Abstract
Abstract
Information on the microbiology of infections after operative ankle fractures, on the details of a treatment protocol used when the ankle joint is preserved, and on the outcome of this protocol will be helpful for the physicians managing patients with this complex problem. We therefore determined the most common pathogen of these infections, the infection recurrence rate, and the amputation rate. We retrospectively reviewed 26 patients of a mean age of 43 years with infections following operative treatment of ankle fractures. Twenty-one of 26 patients (81%) were compromised hosts according to the Cierny-Mader classification. Patients presenting up to 10 weeks postoperatively were treated by débridement and either hardware retention (if implants were judged stable) or hardware removal (if implants were loose). All patients presenting more than 10 weeks postoperatively underwent débridement and hardware removal, with the exception of one patient who underwent below knee amputation. Staphylococcus aureus was identified in 17 patients (65%) and was oxacillin-resistant in six (23%). The infection recurred in five of 18 patients who were followed up for 8 months on average. Three recurrent infections were controlled with repeat débridement. The remaining two patients underwent below-knee amputation, resulting in amputations in 3 of 18 patients. Infection after operative treatment of ankle fractures is a limb-threatening complication, especially in patients with comorbidities, such as diabetes mellitus. Treatment is challenging with high infection recurrence and amputation rates.
Level of Evidence: Level IV, therapeutic study case series. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Infection is a well-known complication of operative treatment of ankle fractures with infection rates ranging from 1% to 8% in large series [10, 14, 16, 17]. Risk factors for infection after open reduction and internal fixation of ankle fractures include diabetes mellitus [2, 4, 7, 8, 12, 13, 15, 18], alcoholism [20], advanced age [1], and high-energy injuries [9]. Patients with diabetes mellitus after operative treatment of ankle fractures have had infection rates ranging from 10% to 60% [7, 13], amputation rates up to 42% in open ankle fractures [21], and mortality up to 11% [18].
In one study infected ankle and pilon fractures were combined [11], the majority of fractures were open, and all infected fractures were treated by arthrodesis of the ankle. The microbiology of these infections and the recurrence of infection after the first procedure were not reported; 2 of 19 patients underwent amputation [11]. Information on the microbiology of these infections, on the details of a treatment protocol used when the ankle joint is preserved, and on the outcome of this protocol will be helpful for the physicians managing patients with the complex problem of infection after operative treatment of ankle fractures.
The purpose of our study was (1) to determine the most common pathogen in infections after open reduction and internal fixation of ankle fractures, (2) to determine the infection recurrence rate, and (3) to determine the amputation rate with the treatment protocol used at our institution.
Material and Methods
We retrospectively reviewed the medical records of 26 patients (20 men and six women) with a mean age of 43 years (range, 21–65 years) treated at our institution from 2000 to 2004 for infections following operative treatment of ankle fractures. We excluded patients with fractures of the tibial plafond or pathologic fractures. Twenty-one of 26 patients (81%) were compromised hosts according to the Cierny-Mader classification [6] with one or more comorbidities. The most common comorbidities were smoking in 14 patients, low albumin (< 3.5 mg/dL) in 14 patients, type 2 diabetes mellitus in five patients, and intravenous drug abuse in five patients. Four patients had hepatitis B and/or C (one of them had developed cirrhosis), four patients reported alcohol abuse, one patient was infected with HIV, one patient had congestive heart failure, and one patient had chronic pulmonary disease. Twenty-two patients (85%) presented with wound drainage and four without drainage but with soft tissue swelling and erythema. The lateral side was involved in 16 patients (62%), the medial in seven, and both sides in three patients. We had prior IRB approval.
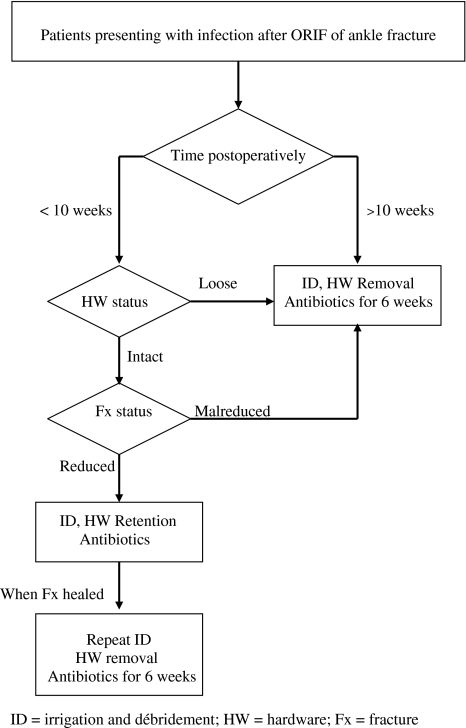
Our treatment protocol was based on the time postsurgery and on the stability provided by the hardware (Fig. 1). Eleven patients presented to us up to 10 weeks postoperatively with a mean time from surgery of 4 weeks (range, 1–9 weeks). These patients were treated by débridement and hardware retention if implants were stable and the fracture well reduced; six patients belonged to this subgroup. This was not intended as definitive treatment, but rather as a temporizing measure, aiming to suppress the infection until fracture healing, to be followed by repeat débridement and hardware removal after fracture healing. If the implants were loose or the fracture was grossly malreduced, patients were treated by débridement and hardware removal; five patients belonged to this subgroup. The 15 patients presenting to us at 11 or more weeks postoperatively (mean time from surgery, 18 months; range, 11 weeks–4.5 years) underwent débridement and hardware removal. Beyond 10 weeks we anticipate sufficient healing to have taken place so that the fracture will not displace following removal of the implants. The exception was one patient with diabetes mellitus, hepatitis B, hepatitis C, cirrhosis, and destruction of the ankle joint who elected to undergo a below-knee amputation, because of the increased surgical risk, increased infection recurrence risk, and questionable anticipated functional outcome.
Fig. 1.
Treatment protocol for infections after ORIF of ankle fractures.
Three of 26 patients (11%) required soft tissue coverage following débridement and the sural flap was used. Culture-specific antibiotic therapy was administered for at least 6 weeks postoperatively; when implants were temporarily retained until fracture healing, antibiotic therapy was continued until 6 weeks after the final débridement with hardware removal. Eight patients did not complete a minimum 6-month followup and the outcome is based on 18 of 26 patients (69%) with a mean followup of 8 months (range, 6–17 months) (Table 1). The 8 patients (6 male and 2 female) that were not followed up had a mean age of 47 years and 6 of 8 patients were compromised hosts (one with diabetes mellitus). The remaining 18 patients (14 male and 4 female) who were followed up had a mean age of 42 years and 15 of 18 patients were compromised hosts (4 with diabetes mellitus).
Table 1.
Demographics, comorbidities, treatment, and outcome data of the 18 patients followed up
| Patient | Gender | Age | Comorbidities | Time of presentation(after ORIF) | HW status | Initial surgery | Intraoperative culture results | Antibiotics | Recurrence of infection | Comments | Amputation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 38 | Low albumin, hypothyroidism | 1 week | Intact | ID, HW retained | S. epidermidis | Daptomycin | Yes (7 weeks after ID) | Ulceration with exposed bone at medial distal tibia | Yes |
| 2 | M | 31 | None | 2 weeks | Intact | ID, HW retained | OSSA | Cefazolin | Yes (8 weeks after ID) | Repeat ID, HW removed | No |
| 3 | M | 53 | Low albumin, smoking, alcohol | 2 weeks | Intact | ID, HW retained | ORSA | Vancomycin, then PO Bactrim | Yes (7 weeks after ID) | Repeat ID, HW removed | No |
| 4 | M | 54 | Low albumin, smoking, alcohol | 7 weeks | Loose | ID, HW removed | OSSA | Oxacillin then PO cefazolin | Yes (14 weeks after ID) | Repeat ID | No |
| 5 | F | 58 | DM, retinopathy, peripheral neuropathy, low albumin | 7 weeks | Loose | ID, HW removed | OSSA | Oxacillin plus rifampin | Yes (3 weeks after ID) | Yes | |
| 6 | M | 21 | Low albumin, smoking | 3 weeks | Intact | ID, HW retained | S. epidermidis, Propionibacterium | Vancomycin | No | Repeat ID, HW removed | No |
| 7 | M | 37 | HIV, hepatitis C, IVDA, smoking | 5 weeks | Intact but fibula malreduced | ID, HW removed | Serratia, Acinetobacter | Vancomycin, levofloxacin, amikacin | No | No | |
| 8 | M | 52 | IVDA, smoking, alcohol | 9 weeks | Loose | ID, HW removed | OSSA | Oxacillin | No | No | |
| 9 | M | 30 | None | 11 weeks | Intact | ID, HW removed | OSSA | Cefazolin plus rifampin | No | No | |
| 10 | M | 40 | Smoking | 13 weeks | Intact | ID, HW removed | S. epidermidis | Vancomycin | No | No | |
| 11 | M | 44 | DM, IVDA, smoking, low albumin | 14 weeks | Intact | ID, HW removed | OSSA | Cefazolin | No | No | |
| 12 | M | 59 | DM, alcohol, low albumin | 15 weeks | Intact | ID, HW removed | Enterobacter cloacae, S. epidermidis | Vancomycin, levofloxacin | No | No | |
| 13 | M | 49 | None | 13 months | Intact | ID, HW removed | ORSA | Vancomycin | No | No | |
| 14 | M | 33 | Low albumin | 44 weeks | Intact | ID, HW removed | ORSA | Vancomycin | No | No | |
| 15 | M | 36 | Smoking | 12 months | Loose | ID, HW removed | ORSA | Vancomycin | No | No | |
| 16 | F | 36 | Smoking | 21 months | Intact | ID, HW removed | OSSA, S. epidermidis | Vancomycin | No | No | |
| 17 | M | 30 | Low albumin | 4.5 years | Loose | ID, HW removed | ORSA | Vancomycin plus rifampin | No | No | |
| 18 | F | 47 | DM, IVDA, hepatitis B, hepatitis C, cirrhosis, low albumin, smoking | 23 months | Loose | Amputation | Not available (S. epidermidis in preoperative cultures) | Ertapenem plus levofloxacin (before amputation) | No | Ulcerations of lateral and medial side of ankle, joint destruction | Yes |
HW = hardware; ORIF = open reduction internal fixation; HIV = human immunodeficiency virus; IVDA = intravenous drug abuse; DM = diabetes mellitus; OSSA = oxacillin sensitive Staphylococcus aureus; ORSA = oxacillin resistant Staphylococcus aureus.
Our culturing routine for determining the microbiology of these infections consisted first of preoperative cultures of the draining fluid upon patient presentation, and second of multiple intraoperative cultures; specifically, samples of fluid, soft tissue, and bone from the site of infection were sent for aerobic, anaerobic, mycobacterial, and fungal cultures. Antibiotics were started upon patient presentation. Preantibiotic blood cultures were not routinely taken.
Patients were followed at 2–4 week intervals. After the final débridement with hardware removal and completion of the antibiotic therapy they were followed up at 6–8 week intervals. Recurrence of infection was defined as development of wound drainage combined with positive cultures, either from the draining fluid or intraoperatively during repeat surgery.
Results
Staphylococcus aureus was the most common pathogen, identified in 17 of 26 patients (65%), and was oxacillin-resistant in six of 26 patients (23%) (Table 2). Staphylococcus epidermidis was identified in six patients (23%), Enterobacter cloacae in two, Propionibacterium acnes in two, and Acinetobacter, Serratia, Pseudomonas aeruginosa, vancomycin-resistant Enterococcus, and diphtheroids in one patient each. Twenty infections (77%) were monomicrobial and six infections (23%) were polymicrobial.
Table 2.
Microbiology of infections following operative treatment of ankle fractures in 26 patients*
| Pathogens | Number |
|---|---|
| Gram positive (n = 25) | |
| Staphylococcus aureus–oxacillin sensitive | 11 |
| Staphylococcus aureus–oxacillin resistant | 6 |
| Staphylococcus epidermidis | 6 |
| Enterococcus faecalis, vancomycin resistant | 1 |
| Diphtheroids | 1 |
| Gram-negative (n = 5) | |
| Enterobacter cloacae | 2 |
| Pseudomonas aeruginosa | 1 |
| Acinetobacter baumannii | 1 |
| Serratia marcescens | 1 |
| Anaerobes (n = 2) | |
| Propionibacterium acnes | 2 |
* The number of pathogens is 32 because 6 infections were polymicrobial.
The infection recurred in five of 18 patients who were followed up. Four of these five patients were compromised hosts. Three recurrences occurred in four patients with followup (six patients in the whole series) when implants were retained; two of these recurrent infections were controlled with repeat débridement and removal of the hardware present, since the fracture was considered healed at the time of repeat surgery. One patient with hypothyroidism and low albumin developed ulceration with exposed bone at the medial aspect of the distal tibia and elected to undergo below-knee amputation instead of further surgical treatment, which would have also required flap coverage. Two recurrences (both on compromised patients) took place in four patients with follow up (five patients in the whole series) when hardware was removed before 10 weeks because of loosening or malreduction. There was no recurrence of infection in the nine patients with follow up (14 patients in the whole series) when hardware was removed for late infections. One recurrence happened in a patient who was a smoker and excessive alcohol user with low albumin and resolved after a repeat débridement. The other recurrence took place in a patient with diabetes mellitus, peripheral neuropathy, and low albumin and was treated with below-knee amputation. Therefore, recurrence of infection despite the completion of the treatment plan took place in two of the 18 patients.
Three of the 18 patients who were followed up underwent below-knee amputation, resulting in an amputation rate of 17% and salvage of the extremity in 15 of 18 patients. One amputation was performed primarily and two were performed secondarily owing to infection recurrence. All three amputations were performed in compromised hosts and two of these three patients had diabetes mellitus. Overall, two of five patients with diabetes mellitus (two of four patients with diabetes mellitus who were followed up) and a postoperative infection after ankle fracture fixation underwent a below-knee amputation.
Discussion
Information on the microbiology of infections after operative treatment of ankle fractures and on the outcome of a treatment protocol used when the ankle joint is preserved will be helpful for physicians managing patients with this complex problem. The current series demonstrates that infection after operative treatment of ankle fractures is most commonly caused by Staphylococcus aureus and is a challenging and limb-threatening complication, especially in patients with diabetes mellitus or other compromising factors.
Our study has several limitations in addition to its retrospective nature. There was a considerable loss to followup, so the outcome data were drawn from 18 of the 26 patients studied. We did not determine any functional outcomes, so it remains unclear if the infectious process resulted in compromised motion of the ankle joint and impaired patient function. It should be noted that infection may recur late and the relatively short 8 month followup in our study may have underestimated the infection recurrence rate. The lack of long-term followup does not allow any assessment of the effect of the infectious process regarding development of osteoarthrosis of the ankle joint. However, the series, while relatively large, allows a short-term evaluation of the outcome of this protocol regarding control of infection and salvage of the extremity.
Our treatment protocol takes into account the time postsurgery, the status of fracture healing, and the stability provided by the hardware. As a general rule implants are retained if the fracture is not healed and the implants are providing stability. This principle has been outlined before by us and other authors [17, 19], but our series is the first, to our knowledge, to evaluate the outcome of this protocol in the treatment of the infected ankle fracture.
The high amputation and infection recurrence rates indicate the challenging nature of infections in patients with comorbidities, who are therefore considered compromised hosts [5, 6]. Two of our three amputations were performed in patients with diabetes mellitus and, overall, two of five patients with diabetes mellitus (two of four patients with diabetes mellitus who were followed up) and a postoperative ankle fracture infection underwent a below-knee amputation. In patients with diabetes amputation rates up to 20% in closed ankle fractures [15] and up to 42% in open ankle fractures [21] have been reported in the literature.
Our infection recurrence rate was 28% (five of 18 patients) but three of these recurrences took place when retaining the hardware, which was not performed as definitive treatment, but as a temporizing measure instead until fracture healing. Infection rates after operative treatment of ankle fractures in patients with diabetes mellitus have ranged from 10% to 60% in the literature [7, 13]. The abnormal glucose metabolism in diabetes results in vasculopathy, neuropathy, inhibition of wound healing, and impaired immune function, thereby predisposing to infectious complications [3, 4, 22]. Difficulty in controlling infection, poor potential for wound healing, and neuropathy may explain the high amputation rate in patients with diabetes mellitus.
Staphylococcus aureus was the most common pathogen and approximately ¼ of all infections in the current series were caused by oxacillin-resistant Staphylococcus aureus. Therefore, empiric antibiotics for postoperative ankle fracture infections should provide coverage for oxacillin-resistant Staphylococcus aureus.
Infection after operative treatment of ankle fractures is a challenging and limb-threatening complication, especially in patients with comorbidities, such as diabetes mellitus. The patient should be informed about the challenging nature of the problem, the potential for multiple procedures, and the risk for amputation.
Acknowledgment
We thank Dr Francis Schiller for his help with the care of these patients.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article. The authors have full control of all primary data and they agree to allow the journal to review their data if requested.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Beauchamp CG, Clay NR, Thexton PW. Displaced ankle fractures in patients over 50 years of age. J Bone Joint Surg Br. 1983;65:329–332. [DOI] [PubMed]
- 2.Bibbo C, Lin SS, Beam HA, Behrens FF. Complications of ankle fractures in diabetic patients. Orthop Clin North Am. 2001;32:113–133. [DOI] [PubMed]
- 3.Bybee JD, Rogers DE. The phagocytic activity of polymorphonuclear leukocytes obtained from patients with diabetes mellitus. J Lab Clin Med. 1964;64:1–13. [PubMed]
- 4.Chaudhary SB, Liporace FA, Gandhi A, Donley BG, Pinzur MS, Lin SS. Complications of ankle fracture in patients with diabetes. J Am Acad Orthop Surg. 2008;16:159–170. [DOI] [PubMed]
- 5.Cierny G, 3rd, DiPasquale D. Periprosthetic total joint infections: staging, treatment, and outcomes. Clin Orthop Relat Res. 2002;403:23–28. [DOI] [PubMed]
- 6.Cierny G 3rd, Mader JT, Penninck JJ. A clinical staging system for adult osteomyelitis. Clin Orthop Relat Res. 2003;414:7–24. [DOI] [PubMed]
- 7.Costigan W, Thordarson DB, Debnath UK. Operative management of ankle fractures in patients with diabetes mellitus. Foot Ankle Int. 2007;28:32–37. [DOI] [PubMed]
- 8.Flynn JM, Rodriguez-del Rio F, Piza PA. Closed ankle fractures in the diabetic patient. Foot Ankle Int. 2000;21:311–319. [DOI] [PubMed]
- 9.Hoiness P, Stromsoe K. The influence of the timing of surgery on soft tissue complications and hospital stay. A review of 84 closed ankle fractures. Ann Chir Gynaecol. 2000;89:6–9. [PubMed]
- 10.Hughes JL, Weber H, Willenegger H, Kuner EH. Evaluation of ankle fractures: non-operative and operative treatment. Clin Orthop Relat Res. 1979;138:111–119. [PubMed]
- 11.Hulscher JB, te Velde EA, Schuurman AH, Hoogendoorn JM, Kon M, van der Werken C. Arthrodesis after osteosynthesis and infection of the ankle joint. Injury. 2001;32:145–152. [DOI] [PubMed]
- 12.Jones KB, Maiers-Yelden KA, Marsh JL, Zimmerman MB, Estin M, Saltzman CL. Ankle fractures in patients with diabetes mellitus. J Bone Joint Surg Br. 2005;87:489–495. [DOI] [PubMed]
- 13.Kristiansen B. Results of surgical treatment of malleolar fractures in patients with diabetes mellitus. Dan Med Bull. 1983;30:272–274. [PubMed]
- 14.Lindsjo U. Operative treatment of ankle fracture-dislocations. A follow-up study of 306/321 consecutive cases. Clin Orthop Relat Res. 1985;199:28–38. [PubMed]
- 15.Low CK, Tan SK. Infection in diabetic patients with ankle fractures. Ann Acad Med Singapore. 1995;24:353–355. [PubMed]
- 16.Mak KH, Chan KM, Leung PC. Ankle fracture treated with the AO principle—an experience with 116 cases. Injury. 1985;16:265–272. [DOI] [PubMed]
- 17.Marsh JL, Saltzman CL. Ankle fractures. In: Rockwood and Green’s Fractures in Adults, Vol. 2. Bucholz RW, Heckman JD, Court-Brown CM, eds. Philadelphia, PA: Lippincott; 2005:2148–2247.
- 18.McCormack RG, Leith JM. Ankle fractures in diabetics. Complications of surgical management. J Bone Joint Surg Br. 1998;80:689–692. [DOI] [PubMed]
- 19.Patzakis MJ, Zalavras CG. Chronic posttraumatic osteomyelitis and infected nonunion of the tibia: current management concepts. J Am Acad Orthop Surg. 2005;13:417–427. [DOI] [PubMed]
- 20.Tonnesen H, Pedersen A, Jensen MR, Moller A, Madsen JC. Ankle fractures and alcoholism. The influence of alcoholism on morbidity after malleolar fractures. J Bone Joint Surg Br. 1991;73:511–513. [DOI] [PubMed]
- 21.White CB, Turner NS, Lee GC, Haidukewych GJ. Open ankle fractures in patients with diabetes mellitus. Clin Orthop Relat Res. 2003;414:37–44. [DOI] [PubMed]
- 22.Wukich DK, Kline AJ. The management of ankle fractures in patients with diabetes. J Bone Joint Surg Am. 2008; 90:1570–1578. [DOI] [PubMed]