Abstract
Sarcoma associated with bone infarct is a rare condition sparsely reported in the literature. Sixty percent of cases arise about the knee and most are malignant fibrous histiocytomas. We report 15 patients; 12 of 15 presented with a tumor around the knee. Treatment was limb salvage in seven patients, amputation in six, and biopsy alone in two. For patients without metastatic disease at presentation, the 2-year disease-free survival rate was 63% (seven of 11). Two patients received chemotherapy and both were continuously disease-free at last followup. When we combined our 15 patients with the 52 previously reported in the literature, 38 of the 67 (57%) died of their disease at an average of 19.2 months after diagnosis; 21 patients (31%) were continuously disease-free for 24 months. Of 13 patients who received chemotherapy, eight (62%) were continuously disease-free at 24 months compared with 24% (13 of 54) of those who did not receive chemotherapy. Overall, prognosis for these patients is poor, but survival in patients without metastatic disease at diagnosis approaches that of other bone sarcomas. There is a trend suggesting adjuvant chemotherapy combined with appropriate surgery may improve patient outcomes.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Secondary sarcoma arising in association with a preexisting bone infarct is extremely rare and much less common than secondary sarcoma arising in association with radiation or Paget’s disease [10, 16]. Bone infarct-associated sarcoma most commonly occurs in the sixth decade of life, and almost 2/3 of the patients are men [17]. Sixty percent of cases arise about the knee and most are malignant fibrous histiocytomas [17]. One-third of patients have an identifiable etiology for their infarct [17]. Bone infarct is a relatively common orthopaedic finding that is often idiopathic but can be seen secondary to an underlying condition such as alcoholism, corticosteroid use, or Caisson’s disease. Also known as aseptic or avascular necrosis of bone, bone infarcts occur anywhere in the skeleton but are predominately seen around the hip and knee.
Furey et al. [8] reported the first case, a fibrosarcoma, in 1960. Since then, several other types of infarct-associated sarcomas have been reported, including malignant fibrous histiocytoma [13], osteosarcoma [15], angiosarcoma [1], and epithelioid hemangioendothelioma [14]. Case studies and a few compilations of the reported data have been published [5, 17], but no more than five cases of sarcoma associated with bone infarct have been documented from one institution [5].
There were two aims of this study: (1) to better characterize the demographics, natural history, and survivorship of this rare condition by comparing and combining our information with that already amassed in the literature, and (2) to determine whether adjuvant chemotherapy is associated with longer patient survival.
Materials and Methods
We retrospectively reviewed our orthopaedic oncology database, a prospectively collected library dating back more than 40 years, to identify all 15 patients with sarcoma associated with bone infarct. No patients were seen specifically for this study. Each patient had a histologic diagnosis of sarcoma originating in bone with a concomitant diagnosis of an associated bone infarct confirmed by histology, radiology, or both (Fig. 1). The average age at diagnosis was 56.4 years (range, 39–75 years), and eleven patients were diagnosed in the fifth or sixth decade of life (Table 1). Two of the 15 patients were lost to followup. One of these patients had a malignant fibrous histiocytoma of the proximal tibia treated with a transfemoral amputation. He had widespread metastases develop at 16 months and then was lost to followup. The other patient had an osteosarcoma of the proximal femur and presented with metastatic disease. After biopsy, he elected to undergo palliative radiation therapy alone before being lost to followup. We obtained prior Institutional Review Board approval for this study,
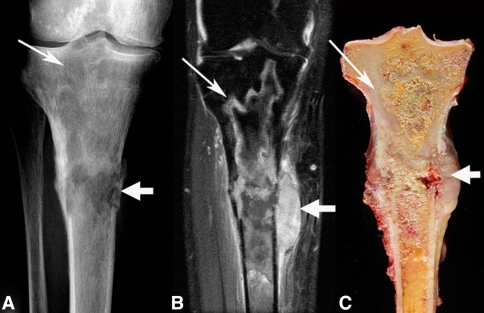
Fig. 1A–C.
An (A) anteroposterior radiograph, (B) coronal T2-weighted fat saturation MR image, and (C) coronal gross section show a proximal tibial malignant fibrous histiocytoma arising in an infarct. Curvilinear radiodensities of the underlying mature bone infarct (narrow arrows) can be seen blending into the permeative destructive areas (wide arrow) of the malignancy associated with a soft tissue mass and pathologic fracture.
Table 1.
Clinical data for patient series
| Age (years)/ Gender | Site of tumor | Stage | Histology | Adjuvant treatment | Surgical treatment | Clinical course |
|---|---|---|---|---|---|---|
| 51/female | Distal femur | IIB | MFH | AKA | CDF 73 months | |
| 67/male | Distal femur | IIB | MFH | Resection and reconstruction AKA after vascular complication | CDF 53 months | |
| 59/ female | Proximal tibia | IIB | MFH | AKA | DOD 1 month | |
| 67/male | Distal femur, proximal tibia | III | MFH | AKA | DOD 1 month | |
| 32/male | Proximal femur | IIB | MFH | Resection and reconstruction | CDF 27 months | |
| 56/female | Proximal tibia | IIB | MFH | Postoperative chemotherapy | Resection and reconstruction with two-stage reimplant for infection | CDF 24 months |
| 60/male | Distal femur | IIA | MFH | Resection and tibial turnback knee fusion | DOC 324 months | |
| 51/female | Distal femur | III | MFH | Biopsy only | DOD 12 months | |
| 48/male | Proximal tibia | IIB | MFH | AKA | Metastasis at 16 months, lost to followup | |
| 61/female | Acetabulum | IIB | MFH | Resection and allograft reconstruction | DOD 12 months | |
| 64/female | Proximal tibia | IIB | MFH | AKA | DOC 24 months (died of bladder cancer) | |
| 61/female | Proximal tibia | IIB | MFH | AKA | DOD 12 months | |
| 55/male | Proximal tibia | IIB | OS | Preoperative chemotherapy | Resection and reconstruction | CDF 58 months |
| 75/female | Distal femur | III | OS | Resection and reconstruction | DOD 5 months | |
| 39/male | Proximal femur | III | OS | Palliative XRT | Biopsy only | Lost to followup 2 weeks |
XRT = radiation therapy; MFH = malignant fibrous histiocytoma; OS = osteosarcoma; AKA = above-knee amputation; CDF = continuously disease-free; DOD = dead of disease; DOC = died of other cause.
All patients had the classic findings of malignant fibrous histiocytoma or osteosarcoma inside, or in direct contact with, a bony infarct. In all instances, the bone infarct was spatially immediately adjacent to the sarcoma (Fig. 2). Tumors with areas of necrosis or dead bone in the substance of the lesion were not included.
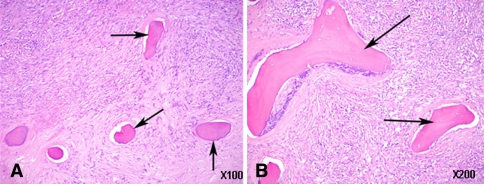
Fig. 2A–B.
(A) Low-power (×10) and (B) high-power (×20) photomicrographs show the interface between necrotic bone from an infarct (arrows) and malignant fibrous histiocytoma (Stain, hematoxylin and eosin).
Surgical treatment included limb salvage in seven patients (five prosthetic reconstructions, one massive allograft, one tibial turnback procedure), amputation in six patients, and biopsy alone in two patients. One patient with a distal femoral reconstruction later underwent amputation after vascular complications. One patient with a proximal tibial reconstruction had an infection develop and underwent successful two-stage removal and reimplantation.
Additional data collected included cause of bony infarct (if any), stage of the lesion according to the Musculoskeletal Tumor Society staging system proposed by Enneking et al. [7], treatment, any adjuvant therapy received by the patient, and survival. This same information was collected from all the cases of bone infarct-associated sarcoma in the reported literature.
We used the Kaplan-Meier method (LIFETEST from SAS® Version 9.1; SAS Institute Inc, Cary, NC) to construct survival curves for patients with Stage II and Stage III lesions using death as an end point (Fig. 3).
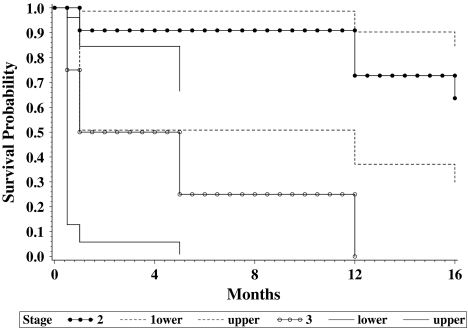
Fig. 3.
Kaplan-Meier survival curves (with 95% confidence intervals) show disease-free survival of Musculoskeletal Tumor Society Stage IIB and Stage III tumors. Survival rates for patients at our institution with Stage IIB disease approached that of other bone sarcomas. Patients with Stage III disease fared extremely poorly.
Results
The most common site of disease was the proximal tibia, in six patients, followed by the distal femur in five. One patient presented with a very large mass around the knee, and it was impossible to determine if the tumor originated in the tibia or femur. Twelve of the 15 patients presented with a tumor around the knee. At presentation the Musculoskeletal Tumor Society stage in 10 patients was IIB, Stage IIA in one, and Stage III (metastatic disease) in four. Surgical management included limb salvage in seven patients (five prosthetic reconstructions, one massive allograft, one tibial turnback procedure), amputation in six patients, and biopsy alone in two patients.
Assuming both of the patients lost to followup died, eight patients (six malignant fibrous histiocytomas, two osteosarcomas) died of their disease. Survival from the time of diagnosis ranged from 1 to 16 months. None of the patients with Stage III disease survived longer than 12 months. Five patients (four with malignant fibrous histiocytomas, one with osteosarcoma) were still alive without evidence of disease at 24, 27, 53, 58, and 73 months. Two patients with malignant fibrous histiocytoma died as a result of a different illness without evidence of tumor return at 2 and 27 years. Overall, the 2-year continuous disease-free survival for our group of patients was 47% (seven of 15 patients). The 2-year continuous disease-free survival for patients without metastatic disease (10 patients with Stage IIB, one with Stage IIA disease) was 63% (Fig. 3). Only two patients received chemotherapy (preoperative in one patient with osteosarcoma, postoperative in one patient with malignant fibrous histiocytoma). These two patients have been continuously disease-free.
Discussion
Sarcomatous degeneration of a bone infarct is extremely rare and has been reported only sporadically, often as isolated case reports. Our purpose was twofold: (1) to better characterize this rare sarcoma, and (2) to ascertain whether a specific treatment was associated with longer survival time. We did this by reviewing our patients and adding these data to those already amassed in the literature (Table 2).
Table 2.
Comparison of current and published series
| Study | Number of patients | Male gender | Mean age (years) | Identifiable cause | Location | Histology | Surgical treatment | Adjuvant chemotherapy | Survival |
|---|---|---|---|---|---|---|---|---|---|
| Current study | 15 | 47% | 56.4 | 27% | Distal femur 33%, proximal tibia 40% | 80% MFH, 20% OS | 47% amputation, 33% limb salvage | 13% | 46% DOD at average of 7 months |
| 1992 to present [1, 2, 4–6, 9, 11, 12, 15] | 14 | 79% | 49.5 | 50% | Distal femur 43%. proximal tibia 14% | 50% MFH, 43% angiosarcoma, 7% OS | 57% amputation, 38% limb salvage | 21% | 42% DOD at average of 16.3 months |
| Torres and Kyriakos [17] | 38 | 68% | 53.4 | 53% | Femur 55%, tibia 37% | 63% MFH, 18% OS | 76% amputation, 21% limb salvage | 21% | 79% DOD at average of 24 months |
| All cases | 67 | 65.7% | 53.3 | 36% | About the knee 60% | 69% MFH, 17% OS, 9% angiosarcoma | 66% amputation, 27% limb salvage | 19% | 57% DOD at average of 19.2 months |
MFH = malignant fibrous histiocytoma; OS = osteosarcoma; DOD = dead of disease.
There are several limitations to our study. The sample size is small, a characteristic common to all the studies addressing secondary sarcomas. These cases occurred over a span of nearly 50 years, during which time medical and surgical management has changed greatly. Data were gathered retrospectively, and two of our patients were lost to followup (although they almost certainly died of their disease). The remainder of the data was collected from published reports that span two decades. At times, the information from the literature was incomplete. However, given the rarity of the condition, this is likely the most effective way of gathering meaningful data.
In 1992, Torres and Kyriakos [17] summarized the data in the existing literature, combining information for 38 patients from 26 reports. Twenty-six (68%) of the patients were males with an average age at diagnosis of 53.4 years. Pain was present in all patients with other presenting symptoms, including a mass, weight loss, or pathologic fracture. Twenty (53%) of the patients had an identifiable cause of infarct, including Caisson’s disease, alcoholism, sickle cell trait or disease, steroid use, and a hereditary condition. The tumors arose most commonly in the femur (21 of 38 [55%]) followed by the tibia (14 of 38 [37%]). Malignant fibrous histiocytoma was the most common diagnosis (24 patients [63%]). Seven patients (18%) had osteosarcoma, and five (13%) patients had fibrosarcoma. Anaplastic and pleomorphic sarcomas also were seen.
Most patients (36 of 38) underwent some form of surgery (two patients had radiation alone). Twenty-five (66%) patients had surgery alone with no other adjuvant treatment. The remainder of the patients had various adjuvant treatments. Five patients had chemotherapy alone, one had radiation alone, and three had radiation and chemotherapy. Eight of 38 (21%) patients received some form of chemotherapy. Overall, survival for the Torres and Kyriakos’ group of patients was poor. One of the seven (14%) patients with osteosarcoma was a long-term survivor, but he had a malignant fibrous histiocytoma develop in the opposite leg. Only 22% of the patients with malignant fibrous histiocytoma were alive and well at 5 years. Survival for the other patients averaged 24 months. Of eight patients (21%) who were long-term survivors, four had surgery alone, three had adjuvant postoperative chemotherapy, and one had preoperative radiation therapy.
Since Torres and Kyriakos’ 1992 publication, 14 additional cases have been documented, mostly as case reports [1, 2, 4–6, 9, 11, 12, 15], which we have reviewed. There were seven patients with malignant fibrous histiocytomas, six with angiosarcomas, and one with osteosarcoma. Eleven of the patients were male (79%) with an average age of 49.5 years at the time of diagnosis. Eight tumors originated about the knee (six distal femurs, two proximal tibias), two at the midshaft femur, two distally in the tibia, and one each in the proximal humerus and midshaft of the tibia. One-half of the patients had a preexisting condition that predisposed to bone infarction; 12 presented with pain and two presented with a pathologic fracture. In this collection, the most common form of treatment was amputation, performed in eight of the 14 patients. Five patients had a resection and some form of reconstruction. Only three patients received chemotherapy.
Of these 14 patients, two had no reported followup. Five of the remaining 12 patients died of their disease with an average survival of 16.3 months. Six patients survived without any evidence of disease at a minimum followup of 11 months (average, 3 years; range, 11 months–7 years). Three of the survivors had received some form of chemotherapy. At last followup, one patient was alive with progressive metastatic disease at 11 months.
Combining our series of 15 patients with those of Torres and Kyriakos [17] and the subsequent case reports, to the best of our knowledge, there have been 67 reported cases of infarct-associated sarcoma. The average age at presentation is 53.3 years, and men accounted for 66% of patients. The sarcoma arose about the knee in 60% of the patients (slightly more common in the distal femur than the proximal tibia). Other less common locations include the proximal femur, the femoral shaft, distal tibia, and proximal humerus. The type of tumor seen was malignant fibrous histiocytoma 69% of the time followed by osteosarcoma (17%) and angiosarcoma (9%). Fibrosarcoma and pleomorphic sarcoma also were represented. Thirty-six percent of the patients had an identifiable cause for bone infarction, mostly Caisson’s and sickle cell disease.
Treatment for these patients was almost always surgical (the exceptions include one patient who had biopsy alone and three who had biopsy and radiation therapy); 44 of the 67 patients (66%) were treated by amputation. Only 18 (27%) patients underwent limb salvage and had some type of reconstruction. Moreover, only 13 patients received chemotherapy, and seven patients were treated with radiation therapy.
The prognosis for these patients with a sarcoma associated with bone infarct appears relatively poor. Thirty-eight of 67 (57%) patients died of their disease at an average of 19.2 months after diagnosis. Only 21 of the 67 (31%) patients were continuously disease-free for at least 2 years. The remaining 12 patients had incomplete followup. Of the 13 patients who received chemotherapy, eight were long-term survivors (62%). In contrast, of the 54 patients who did not receive chemotherapy, only 13 survived (24%).
Although it is difficult to draw definitive conclusions from this type of analysis of few patients from multiple reports, we believe several trends common to the groups of patients studied have emerged. Bone infarct-associated sarcomas typically arise about the knee in patients in their 50s and 60s. A definitive cause of infarction is elucidated just over 1/3 of the time. More than 50% of patients die of their disease, and the majority of patients who die do so within 2 years.
However, in patients presenting without metastasis, aggressive treatment may lead to longer-term survivorship. In our series, the 2-year disease-free survival rate was 63%. Similar findings were reported by Shaheen et al. [16] with aggressive treatment of nonmetastatic radiation-induced sarcoma of bone. In that study, there was a subset of 10 patients who presented without metastases and were treated aggressively with chemotherapy and surgery. For that group, the 2-year disease-free survival rate was 70%, which is similar to the survival rate reported for patients with primary localized sarcoma of bone treated with chemotherapy and surgery (50% to 80%) [3]. In addition, Shaheen et al. [16] reported a trend suggesting optimal outcomes were achieved more commonly in patients who received chemotherapy. Many patients with radiation-induced sarcoma of the bone, because of age or comorbidities, are treated with lower drug doses and shorter duration of chemotherapy. Seven patients in the study by Shaheen et al. had received incomplete chemotherapy, but two of these patients remained alive without detectable disease at 52 and 65 months, leading the authors to conclude even incomplete chemotherapy was better than none.
Sarcoma secondary to bone infarct is a rare and challenging clinical entity for physicians and patients. Most of the data suggest this is an aggressive disease, but there is a paucity of information to help guide management. Because of the rarity of this disease, it is unlikely prospective studies will reveal the optimal treatment regimen. Based on our small, single-institution series, patients without metastatic disease and treated with wide surgical resection have a reasonable chance (63%) of surviving longer than 2 years. By combining the data from all reported cases of bone infarct-associated sarcoma, patients treated with any form of chemotherapy had a 62% 2-year disease-specific survival rate compared with the 24% rate observed in those not treated with chemotherapy. These findings suggest chemotherapy should be considered as a potentially beneficial adjuvant treatment in the management of this disease. Perhaps, with more aggressive treatment, survival for patients with this rare sarcoma can be improved.
Acknowledgments
We thank Joanne Clarke for editorial assistance in preparing the manuscript for publication.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution either has waived or does not require approval for the reporting of this case and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Abdelwahab IF, Kenan S, Klein MJ, Lewis MM. Case report: angiosarcoma occurring in a bone infarct. Clin Radiol. 1992;45:412–414. [DOI] [PubMed]
- 2.Abdelwahab IF, Klein MJ, Hermann G, Springfield D. Angiosarcomas associated with bone infarcts. Skeletal Radiol. 1998;27:546–551. [DOI] [PubMed]
- 3.Bielack S, Kempf-Bielack B, Delling G, Exner G, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jurgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2002;20:776–790. [DOI] [PubMed]
- 4.Cerilli LA, Fechner RE. Angiosarcoma arising in a bone infarct. Ann Diagn Pathol. 1999;3:370–373. [DOI] [PubMed]
- 5.Desai P, Perino G, Present D, Steiner GC. Sarcoma in association with bone infarcts: report of five cases. Arch Pathol Lab Med. 1996;120:482–489. [PubMed]
- 6.Duong S, Sallis JG, Zee SY. Malignant fibrous histiocytoma arising within a bone infarct in a patient with sickle cell trait. Int J Surg Pathol. 2004;12:67–73. [DOI] [PubMed]
- 7.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed]
- 8.Furey JG, Ferrer-Torrer-Torells M, Reagan JW. Fibrosarcoma arising at the site of bone infarcts: a report of 2 cases. J Bone Joint Surg Am. 1960;42:802–810. [PubMed]
- 9.Gaucher AA, Regent DM, Gillet PM, Pere PG, Aymard BM, Clement V. Case report 656: malignant fibrous histiocytoma in a previous bone infarct. Skeletal Radiol. 1991;20:137–140. [DOI] [PubMed]
- 10.Healey JH, Buss D. Radiation and pagetic osteogenic sarcomas. Clin Orthop Relat Res. 1991;270:128–134. [PubMed]
- 11.Kenan S, Abdelwahab IF, Hermann G, Klein MJ. Malignant fibrous histiocytoma associated with a bone infarct in a patient with hereditary bone dysplasia. Skeletal Radiol. 1998;27:463–467. [DOI] [PubMed]
- 12.Matsuno T, Kaneda K, Takeda N. Development of angiosarcoma at the site of a bone infarct. Clin Orthop Relat Res. 1996;327:259–263. [DOI] [PubMed]
- 13.Michael RH, Dorfman HD. Malignant fibrous histiocytoma associated with bone infarcts: report of a case. Clin Orthop Relat Res. 1976;118:180–183. [PubMed]
- 14.Pins MR, Mankin HJ, Xavier RJ, Rosenthal DI, Dickersin GR, Rosenberg AE. Malignant epithelioid hemangioendothelioma of the tibia associated with a bone infarct in a patient who had Gaucher disease: a case report. J Bone Joint Surg Am. 1995;77:777–781. [DOI] [PubMed]
- 15.Resnik CS, Aisner SC, Young JW, Levine A. Case Report 767: osteosarcoma arising in bone infarction. Skeletal Radiol. 1993;22:58–61. [DOI] [PubMed]
- 16.Shaheen M, Deheshi BM, Riad S, Werier J, Holt GE, Ferguson PC, Wunder JS. Prognosis of radiation-induced bone sarcoma is similar to primary osteosarcoma. Clin Orthop Relat Res. 2006;450:76–81. [DOI] [PubMed]
- 17.Torres FX, Kyriakos M. Bone infarct-associated osteosarcoma. Cancer. 1992;70:2418–2430. [DOI] [PubMed]