we excluded patients with superficial wound drainage who did not have positive culture specimen. Even with loss to followup representing a possible limitation of the study design, we believe this does not materially impact the finding that UTIs rarely, if ever, seed a recently implanted prosthesis.
Another limitation could be the fact that the difference between the groups may be the result of a Type II statistical error. However, joint infection is a rare condition (ie, only 58 cases from a sample of nearly 20,000 cases), which would impose practical obstacles in generating an adequate sample size. Therefore, we chose to study smaller samples, which necessarily meant low power. Our current sample would have a 25% power to detect a significant (p = 0.05) effect size. In other words, our study would have been able to detect a major effect only if the rate of urosepsis in the patients with joint infections was at least approximately 17% (ie, approximately 10% higher than that of the control subjects). Stated differently, to have an 80% power, we would need to have approximately 260 patients with joint infections and a similar number of control subjects (cases without joint infection). Extrapolating from our current sample, one would have to survey a data set of 80,000 to 100,000 cases to be able to detect such a number of joint infections. This is difficult given practical constraints.
The associations we found should be interpreted in the context of the given power (ie, it may mean either presence of major differences but not detected because of limited power or absence of a true difference). Motivated from this limitation, we attempted to investigate the actual microbes responsible for the urosepsis and joint infection; the fact that they were different is a strong argument in favor of the second interpretation rather than the first. Also, in the complete absence of relevant literature, even knowledge of the proportions of urosepsis in patients with joint infections and control subjects is of major interest.
We used the Centers for Disease Control and Prevention [19] criteria for classifying wound infection. Few studies adhere to these criteria [3, 30, 35, 37]. This requires 12-month followup and we were able to classify cases into deep and superficial infection based on the Centers for Disease Control and Prevention criteria. Some studies include only deep infection, which may underestimate the true incidence of wound infections. In addition, we used matched pairs from a randomized subset of the entire database, which enabled us to compare the risks of UTI in patients with and without wound infections. This is a large consecutive group of almost 20,000 patients and strict matching by age, surgeon, year of surgery, and joint in the control group was followed. Urinary catheter and UTI are reliably documented in the medical records and can be studied by a case-control methodology [25].
There are no clear guidelines to support either delaying or postponing joint arthroplasty because of the presence of bacteriuria with no systemic symptoms [2, 9, 13, 15]. There also are no prospective studies documenting any correlation between the preoperative bacteriuria and deep joint infection [1, 15, 36, 43] (Table 4). However, several case reports and small case series suggest an association between postoperative UTI and joint infection [7, 8, 17, 21, 28]. In these cases, the same pathogens were isolated in both infections. Most of the reports [7, 8, 17, 21, 28] did not specify whether prophylactic antibiotic therapy had been used.
Table 4.
Studies of UTI and infected joint arthroplasties
| Study | Prospective | CDC criteria | Length of followup | Interval between UTI and joint infection | Control groups | UTI rate | Antibiotics for UTI |
|---|---|---|---|---|---|---|---|
| Maderazo et al. [28] | Preoperative 21% (same phage urine-joint) | ||||||
| Wroblewski and del Sel [52] | 2.1 years | No UTI only catheter preoperatively (15–36 months) | 6.2% (no phage typing done) | Yes | |||
| Schmalzried et al. [38] | 61 months | NA | Yes | ||||
| Cruess et al. [7] | Case report | 2 years | 4 weeks | No phage typing done | Yes | ||
| Peersman et al. [33] | 3 months | Yes; UTI not recorded | 10% (no phage typing done) | ||||
| Hall [17] | Case report | 4 years | 3 weeks | Phage typing done | Yes | ||
| Poss et al. [34] | 3.5 years | NA | 11% (phage typing was made) | Yes | |||
| Fitzgerald et al. [13] | Yes | 2 years | 3 months | No correlation was made | NA | ||
| Donovan et al. [11] | 359 patients retrospective; 67 patients prospective | 3 months? | 3.6% in retrospective; 2.9% in prospective; one deep infection; no phage typing done | NA | |||
| D`Ambrosia et al. [8] | Case report | 4 weeks | Phage typing done from UTI and decubitus ulcer | NA | |||
| Glynn and Sheehan [15] | No correlation between bacteriuria and deep infection | Yes | |||||
| Hunter and Dandy [20] | NA | NA | Rate? Phage typing done | NA | |||
| Surin et al. [46] | 3–10 years | 11%; no correlation with deep infection | NA | ||||
| Berbari et al. [3] | Yes | NA | Yes | No association with deep infection | NA | ||
| Saleh et al. [37] | Yes | 30 days | Yes | No associations | NA | ||
| Irvine et al. [21] | NA | 5 UTIs; in 3, phage typing done | NA | ||||
| Kaandorp et al. [22] | 7 UTIs in 188 deep infections; 5 patients were immunocom-promised | NA | |||||
| Ainscow and Denham [1] | Yes | 6 years | NA | 11%; no joint infection developed | |||
| Krijnen et al. [24] | Yes | 3 years | 2 UTIs in 37; deep infections in 4907 patients |
UTI = urinary tract infection; CDC = Centers for Disease Control and Prevention; NA = not available.
In a study using strict definitions of patients with infections and control subjects and with standardized prospective surveillance methods, perioperative UTI was not identified as a potential risk factor for prosthetic joint infection [3]. Also, other authors reported results from a 20-year surveillance program for the predictors of wound infection after joint arthroplasty [37]. No causal relationship was determined between the UTI and the prosthetic joint infection. In a prospective study of 1497 newly catheterized patients, 235 acquired a UTI yet only one patient had sepsis develop [47]. Furthermore, in a randomized study of 100 patients who were catheterized after joint arthroplasty, there was no increase in the rate of UTI and no incidence of wound infection [29]. Sharrock and Finerty [41] reported, in a population of 2621 patients who routinely were catheterized after the joint arthroplasty, there were 23 UTIs and three joint infections. The bacteria isolated from the joint were not urinary tract pathogens [41].
We designed a case-control study to compare the rate of UTIs in patients with joint infection and in patients without joint infection. We found no association between UTI and joint sepsis after joint arthroplasty. We found only one of 19,735 patients (0.005%) who had a UTI and wound infection develop and the pathogen was the same for both infections. This patient had a postoperative UTI. No association was determined between the preoperative or postoperative UTI and wound infection in patients with infections and control subjects. It theoretically is possible a pathogen from the urinary tract would cause bacteremia and seed the hip without causing UTI. However, in the majority (80%), all wound infections were not enteric pathogens. In addition, it is possible a UTI could influence the patient’s immune system predisposing the patient to wound infection from another source. However, we found no evidence for this from the control subjects matched with the patients with infections.
Our data suggested no clear evidence between postoperative UTI and joint infection, which is consistent with the literature [22, 24, 36, 41]. The majority of bacteria we observed were not enteric pathogens. Furthermore, identical bacteria were not isolated in both locations. The rate of joint infections in our patients is 0.29%. In the literature, the rates of joint infection range from 0.38% to 2.3% [28, 35, 39]. These data suggest treated UTI is a minimal risk factor for prosthetic joint infection.
Acknowledgments
We thank Dr. Scarmea for his valuable help with the statistics of this study.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Ainscow DA, Denham RA. The risk of haematogenous infection in total joint replacements. J Bone Joint Surg Br. 1984;66:580–582. [DOI] [PubMed]
- 2.American Urological Association; American Academy of Orthopaedic Surgeons. Antibiotic prophylaxis for urological patients with total joint replacements. J Urol. 2003;169:1796–1797. [DOI] [PubMed]
- 3.Berbari EF, Hanssen AD, Duffy MC, Steckelberg JM, Ilstrup DM, Harmsen WS, Osmon DR. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis. 1998;27:1247–1254. [DOI] [PubMed]
- 4.Bozic KJ, Ries MD. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg Am. 2005;87:1746–1751. [DOI] [PubMed]
- 5.Burke JP. Infection control: a problem for patient safety. N Engl J Med. 2003;348:651–656. [DOI] [PubMed]
- 6.Crockarell JR, Hanssen AD, Osmon DR, Morrey BF. Treatment of infection with debridement and retention of the components following hip arthroplasty. J Bone Joint Surg Am. 1998;80:1306–1313. [DOI] [PubMed]
- 7.Cruess RL, Bickel WS, vonKessler KL. Infections in total hips secondary to a primary source elsewhere. Clin Orthop Relat Res. 1975;106:99–101. [DOI] [PubMed]
- 8.D’Ambrosia RD, Shoji H, Heater R. Secondarily infected total joint replacements by hematogenous spread. J Bone Joint Surg Am. 1976;58:450–453. [PubMed]
- 9.David TS, Vrahas MS. Perioperative lower urinary tract infections and deep sepsis in patients undergoing total joint arthroplasty. J Am Acad Orthop Surg. 2000;8:66–74. [DOI] [PubMed]
- 10.Deacon JM, Pagliaro AJ, Zelicof SB, Horowitz HW. Prophylactic use of antibiotics for procedures after total joint replacement. J Bone Joint Surg Am. 1996;78:1755–1770. [DOI] [PubMed]
- 11.Donovan TL, Gordon RO, Nagel DA. Urinary infections in total hip arthroplasty: influences of prophylactic cephalosporins and catheterization. J Bone Joint Surg Am. 1976;58:1134–1137. [PubMed]
- 12.Douglas P, Asimus M, Swan J, Spigelman A. Prevention of orthopaedic wound infections: a quality improvement project. J Qual Clin Pract. 2001;21:149–153. [DOI] [PubMed]
- 13.Fitzgerald RH Jr, Nolan DR, Ilstrup DM, Van Scoy RE, Washington JA 2nd, Coventry MB. Deep wound sepsis following total hip arthroplasty. J Bone Joint Surg Am. 1977;59:847–855. [PubMed]
- 14.Gaine WJ, Ramamohan NA, Hussein NA, Hullin MG, McCreath SW. Wound infection in hip and knee arthroplasty. J Bone Joint Surg Br. 2000;82:561–565. [DOI] [PubMed]
- 15.Glynn MK, Sheehan JM. The significance of asymptomatic bacteriuria in patients undergoing hip/knee arthroplasty. Clin Orthop Relat Res. 1984;185:151–154. [PubMed]
- 16.Grogan TJ, Dorey F, Rollins J, Amstutz HC. Deep sepsis following total knee arthroplasty: ten-year experience at the University of California at Los Angeles Medical Center. J Bone Joint Surg Am. 1986;68:226–234. [PubMed]
- 17.Hall AJ. Late infection about a total knee prosthesis: report of a case secondary to urinary tract infection. J Bone Joint Surg Br. 1974;56:144–147. [PubMed]
- 18.Hanssen AD, Osmon DR, Nelson C. Prevention of deep periprosthetic joint infection. J Bone Joint Surg Am. 1996;78:458–471. [PubMed]
- 19.Horan TC. Surveillance of nosocomial infections. In: Mayhall CG, ed. Hospital Epidemiology and Infection Control. 3rd ed. Philadelphia, PA: Williams & Wilkins; 2004.
- 20.Hunter G, Dandy D. The natural history of the patient with an infected total hip replacement. J Bone Joint Surg Br. 1977;59:293–297. [DOI] [PubMed]
- 21.Irvine R, Johnson BL Jr, Amstutz HC. The relationship of genitourinary tract procedures and deep sepsis after total hip replacements. Surg Gynecol Obstet. 1974;139:701–706. [PubMed]
- 22.Kaandorp CJ, Dinant HJ, van de Laar MA, Moens HJ, Prins AP, Dijkmans BA. Incidence and sources of native and prosthetic joint infection: a community based prospective survey. Ann Rheum Dis. 1997;56:470–475. [DOI] [PMC free article] [PubMed]
- 23.Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725–730. [DOI] [PubMed]
- 24.Krijnen P, Kaandorp CJ, Steyerberg EW, van Schaardenburg D, Moens HJ, Habbema JD. Antibiotic prophylaxis for haematogenous bacterial arthritis in patients with joint disease: a cost effectiveness analysis. Ann Rheum Dis. 2001;60:359–366. [DOI] [PMC free article] [PubMed]
- 25.Kritchevsky SB, Braun BI, Wong ES, Solomon SL, Steele L, Richards C, Simmons BP. Impact of hospital care on incidence of bloodstream infection: the evaluation of processes and indicators in infection control study. Emerg Infect Dis. 2001;7:193–196. [DOI] [PMC free article] [PubMed]
- 26.Leape LL, Brennan TA, Laird N, Lawthers AG, Localio AR, Barnes BA, Hebert L, Newhouse JP, Weiler PC, Hiatt H. The nature of adverse events in hospitalized patients: results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324:377–384. [DOI] [PubMed]
- 27.Lentino JR. Prosthetic joint infections: bane of orthopedists, challenge for infectious disease specialists. Clin Infect Dis. 2003;36:1157–1161. [DOI] [PubMed]
- 28.Maderazo EG, Judson S, Pasternak H. Late infections of total joint prostheses: a review and recommendations for prevention. Clin Orthop Relat Res. 1988;229:131–142. [PubMed]
- 29.Michelson JD, Lotke PA, Steinberg ME. Urinary-bladder management after total joint-replacement surgery. N Engl J Med. 1988;319:321–326. [DOI] [PubMed]
- 30.Minnema B, Vearncombe M, Augustin A, Gollish J, Simor AE. Risk factors for surgical-site infection following primary total knee arthroplasty. Infect Control Hosp Epidemiol. 2004;25:477–480. [DOI] [PubMed]
- 31.Nichols RL. Preventing surgical site infections: a surgeon’s perspective. Emerg Infect Dis. 2001;7:220–224. [DOI] [PMC free article] [PubMed]
- 32.Olson ES, Cookson BD. Do antimicrobials have a role in preventing septicaemia following instrumentation of the urinary tract? J Hosp Infect. 2000;45:85–97. [DOI] [PubMed]
- 33.Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;392:15–23. [DOI] [PubMed]
- 34.Poss R, Thornhill TS, Ewald FC, Thomas WH, Batte NJ, Sledge CB. Factors influencing the incidence and outcome of infection following total joint arthroplasty. Clin Orthop Relat Res. 1984;182:117–126. [PubMed]
- 35.Ridgeway S, Wilson J, Charlet A, Kafatos G, Pearson A, Coello R. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87:844–850. [DOI] [PubMed]
- 36.Ritter MA, Fechtman RW. Urinary tract sequelae: possible influence on joint infections following total joint replacement. Orthopedics. 1987;10:467–469. [DOI] [PubMed]
- 37.Saleh K, Olson M, Resig S, Bershadsky B, Kuskowski M, Gioe T, Robinson H, Schmidt R, McElfresh E. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res. 2002;20:506–515. [DOI] [PubMed]
- 38.Schmalzried TP, Amstutz HC, Au MK, Dorey FJ. Etiology of deep sepsis in total hip arthroplasty: the significance of hematogenous and recurrent infections. Clin Orthop Relat Res. 1992;280:200–207. [PubMed]
- 39.Schutzer SF, Harris WH. Deep-wound infection after total hip replacement under contemporary aseptic conditions. J Bone Joint Surg Am. 1988;70:724–727. [PubMed]
- 40.Sculco TP. The economic impact of infected joint arthroplasty. Orthopedics. 1995;18:871–873. [PubMed]
- 41.Sharrock NE, Finerty E. Hip replacement, hip seeding, and epidural anaesthesia. Lancet. 2005;365:1011–1012. [DOI] [PubMed]
- 42.Sia IG, Berbari EF, Karchmer AW. Prosthetic joint infections. Infect Dis Clin North Am. 2005;19:885–914. [DOI] [PubMed]
- 43.Spelman DW. Hospital-acquired infections. Med J Aust. 2002;176:286–291. [DOI] [PubMed]
- 44.Stamm WE. Catheter-associated urinary tract infections: epidemiology, pathogenesis, and prevention. Am J Med. 1991;91:65S–71S. [DOI] [PubMed]
- 45.Stephan F, Sax H, Wachsmuth M, Hoffmeyer P, Clergue F, Pittet D. Reduction of urinary tract infection and antibiotic use after surgery: a controlled, prospective, before-after intervention study. Clin Infect Dis. 2006;42:1544–1551. [DOI] [PubMed]
- 46.Surin VV, Sundholm K, Backman L. Infection after total hip replacement: with special reference to a discharge from the wound. J Bone Joint Surg Br. 1983;65:412–418. [DOI] [PubMed]
- 47.Tambyah PA, Knasinski V, Maki DG. The direct costs of nosocomial catheter-associated urinary tract infection in the era of managed care. Infect Control Hosp Epidemiol. 2002;23:27–31. [DOI] [PubMed]
- 48.Thomas C, Cadwallader HL, Riley TV. Surgical-site infections after orthopaedic surgery: statewide surveillance using linked administrative databases. J Hosp Infect. 2004;57:25–30. [DOI] [PubMed]
- 49.Ure KJ, Amstutz HC, Nasser S, Schmalzried TP. Direct-exchange arthroplasty for the treatment of infection after total hip replacement: an average ten-year follow-up. J Bone Joint Surg Am. 1998;80:961–968. [DOI] [PubMed]
- 50.Warren JW. The catheter and urinary tract infection. Med Clin North Am. 1991;75:481–493. [DOI] [PubMed]
- 51.Warren JW. Catheter-associated urinary tract infections. Infect Dis Clin North Am. 1997;11:609–622. [DOI] [PubMed]
- 52.Wroblewski BM, del Sel HJ. Urethral instrumentation and deep sepsis in total hip replacement. Clin Orthop Relat Res. 1980;146:209–212. [PubMed]