Abstract
Warfarin dosing algorithms do not account for genetic mutations that can affect anticoagulation response. We retrospectively assessed to what extent the VKORC1 variant genotype would alter the likelihood of being a hyperresponder or hyporesponder to warfarin in patients undergoing total joint arthroplasty. We used the international normalized ratio (INR) on the third postoperative day of 3.0 or greater to define warfarin hyperresponders and 1.07 or less to define hyporesponders. A control group of normal responders was identified. From a cohort of 1125 patients receiving warfarin thromboprophylaxis, we identified 30 free of predisposing factors that could affect warfarin response: 10 hyperresponders, eight hyporesponders, and 12 normal responders. Homozygous carriers of the VKORC1 mutant AA genotype were more likely (compared with carriers of GA or GG genotypes) to be hyperresponders (odds ratio, 7.5; 95% confidence interval, 1.04–54.1). Homozygous carriers of the GG (normal) genotype were more likely (compared with carriers of AA or GA genotypes) to be hyporesponders (odds ratio, 9; 95% confidence interval, 1.14–71). Preoperative screening for the VKORC-1 genotype could identify patients with a greater potential for being a hyperresponder or hyporesponder to warfarin. This may allow an adjusted pharmacogenetic-based warfarin dose to optimize anticoagulation, reducing postoperative risks of bleeding and thrombosis or embolism.
Level of Evidence: Level III, diagnostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Pharmacologic thromboprophylaxis with warfarin is commonly used after THA and TKA [11]. A survey of the members of the American Association of Hip and Knee Surgeons in 2001 revealed warfarin was the most frequent form of pharmacologic thromboprophylaxis used at that time in 66% of THAs and 59% of TKAs [22]. Large individual dosage variations of warfarin are required to achieve prophylactic and therapeutic ranges. Because of warfarin’s low therapeutic index and frequent complications of prophylaxis and/or therapy [17], the INR must be measured regularly and dosage constantly adjusted for the duration of postoperative pharmacologic thromboprophylaxis. Excessive anticoagulation can lead to major local or systemic bleeding [16], which can be fatal [17, 23]. There is a greater probability of thrombosis or embolism if patients do not receive optimal anticoagulation [3, 6].
Patient-specific factors such as advanced age, low body mass index (BMI), Asian race, diet, certain comorbidities, and medications alter the response to warfarin (predisposing factors) [14, 23, 42]. Recent investigations have focused on genetic determinants that alter the expression or function of enzymes involved in vitamin K and warfarin metabolism and action [18, 41]. These mutations further explain interindividual variations in warfarin response. Warfarin inhibits the posttranslational carboxylation of glutamate residues on proteins dependent on vitamin K [9]. This carboxylation requires the reduced form of vitamin K. Warfarin inhibits vitamin K epoxide reductase (VKOR), the enzyme responsible for recycling of vitamin K. Mutations of the gene VKORC1, that codifies for warfarin’s target in the liver, the enzyme VKOR, have been associated with warfarin resistance or hyperresponse [7]. VKOR reduces vitamin K, which is required for synthesis of clotting factors II, VII, IX, and X and protein C and protein S [24]. Warfarin inhibits VKOR, thus impairing the synthesis of clotting factors. The gene for VKOR was mapped to human chromosome 16p12-q21 [9]. A specific target site of warfarin in VKOR was identified as subunit 1 (VKORC1) [34, 35]. VKORC1 has approximately 4 kb, consists of three exons, and encodes a 163-amino acid enzyme located in the endoplasmic reticulum (NP_076869). Recent studies reported genetic variants of VKORC1 affect warfarin response and dosing [7, 15, 18, 32]. Different mutations (missense mutation, synonymous mutations, and polymorphism) in the VKORC1 gene alleles have been identified [7]. Homozygous or heterozygous forms of polymorphism in the alleles affect warfarin anticoagulant activity after standardized dosage (GG, normal; GA, heterozygous; AA, homozygous). Genotypic analysis of VKORC1 was checked for polymorphism in alleles. Genotype-based frequencies of the AA mutation have been estimated to be 11%, GA heterozygosity 40%, and GG, wild-type normal, 49% [24].
We asked to what extent the VKORC1 variant genotype altered the likelihood of being a hyperresponder or hyporesponder to warfarin in patients undergoing total joint arthroplasty (TJA) who had a supratherapeutic or subtherapeutic INR develop without known clinical or medication predisposing factors [14, 42].
Materials and Methods
We retrospectively reviewed 3529 patients having THAs or TKAs and found 1125 (466 THAs [41.4%], 659 TKAs [58.6%]) received warfarin as pharmacologic thromboprophylaxis in the context of a multimodal prophylaxis protocol [4, 12, 37] from July 1, 2006, to March 31, 2007. The multimodal prophylaxis [12] protocol includes preoperative discontinuation of procoagulant medications, autologous blood donation, hypotensive epidural anesthesia, intravenous heparin before femoral preparation in THA, lavage and aspiration of intramedullary contents, pneumatic compression, knee-high elastic stockings, early mobilization, and chemoprophylaxis for 4 to 6 weeks. The study was approved by the Institutional Review Board of our institution and was performed with signed informed consent.
We administered the first dose of warfarin, 5 mg [2], the night of the day of surgery with the goal of achieving and maintaining an INR of 2 by the third postoperative day. A standardized algorithm was used for warfarin dosing on the first and second postoperative days [2]. Warfarin effect and dosing were determined by daily INR monitoring during the hospital stay and by the medical doctor after discharge.
We used a previous study of 219 serially operated patients who had TJAs [2] to establish third postoperative day INR percentiles and, from these percentiles, to characterize response categories for the current study. In these 219 patients, the 95th percentile for third postoperative day INR was 3 and the 5th percentile was 1.07; in the current study, we therefore defined hyperresponders by an INR of 3 or greater and hyporesponders by an INR of 1.07 or less.
Patients with a history of liver disease, irritable bowel syndrome, inflammatory bowel disease, or any clinical condition or medications that are known to alter the response to warfarin [13] were excluded. These exclusions provided a cohort in which potential effects of the VKORC1 mutation might be understood in the absence of clinical and environmental factors that would substantially affect INR. In the 1125 patients undergoing TJA, there were 64 hyperresponders, but only 10 (16%) had no predisposing factors that could alter the warfarin response. There were 44 hyporesponders, but only eight (18%) had no predisposing factors that could alter the warfarin response. Thus, the study group consisted of 18 patients: eight hyporesponders and 10 hyperresponders (Tables 1, 2).
Table 1.
Patient demographics
| Characteristic | Hyperresponders (n = 10) | Hyporesponders (n = 8) | Control subjects (n = 12) | Group differences |
|---|---|---|---|---|
| Age (years)* | 64 (29–86) | 59 (45–68) | 70 (38–87) | Hyporesponders were younger than control subjects (p = 0.025) |
| Gender (female:male) | 9:1 | 3:5 | 10:2 | Hyperresponder group had more women than hyporesponders (p = 0.043) |
| Body mass index* | 28 (19–40) | 26 (22–36) | 28 (24–48) | p > 0.05 |
| Ethnicity (US white:Hispanic) | 9:1 | 6:2 | 11:1 | p > 0.05 |
| THA:TKA | 6:4 | 5:3 | 4:8 | p > 0.05 |
*Values expressed as means, with ranges in parentheses.
Table 2.
Patients’ PCR results and INR data
| Group/Patient | INR POD1 | INR POD2 | INR POD3 | Race | Surgery | VKORC1 |
|---|---|---|---|---|---|---|
| Hyperresponder | ||||||
| 1 | 0.97 | 1.36 | 3.99 | US white | TKA | AA |
| 2 | 1.10 | 2.45 | 3.48 | US white | THA | AA |
| 3 | 1.12 | 1.68 | 7.61 | Hispanic | TKA | GA |
| 4 | 1.12 | 2.47 | 3.52 | US white | TKA | AA |
| 5 | 1.14 | 4.81 | 5.46 | US white | TKA | GA |
| 6 | 1.21 | 3.06 | 5.18 | US white | THA | AA |
| 7 | 1.07 | 1.64 | 3.17 | US white | RTHA | AA |
| 8 | 1.13 | 2.43 | 3.29 | US white | THA | GA |
| 9 | 1.19 | 1.79 | 4.17 | US white | THA | GG |
| 10 | 1.05 | 2.16 | 3.02 | US white | THA | AA |
| Group mean ± SD | 1.11 ± 0.07 | 2.39 ± 0.99 | 3.96 ± 0.78 | |||
| Hyporesponder | ||||||
| 1 | 1.00 | 1.00 | 0.97 | Hispanic | TKA | GG |
| 2 | 1.02 | 1.04 | 0.99 | US white | TKA | GG |
| 3 | 1.18 | 1.27 | 1.04 | US white | THA | GA |
| 4 | 1.04 | 1.13 | 1.07 | US white | THA | GG |
| 5 | 0.99 | 1.00 | 0.98 | US white | TKA | GG |
| 6 | 1.08 | 1.02 | 0.95 | US white | THA | GG |
| 7 | 1.04 | 1.14 | 0.99 | Hispanic | THA | GA |
| 8 | 1.05 | 1.21 | 1.06 | US white | RTHA | GG |
| Group mean ± SD | 1.05 ± 0.05 | 1.12 ± 0.10 | 1.05 ± 0.08 | |||
| Control | ||||||
| 1 | 1.03 | 1.57 | 1.60 | US white | TKA | GG |
| 2 | 1.70 | 1.59 | 1.64 | US white | THA | GA |
| 3 | 1.05 | 1.50 | 1.52 | US white | TKA | GA |
| 4 | 1.10 | 1.78 | 1.54 | US white | TKA | GA |
| 5 | 1.09 | 1.46 | 1.72 | US white | RTHA | AA |
| 6 | 1.15 | 1.47 | 1.87 | US white | THA | GA |
| 7 | 1.36 | 1.45 | 1.85 | Hispanic | TKA | GA |
| 8 | 1.04 | 1.38 | 1.60 | US white | TKA | GG |
| 9 | 1.03 | 1.60 | 2.01 | US white | TKA | AA |
| 10 | 1.10 | 1.64 | 1.63 | US white | TKA | GA |
| 11 | 1.15 | 1.64 | 1.56 | US white | THA | GA |
| 12 | 1.19 | 1.77 | 1.73 | US white | TKA | GG |
| Group mean ± SD | 1.17 ± 0.19 | 1.57 ± 0.13 | 1.63 ± 0.22 | |||
INR = international normalized ratio; POD1, 2, 3 = postoperative days 1, 2, and 3; RTHA = revision of previous THA; SD = standard deviation.
Normal responders were arbitrarily identified by INRs of 1.51 to 2.10, which were the 50th and 85th percentiles of the distribution of the previously studied 219 subjects, with a third postoperative day INR of 2 as the clinical goal [2]. Using these cutoffs, from the cohort of 3529 patients who had TJAs, there were 410 normal responders and 12 (3%) were used as control subjects for hyporesponders and hyperresponders (Tables 1, 2).
Hyperresponder, hyporesponder, and control groups were similar (p > 0.05) in the three major matching criteria: BMI, ethnicity, and surgical procedure (Table 1). Hyporesponders were younger (p = 0.025) than control subjects, and there were more (p = 0.043) women in the hyperresponder group than in the hyporesponder group (Table 1).
Assuming the VKORC1 mutation accounted for a 25% ± 20% difference in INR response to warfarin [7, 33, 41], with alpha = 0.05 and beta = 0.2, we estimated 12 patients with at least one A allele and 12 with at least one G allele would be needed to discern a major group difference. The selection of 12 control subjects was dictated by the power analysis. We considered this study a nested case-control study and calculated the odds ratio for the hyperresponse or hyporesponse (Table 2).
We collected peripheral blood for genetic testing of VKORC1 mutations by the Molecular Diagnostics Laboratories (Cincinnati, OH). DNA was analyzed for the G/A polymorphism of the VKORC1 gene at −1639 in the promoter region of the VKORC1 using the Roche Diagnostics LightCycler® (Roche Applied Science, Indianapolis, IN) and hybridization probes. Nomenclature for the VKORC1 genotypes is as follows: GG = wild-type normal, GA = heterozygous, and AA = homozygous.
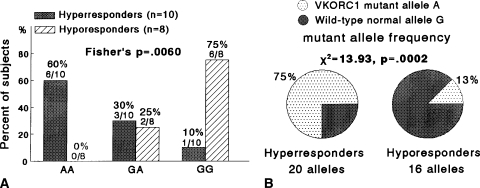
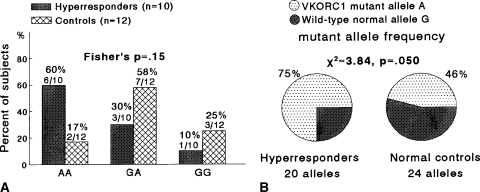
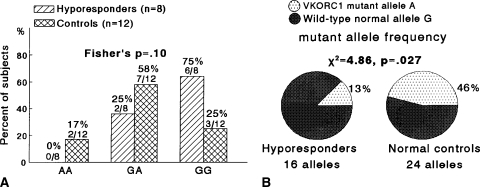
We used Fisher’s exact tests to compare the distributions of AA, AG, and GG genotypes in two by three tables in hyperresponders versus hyporesponders (Fig. 1), in hyperresponders versus control subjects (Fig. 2), and in hyporesponders versus control subjects (Fig. 3). Fisher’s exact test was used because Chi square may not be a valid test when some cells had expected counts less than five. Chi square tests in two by two tables (with 1-degree-of-freedom) were used to compareVKORC1 mutant A allele frequencies and wild-type normal G allele frequencies in hyperresponders versus hyporesponders (Fig. 1B), in hyperresponders versus control subjects (Fig. 2B), and in hyporesponders versus controls (Fig. 3B). Odds ratios were calculated to assess the extent VKORC1 variant genotypes altered patients’ odds of being hyperresponders or hyporeactive to warfarin (Table 3).
Fig. 1A–B.
(A) The distribution of the VKORC1 mutant allele A and wild-type normal allele G differed between the groups. Hyperresponders were more likely to have AA homozygosity and hyporesponders were more likely to have GG homozygosity (Fisher’s p = 0.006). (B) The VKORC1 mutant allele A frequency was greater in hyperresponders versus hyporesponders (p = 0.0002).
Fig. 2A–B.
(A) The distribution of the VKORC1 mutant allele A and wild-type normal allele G did not differ (Fisher’s p = 0.15), comparing hyperresponders versus control subjects. (B) The VKORC1 mutant allele A frequency was greater (p = 0.05) in hyperresponders than in control subjects.
Fig. 3A–B.
(A) The distribution of the VKORC1 mutant allele A and wild-type normal allele G did not differ (Fisher’s p = 0.10), comparing hyporesponders versus control subjects. (B) The VKORC1 mutant allele A frequency was less in hyporesponders than in control subjects (p = 0.027).
Table 3.
Extent of alteration attributable to VKORC1 variant genotype
| Group | AA | GA + GG | Odds ratio | 95% confidence interval |
|---|---|---|---|---|
| Hyperresponder | 6 | 4 | 7.5 | 1.04–54.1 |
| Control | 2 | 10 | ||
| Hyporesponder | 0 | 8 | * | * |
| Control | 2 | 10 | ||
| Group | GG | AA + GA | Odds ratio | 95% confidence interval |
| Hyporesponder | 6 | 2 | 9 | 1.14–71.0 |
| Control | 3 | 9 |
VKORC1 genotypes: GG = normal; GA = heterozygous; AA = homozygous; *cannot be calculated because one cell = 0.
Results
Compared with carriers of the GG or GA genotypes, carriers of the VKORC1 AA genotype were more likely (odds ratio, 7.5; 95% confidence interval, 1.04–54.1) to be hyperresponders (Table 3). Compared with carriers of the AA or GA genotypes, carriers of the VKORC1 GG genotype were more likely (odds ratio, 9; 95% confidence interval,1.14–71.0) to be hyporesponders (Table 3).
When comparing hyperresponders versus hyporesponders, the VKORC1 mutant allele A frequency was greater (p = 0.0002) in hyperresponders and the distribution of the mutant AA and wild-type normal GG homozygosity differed, with hyperresponders more likely to have AA homozygosity and hyporesponders more likely to have GG homozygosity (Fisher’s p = 0.006) (Fig. 1). Comparing hyperresponders with normal control subjects, the VKORC1 mutant allele A frequency was greater (p = 0.05) in hyperresponders (Fig. 2). Hyporesponders had a lower (p = 0.027) VKORC1 mutant allele frequency than normal control subjects (Fig. 3).
Discussion
Warfarin dosing algorithms do not account for genetic mutations that can affect anticoagulation response. We hypothesized, in patients undergoing TJA and receiving warfarin thromboprophylaxis, the VKORC1 mutant A allele would alter the patients’ odds of being a hyperresponder or hyporesponder to warfarin.
Study limitations include imprecise effect measures related to small sample size, and clinical relevance limited by the retrospective study design versus a prospective study [24] in which preoperative VKCORC1 testing might optimize warfarin dosing, possibly reducing the risk of thrombosis in hyporesponders and bleeding in hyperresponders. A large percentage of patients having TJAs, 54 of 64 (90%) hyperresponders and 41 of 49 (82%) hyporesponders, had known predisposing clinical or pharmacologic factors [14, 42] that could affect the response to warfarin. Thus, a large percentage of patients would be helped if their known clinical factors [14, 42] were addressed before initiation of warfarin prophylaxis. The biology of coagulation is extremely complex and other genetic mutations, including mutations in vitamin K-dependent proteins such as factors II and VII, also can alter the warfarin response. We did, however, strictly select patients without clinical factors that predispose to variability in response to warfarin and therefore believe the data allow one to determine in a preliminary way the ability to assess the extent to which VKORC1 variant genotypes alter the patients’ odds of being hyperresponsive or hyporesponsive to warfarin. Given the preliminary nature of our study, we estimate 75 hyperresponders and 66 hyporesponders without exclusions for predisposing factors that could alter the warfarin response would be required to made definitive statements regarding the extent to which VKORC1 variant genotypes alter patients’ odds of being hyperresponsive or hyporesponsive to warfarin.
Despite the use of standardized nomograms (without PCR measures of VKORC1 mutations) to guide warfarin dosing after TJA [2], in a previous study, 106 patients received postoperative warfarin thromboprophylaxis at a dose determined by a nomogram that did not include genetic variables. Five of the 106 patients had a supraprophylactic INR (> 3.0, > 2 standard deviations above the mean) and seven had a subprophylactic INR (< 1.1, > 2 standard deviations below the mean) develop. This observation, coupled with advancements in the understanding of genetic determinants of warfarin response [7, 15, 18, 30–32, 34, 35, 39, 41, 43], led us to hypothesize VKORC1 variant genotypes could alter the warfarin response in patients undergoing TJA.
When entered into the algorithm of Sconce et al. [39], PCR results for VKORC1 provide a pharmacogenetic-based warfarin dose, which should optimize anticoagulation of patients undergoing TJA, reducing the risk of bleeding and thrombosis or embolism. In this frame of reference, the US Food and Drug Administration [8] recently approved updated labeling for warfarin to highlight “…the opportunity for healthcare providers to use genetic tests to improve their initial estimate of what is a reasonable warfarin dose for individual patients.”
Investigations evaluating the association among VKORC1 mutations, responsiveness to warfarin, and bleeding complications have focused predominantly on medical rather than surgical patients [7, 15, 18, 30–32, 34, 35, 39, 41, 43]. Postoperative patients who have undergone TJA are a different population from medical patients because thrombosis is promoted by the operative procedure, and postoperative risks of major bleeding and thrombosis or embolism [10, 25] are of utmost concern [5, 27, 29, 36, 38]. In large series of patients having TJAs who received warfarin thromboprophylaxis without preoperative VKORC1 or CYP2C9/CYP3C9 testing, bleeding complications ranged from 2.3% to 4.1% [1, 19, 20, 28]. The prevalence of these bleeding complications was as high as the reported incidence of venous thrombosis or pulmonary embolism warfarin was intended to prevent.
Millican et al. [24] studied 92 patients undergoing primary or revision THAs and TKAs with warfarin thromboprophylaxis. For each patient, the authors prospectively collected blood samples, clinical variables, current medications, and preoperative and postoperative laboratory values. Polymorphisms in the VKORC1 and CYP2C9 genes were determined. The VKORC1 haplotype A, CYP2C9*2, and CYP2C9*3 mutations predicted altered warfarin response. Millican et al. [24] reported clinical dosage algorithms could explain 51% to 60% of INR variability. However, when they included genetic testing for VKORC1 and CYP2C9, they could account for 80% of the INR variability, with the genetic testing explaining 20% of the INR variability. Millican et al. [24] suggested large clinical trials be done before recommending routine genetic testing. Using stepwise regression analysis, Millican et al. [24] developed an algorithm that allows warfarin dose adjustment after the third postoperative day. This algorithm requires clinical validation.
The number of TJAs performed each year in the United States continues to increase [21], and the majority of patients receive warfarin for postoperative thromboprophylaxis [22] despite its narrow range of therapeutic efficacy and a perplexing tendency for the INR to oscillate in and out of the therapeutic target range. Effective prophylactic anticoagulation occurs only after a few days of administration, and there is no protection during the immediate postoperative period when thrombogenesis is maximally activated [40]. The difficulty in achieving a therapeutic INR is emphasized by our study in which on the third postoperative day, 64 (5.6% of 1125) hyperresponders were overanticoagulated (mean INR, 3.96) and at risk of bleeding. Conversely, 44 (3.9% of 1125) hyporesponders were underanticoagulated (mean INR, 1.05) and unprotected from thromboembolic complications. Although our study of Caucasian and New World Hispanic patients suggested a mutant VKORC1 allele has relevance to a warfarin-dosing algorithm, similar relevance may not pertain to other gene pools, including those of African-Americans [26].
Currently, pharmacogenetic tests are costly and rarely available, but with increased test volumes, costs will decrease and availability will expand. However, the total cost of these PCR analyses must be considered in relation to the potential cost and adverse outcome of bleeding and thromboembolic complications.
We found the mutant VKORC1 allele was more prevalent in patients who were hyperresponders to warfarin than in the control group. It was less prevalent in the hyporesponders than in the control group and hyperresponders. Preoperative identification of VKORC1 mutations may help physicians adjust warfarin dosing to reduce the extremes of variable response to postoperative warfarin prophylaxis.
Footnotes
One or more of the authors (EAS, AGDV, CJG) have received funding from a Knee Society grant.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Amstutz HC, Friscia DA, Dorey F, Carney BT. Warfarin prophylaxis to prevent mortality from pulmonary embolism after total hip replacement. J Bone Joint Surg Am. 1989;71:321–326. [PubMed]
- 2.Asnis PD, Gardner MJ, Ranawat A, Leitzes AH, Peterson MG, Bass AR. The effectiveness of warfarin dosing from a nomogram compared with house staff dosing. J Arthroplasty. 2007;22:213–218. [DOI] [PubMed]
- 3.Beksac B, Gonzalez Della Valle A, Anderson J, Sharrock NE, Sculco TP, Salvati EA. Symptomatic thromboembolism after one-stage bilateral THA with a multimodal prophylaxis protocol. Clin Orthop Relat Res. 2007;463:114–119. [PubMed]
- 4.Beksac B, Gonzalez Della Valle A, Salvati EA. Thromboembolic disease after total hip arthroplasty: who is at risk? Clin Orthop Relat Res. 2006;453:211–224. [DOI] [PubMed]
- 5.Butt AJ, McCarthy T, Kelly IP, Glynn T, McCoy G. Sciatic nerve palsy secondary to postoperative haematoma in primary total hip replacement. J Bone Joint Surg Br. 2005;87:1465–1467. [DOI] [PubMed]
- 6.Caprini JA, Arcelus JI, Motykie G, Kudrna JC, Mokhtee D, Reyna JJ. The influence of oral anticoagulation therapy on deep vein thrombosis rates four weeks after total hip replacement. J Vasc Surg. 1999;30:813–820. [DOI] [PubMed]
- 7.D’Andrea G, D’Ambrosio RL, Di Perna P, Chetta M, Santacroce R, Brancaccio V, Grandone E, Margaglione M. A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin. Blood. 2005;105:645–649. [DOI] [PubMed]
- 8.FDA News: August 16, 2007. Available at: www.fda.gov/bbs/topics/NEWS/2007/NEW01684.html. Accessed August 12, 2008.
- 9.Fregin A, Rost S, Wolz W, Krebsova A, Muller CR, Oldenburg J. Homozygosity mapping of a second gene locus for hereditary combined deficiency of vitamin K-dependent clotting factors to the centromeric region of chromosome 16. Blood. 2002;100:3229–3232. [DOI] [PubMed]
- 10.Friedman RJ. Optimal duration of prophylaxis for venous thromboembolism following total hip arthroplasty and total knee arthroplasty. J Am Acad Orthop Surg. 2007;15:148–155. [DOI] [PubMed]
- 11.Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, Ray JG. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 suppl):338S–400S. [DOI] [PubMed]
- 12.Gonzalez Della Valle A, Serota A, Sorriaux G, Go G, Sharrock N, Sculco TP, Salvati E. Venous thromboembolism is rare after total hip arthroplasty with a multimodal prophylaxis protocol. Clin Orthop Relat Res. 2006;443:146–153. [DOI] [PubMed]
- 13.Goodman LS, Gilman A, Brunton JS, Lazo JS, Parker KL. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. New York, NY: McGraw-Hill; 2006.
- 14.Greenblatt DJ, von Moltke LL. Interaction of warfarin with drugs, natural substances, and foods. J Clin Pharmacol. 2005;45:127–132. [DOI] [PubMed]
- 15.Harrington DJ, Underwood S, Morse C, Shearer MJ, Tuddenham EG, Mumford AD. Pharmacodynamic resistance to warfarin associated with a Val66Met substitution in vitamin K epoxide reductase complex subunit 1. Thromb Haemost. 2005;93:23–26. [DOI] [PubMed]
- 16.Imperiale TF, Speroff T. A meta-analysis of methods to prevent venous thromboembolism following total hip replacement. JAMA. 1994;271:1780–1785. [DOI] [PubMed]
- 17.Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med. 1993;95:315–328. [DOI] [PubMed]
- 18.Li T, Lange LA, Li X, Susswein L, Bryant B, Malone R, Lange EM, Huang TY, Stafford DW, Evans JP. Polymorphisms in the VKORC1 gene are strongly associated with warfarin dosage requirements in patients receiving anticoagulation. J Med Genet. 2006;43:740–744. [DOI] [PMC free article] [PubMed]
- 19.Lieberman JR, Sung R, Dorey F, Thomas BJ, Kilgus DJ, Finerman GA. Low-dose warfarin prophylaxis to prevent symptomatic pulmonary embolism after total knee arthroplasty. J Arthroplasty. 1997;12:180–184. [DOI] [PubMed]
- 20.Lieberman JR, Wollaeger J, Dorey F, Thomas BJ, Kilgus DJ, Grecula MJ, Finerman GA, Amstutz HC. The efficacy of prophylaxis with low-dose warfarin for prevention of pulmonary embolism following total hip arthroplasty. J Bone Joint Surg Am. 1997;79:319–325. [DOI] [PubMed]
- 21.Memtsoudis SG, Gonzalez Della Valle A, Besculides MC, Gaber L, Laskin R. Trends in demographics, comorbidity profiles, in-hospital complications and mortality associated with primary knee arthroplasty 3 830 420 hospital discharges in the United States between 1990 and 2004. J Arthroplasty. 2008 April 14. [Epub ahead of print] [DOI] [PubMed]
- 22.Mesko JW, Brand RA, Iorio R, Gradisar I, Heekin R, Leighton R, Thornberry R. Venous thromboembolic disease management patterns in total hip arthroplasty and total knee arthroplasty patients: a survey of the AAHKS membership. J Arthroplasty. 2001;16:679–688. [DOI] [PubMed]
- 23.Messieh M, Huang Z, Johnson LJ, Jobin S. Warfarin responses in total joint and hip fracture patients. J Arthroplasty. 1999;14:724–729. [DOI] [PubMed]
- 24.Millican E, Jacobsen-Lenzini PA, Milligan PE, Grosso L, Eby C, Deych E, Grice G, Clohisy JC, Barrack RL, Burnett RS, Voora D, Gatchel S, Tiemeier A, Gage BF. Genetic-based dosing in orthopaedic patients beginning warfarin therapy. Blood. 2007; 110:1511–1515. [DOI] [PMC free article] [PubMed]
- 25.Miyagi J, Funabashi N, Suzuki M, Asano M, Kuriyama T, Komuro I, Moriya H. Predictive indicators of deep venous thrombosis and pulmonary arterial thromboembolism in 54 subjects after total knee arthroplasty using multislice computed tomography in logistic regression models. Int J Cardiol. 2007;119:90–94. [DOI] [PubMed]
- 26.Momary KM, Shapiro NL, Viana MA, Nutescu EA, Helgason CM, Cavallari LH. Factors influencing warfarin dose requirements in African-Americans. Pharmacogenomics. 2007;8:1535–1544. [DOI] [PubMed]
- 27.Paavolainen P, Pukkala E, Pulkkinen P, Visuri T. Causes of death after total hip arthroplasty: a nationwide cohort study with 24,638 patients. J Arthroplasty. 2002;17:274–281. [DOI] [PubMed]
- 28.Paiement GD, Wessinger SJ, Hughes R, Harris WH. Routine use of adjusted low-dose warfarin to prevent venous thromboembolism after total hip replacement. J Bone Joint Surg Am. 1993;75:893–898. [DOI] [PubMed]
- 29.Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89:33–38. [DOI] [PubMed]
- 30.Quteineh L, Verstuyft C, Descot C, Dubert L, Robert A, Jaillon P, Becquemont L. Vitamin K epoxide reductase (VKORC1) genetic polymorphism is associated to oral anticoagulant overdose. Thromb Haemost. 2005;94:690–691. [DOI] [PubMed]
- 31.Reitsma PH. Genetic heterogeneity in hereditary thrombophilia. Haemostasis. 2000;30:1–10. [DOI] [PubMed]
- 32.Reitsma PH, van der Heijden JF, Groot AP, Rosendaal FR, Buller HR. A C1173T dimorphism in the VKORC1 gene determines coumarin sensitivity and bleeding risk. PLoS Med. 2005;2:e312. [DOI] [PMC free article] [PubMed]
- 33.Rieder MJ, Reiner AP, Gage BF, Nickerson DA, Eby CS, McLeod HL, Blough DK, Thummel KE, Veenstra DL, Rettie AE. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med. 2005;352:2285–2293. [DOI] [PubMed]
- 34.Rost S, Fregin A, Hunerberg M, Bevans CG, Muller CR, Oldenburg J. Site-directed mutagenesis of coumarin-type anticoagulant-sensitive VKORC1: evidence that highly conserved amino acids define structural requirements for enzymatic activity and inhibition by warfarin. Thromb Haemost. 2005;94:780–786. [DOI] [PubMed]
- 35.Rost S, Fregin A, Ivaskevicius V, Conzelmann E, Hortnagel K, Pelz HJ, Lappegard K, Seifried E, Scharrer I, Tuddenham EG, Muller CR, Strom TM, Oldenburg J. Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature. 2004;427:537–541. [DOI] [PubMed]
- 36.Sachs RA, Smith JH, Kuney M, Paxton L. Does anticoagulation do more harm than good? A comparison of patients treated without prophylaxis and patients treated with low-dose warfarin after total knee arthroplasty. J Arthroplasty. 2003;18:389–395. [DOI] [PubMed]
- 37.Salvati EA, Sharrock NE, Westrich G, Potter HG, Gonzalez Della Valle A, Sculco TP. The 2007 ABJS Nicolas Andry Award: three decades of clinical, basic, and applied research on thromboembolic disease after THA: rationale and clinical results of a multimodal prophylaxis protocol. Clin Orthop Relat Res. 2007;459:246–254. [DOI] [PubMed]
- 38.Sanchez-Ballester J, Smith M, Hassan K, Kershaw S, Elsworth CS, Jacobs L. Wound infection in the management of hip fractures: a comparison between low-molecular weight heparin and mechanical prophylaxis. Acta Orthop Belg. 2005;71:55–59. [PubMed]
- 39.Sconce EA, Khan TI, Wynne HA, Avery P, Monkhouse L, King BP, Wood P, Kesteven P, Daly AK, Kamali F. The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood. 2005;106:2329–2333. [DOI] [PubMed]
- 40.Sharrock NE, Go G, Harpel PC, Ranawat CS, Sculco TP, Salvati EA. The John Charnley Award. Thrombogenesis during total hip arthroplasty. Clin Orthop Relat Res. 1995;319:16–27. [PubMed]
- 41.Wadelius M, Chen LY, Downes K, Ghori J, Hunt S, Eriksson N, Wallerman O, Melhus H, Wadelius C, Bentley D, Deloukas P. Common VKORC1 and GGCX polymorphisms associated with warfarin dose. Pharmacogenomics J. 2005;5:262–270. [DOI] [PubMed]
- 42.Wu AH. Use of genetic and nongenetic factors in warfarin dosing algorithms. Pharmacogenomics. 2007;8:851–861. [DOI] [PubMed]
- 43.Yuan HY, Chen JJ, Lee MT, Wung JC, Chen YF, Charng MJ, Lu MJ, Hung CR, Wei CY, Chen CH, Wu JY, Chen YT. A novel functional VKORC1 promoter polymorphism is associated with inter-individual and inter-ethnic differences in warfarin sensitivity. Hum Mol Genet. 2005;14:1745–1751. [DOI] [PubMed]