Abstract
Various reports confirm elevations in serum markers associated with skeletal muscle injury after orthopaedic surgery in the absence of overt clinical manifestations of myocardial injury. We therefore measured the influence surgical approach has on these serum markers after primary THA. We nonrandomly enrolled 30 nonconsecutive patients undergoing THA in three groups of 10 based on current surgical approaches used at our facility: (1) minimally invasive (MIS) modified Watson Jones approach; (2) miniposterior transmuscular approach (MIS-I); and (3) MIS-II incision. Blood samples for hemoglobin, hematocrit, cardiac troponin I, total creatine kinase, creatine phosphokinase, and serum myoglobin were obtained the morning before surgery as a baseline, immediately postoperatively, and 72 hours thereafter. We found reproducible trends in serum enzyme levels consistent with skeletal muscle damage resulting from primary THA. Troponin I remained normal in all but one patient indicating no myocardial contribution to measured serum enzyme levels. All three procedures resulted in similar trends in serum enzyme markers relevant to primary THA. Our preliminary data suggest no surgical approach appears to affect the degree of muscle trauma more or less than another.
Introduction
Recent developments in orthopaedic surgery indicate primary THA can be completed successfully using MIS approaches [1, 3, 5, 6, 8, 26, 27]. The theoretical benefits of these approaches include less tissue dissection, decreased surgical blood loss, decreased postoperative pain, decreased time to hospital discharge, decreased time to the return of activities of daily living, and the potential for cost savings across the entire continuum of care [6, 26]. Although MIS surgical approaches may be applied to various procedures across all disciplines of orthopaedics, and the intuitive benefits of a smaller incision length are well communicated across all public media, there still exists conflicting results in the medical literature regarding their overall benefit [10, 25, 30].
Hemoglobin and hematocrit are commonly used measurements of the effect of bleeding during and after surgical procedures. Serial assays of creatine kinase (CK), creatine phosphokinase (CPK), serum myoglobin, and cardiac troponin I are laboratory tests of choice for the diagnosis of myocardial injury. Various authors report changes in these serum markers as a result of skeletal muscle injury, which also correlates with surgical incision length, surgical approach, and operative time [12, 16, 18, 20, 21, 24]. These reports confirm elevations in CK, CPK, and serum myoglobin can occur after orthopaedic surgery in the absence of overt clinical manifestations of myocardial injury.
In our preliminary study, we addressed the following questions: (1) Is there a measurable effect of surgery related to primary THA and defined serum markers associated with skeletal muscle injury? If there is a measurable effect of THA on these serum markers, (2) what is their relation with time postoperatively after primary THA? (3) How do these results compare with reported serum levels related to other surgical procedures, systemic failures, or trauma?
Materials and Methods
We nonrandomly enrolled 10 nonconsecutive subjects in each of three groups based on surgical techniques currently used by the two surgeon authors (RGC, JAK) in our practice for primary THA. Patients must have met standard primary THA inclusion criteria to be eligible for study participation, which included failure of nonoperative treatment for a severely disabled joint secondary to painful osteoarthritis, rheumatoid arthritis, posttraumatic arthritis, or displaced femoral neck fracture that warranted primary THA, and stable health as warranted for performing THA. The combined demographics of the patients recruited included 14 (47%) males and 16 (53%) females with an average age of 74.8 ± 7.5 (range, 55–86 years) and an average body mass index of 28.0 ± 4.5 kg/m2 (range, 22–39 kg/m2) (Tables 1, 2). We had prior Institutional Review Board approval and informed consent for the study.
Table 1.
Patient demographics by surgical incision group
| Group | Gender | Age (years)* | Body mass index (kg/m2)* | |
|---|---|---|---|---|
| Males | Females | |||
| MIS modified Watson Jones | 3 | 7 | 74.8 ± 8.4 | 28.0 ± 4.6 |
| 61–86 | 21.8–37.3 | |||
| Miniposterior THA | 4 | 6 | 73.0 ± 5.5 | 26.9 ± 4.0 |
| 66–82 | 22.1–32.5 | |||
| MIS II incision THA | 7 | 3 | 69.5 ± 8.1 | 27.6 ± 5.2 |
| 55–83 | 21.6–38.6 | |||
* Mean ± standard deviation and range.
Table 2.
Intraoperative data by surgical group
| Group | Operative time (minutes) | Incision length (cm) | Estimated blood loss (cc) |
|---|---|---|---|
| MIS modified Watson Jones THA | 46.5 ± 5.8 | 10.4 ± 2.1 | 115.0 ± 66.9 |
| 40–55 | 9.0–15.0 | 50–300 | |
| Miniposterior THA | 66.4 ± 7.0 | 8.8 ± 2.3 | 211.1 ± 116.7 |
| 60–75 | 6.0–13.0 | 100–500 | |
| MIS II incision (posterior incision) | NA | 3.4 ± 0.5 | NA |
| 3.0–4.0 | |||
| MIS II incision (anterior incision) | NA | 6.0 ± 0.7 | NA |
| 5.0–7.0 | |||
| MIS II (combined incision length) | 63.7 ± 12.0 | 9.4 ± 0.9 | 135.0 ± 62.6 |
| 45–83 | 8.0–11.0 | 100–300 |
Values are mean ± standard deviation and range; NA = not available.
The three surgical approach groups included an MIS modified Watson Jones approach, a miniposterior approach, and an MIS-II incision approach. The MIS modified Watson Jones approach is an anterior approach that traverses the tensor fascia and gluteus medius without any muscle detachment. It therefore is an intermuscular approach that requires retraction against two muscle bellies. The miniposterior approach uses a familiar approach to the hip through a smaller incision but with less muscle detachment than its standard incision counterpart. The clinical advantage is a more intact soft tissue envelope with greater stability, less muscle and tissue dissection, and easier recovery for the patient. The MIS-II incision THA requires a direct anterior approach to the acetabulum through an intermuscular plane between the sartorius and tensor fascia. The posterior incision is for placement of the femoral component, requiring passage of instruments through the belly of the gluteus maximus and posterior aspect of the gluteus medius. Data collected intraoperatively included incision length, operative time (skin to skin), and estimated blood loss. We also recorded incision length associated with these approaches to see if this had any effect on the overall results.
Blood samples for total CK, CPK, and serum myoglobin were obtained at screening and the morning before surgery as a baseline. Thereafter, blood samples were drawn immediately postoperatively in the recovery room and 8, 16, 24, 36, 48, and 72 hours postoperatively. We obtained hemoglobin (g/dL) and hematocrit (%) preoperatively and 16, 36, and 72 hours (± 6 hours) postoperatively. Cardiac troponin I (ng/mL) was measured the morning of surgery (preoperatively) and 16 hours after surgery to monitor any contributory effect of myocardial injury.
We collected serum specimens and screened for hemolysis and lipemia before freezing at −20°C for batch analysis to avoid interassay variation. Lipemic specimens were ultracentrifuged before freezing. We analyzed batched frozen specimens within 30 days of first draw. Creatine kinase (serum) was analyzed using the reverse reaction and activation by NAC as recommended by the International Federation of Clinical Chemistry and Laboratory Medicine using Roche Hitachi 912 instrumentation (F. Hoffmann-La Roche, Ltd, Basel, Switzerland). Creatine kinase values were not adjusted for physical activity level or patient race. We determined serum myoglobin levels using a solid-phase, two-site chemiluminescent enzyme immunometric assay (Immunolite 2000; DPC, Los Angeles, CA). Any assay results greater than 1000 ng/mL were repeated after dilution.
Standard demographics were collected on all patients while maintaining confidentiality. We used simple descriptive statistics to describe the sample populations. Statistical power for further study recommendations was calculated from our preliminary observations for the particular number of cases to achieve 90% power.
Results
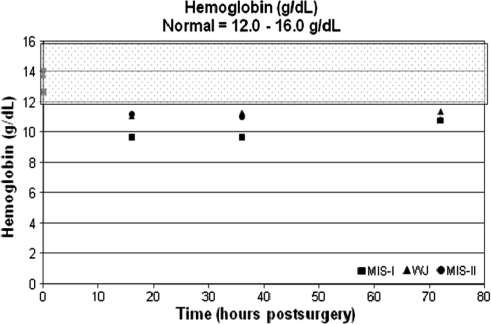
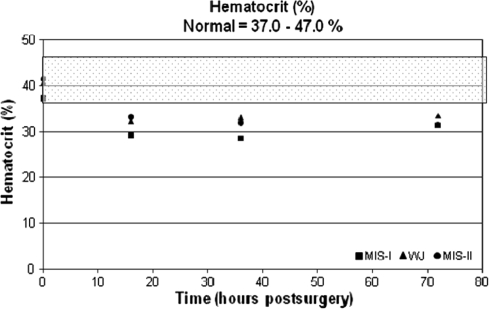
The average hemoglobin, percent hematocrit, and troponin I values were within normal ranges preoperatively across all groups. The average hemoglobin and percent hematocrit decreased and remained at these levels through 72 hours postoperatively (Table 3; Figs. 1, 2). The troponin I levels for patients in the MIS modified Watson Jones and MIS-II incision groups were 0.2 ng/mL at baseline and remained unchanged through 72 hours. All patients in the miniposterior group had a baseline troponin I level of 0.2 ng/mL; however, two patients in the miniposterior group had elevated troponin I levels at 72 hours (1.0 and 1.5 ng/mL).
Table 3.
Average serum values (± standard deviation) by incision type preoperatively and for postoperative values*
| Blood serum/ surgical approach | Preoperative | 1 hour | 8 hours | 16 hours | 24 hours | 36 hours | 48 hours | 72 hours |
|---|---|---|---|---|---|---|---|---|
| Hematocrit | ||||||||
| WJ | 40.5 (4.5) | — | — | 32.2 (4.2) | — | 33.0 (3.1) | — | 33.4 (4.2) |
| MIS-I | 37.0 (3.7) | — | — | 28.9 (3.9) | — | 28.5 (3.1) | — | 31.3 (2.6) |
| MIS-II | 41.2 (3.2) | — | — | 33.0 (2.9) | — | 31.8 (3.8) | — | 31.5 (2.5) |
| Hemoglobin | ||||||||
| WJ | 13.8 (1.6) | — | — | 11.1 (1.5) | — | 11.3 (1.0) | — | 11.4 (1.6) |
| MIS-I | 12.7 (1.4) | — | — | 9.7 (1.5) | — | 9.7 (1.2) | — | 10.8 (0.9) |
| MIS-II | 14.1 (1.2) | — | — | 11.2 (1.0) | — | 11.0 (1.5) | — | 10.8 (1.0) |
| CK | ||||||||
| WJ | 3.4 (1.8) | 5.4 (3.2) | 10.2 (6.5) | 7.0 (4.2) | 5.4 (2.4) | 5.1 (2.0) | 4.2 (2.0) | 3.0 (2.0) |
| MIS-I | 3.4 (2.1) | 4.6 (2.7) | 9.6 (6.8) | 8.0 (4.8) | 6.7 (4.2) | 5.4 (2.9) | 5.1 (3.9) | 4.4 (3.2) |
| MIS-II | 3.5 (2.1) | 4.5 (2.2) | 9.9 (5.5) | 8.5 (3.1) | 7.4 (3.8) | 5.0 (2.1) | 4.4 (1.6) | 3.4 (1.3) |
| CPK | ||||||||
| WJ | 99.2 (66.6) | 158.3 (85.5) | 466.7 (206.3) | 403.0 (183.0) | 426.0 (189.5) | 378.1 (250.2) | 348.6 (230.8) | 250.4 (196.2) |
| MIS-I | 88.8 (70.3) | 119.5 (60.5) | 327.6 (177.4) | 300.9 (225.6) | 338.3 (213.5) | 318.7 (168.4) | 296.1 (162.4) | 241.9 (136.0) |
| MIS-II | 79.9 (53.4) | 242.7 (266.7) | 512.4 (173.3) | 515.4 (248.1) | 470.2 (231.6) | 511.6 (278.4) | 508.8 (231.4) | 441.6 (253.1) |
| SM | ||||||||
| WJ | 45.4 (41.1) | 367.3 (250.6) | 346.4 (144.2) | 226.1 (88.1) | 180.6 (75.9) | 115.2 (27.6) | 86.2 (29.5) | 60.8 (21.9) |
| MIS-I | 39.6 (23.5) | 298.4 (175.4) | 289.4 (259.0) | 225.8 (209.6) | 157.7 (75.1) | 146.7 (86.6) | 100.2 (37.9) | 72.6 (28.9) |
| MIS-II | 37.1 (17.2) | 241.3 (155.5) | 427.6 (247.3) | 320.9 (174.1) | 220.6 (119.4) | 168.0 (91.2) | 118.2 (49.7) | 79.8 (38.7) |
* Normal range and units: hematocrit, 37%–47%; hemoglobin, 12–16 g/dL; creatine kinase (CK), 0.2–4.8 ng/mL; creatine phosphokinase (CPK), 38–174 U/L; serum myoglobin (SM), 0.5–70.0 ng/mL; WJ = modified Watson Jones.
Fig. 1.
The graph shows hemoglobin (g/dL) versus time (hours). The normal range (12.0–16.0 g/dL) is highlighted by the shaded overlay. MIS = minimally invasive surgery; WJ = modified Watson Jones.
Fig. 2.
Hematocrit (%) versus time (hours) is shown in this graph. The normal range (37.0%–47.0%) is highlighted by the shaded overlay. MIS = minimally invasive surgery; WJ = modified Watson Jones.
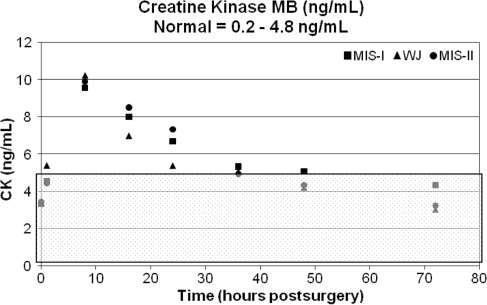
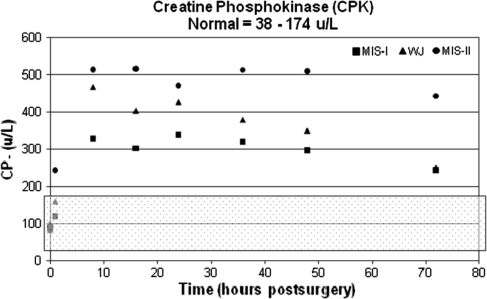
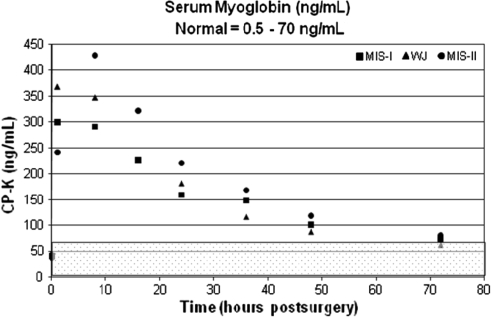
The postoperative results for CK, CPK, and serum myoglobin reached peak values at 8 hours. The CK and serum myoglobin values gradually decreased toward normal after the observed peak measurements. The average CK levels returned to normal 48 hours postoperatively and the average serum myoglobin values returned to normal 72 hours postoperatively. However, the CPK values remained elevated through 48 hours postoperatively and showed a slight downward trend 72 hours postoperatively (Figs. 3, 4, 5). When compared with the preoperative baseline values, all serum markers were elevated (Table 3).
Fig. 3.
The graph shows creatine kinase (CK; ng/mL) versus time (hours). The normal range (0.2–4.8 ng/mL) is highlighted by the shaded overlay. MIS = minimally invasive surgery; WJ = modified Watson Jones.
Fig. 4.
Creatine phosphokinase (U/L) versus time (hours) is shown. The normal range (38–174 U/L) is highlighted by the shaded overlay. MIS = minimally invasive surgery; WJ = modified Watson Jones.
Fig. 5.
The graph shows serum myoglobin (ng/mL) with time. The normal range (0.5–70.0 ng/mL) is highlighted by the shaded overlay. MIS = minimally invasive surgery; WJ = modified Watson Jones.
Discussion
Reported use of blood enzyme markers is common throughout the surgical literature [9, 12, 16–18, 20–24]. Rhabdomyolysis has numerous etiologies and includes local crush injury, vascular compromise, soft tissue infections, electrical injuries, excessive steroid use, seizures, surgery, and heat stroke [1, 19, 22]. Whether these changes occur after differing hip approaches is unknown. We therefore raised the following questions: (1) Is there a measurable effect of surgery related to primary THA and defined serum markers associated with skeletal muscle injury? If there is a measurable effect of THA on these serum markers, (2) what is their relation with time postoperatively after primary THA? (3) How do these results compare with reported serum levels related to other surgical procedures, systemic failures, or trauma?
Our serum results were less than those reported after other surgical procedures, systemic failures, or trauma, but we had no contribution of serum levels from cardiac injury. We were unable to show serum differences across the three surgical groups owing to lack of statistical power because of the small numbers in each group. The measured marker values for each surgical group showed small differences but parallel patterns with time. From our preliminary findings we calculated that to achieve statistical power of 90%, we would need to enroll 65 patients in each of the three surgical groups.
Direct muscle compression resulting from trauma is a common mechanism for liberation of the components of injured skeletal muscle into the circulation, and serum CK becomes considerably elevated with a peak occurring within 24 hours of the injury [28]. The serum enzymes measured for our study have a circulating half-life of approximately 12 hours. Additionally, the use of measured cardiac proteins such as troponin I is specific for coronary injury and predicts adverse events after possible acute coronary syndrome [23]. For this study, we used troponin I to rule out any coronary injury that may confound our results.
The introduction of various less invasive surgical techniques for primary THA has led to much discussion, and frequently, contradictory results. The determination of the justification of various MIS procedures usually involves standard postoperative radiographic assessments of component position and well-accepted pain and functional scoring systems [14]. Current scoring systems used may not be sensitive enough to detect consistent differences among various MIS approaches postoperatively. Unfortunately, use of subjective measurements of outcome may contribute to contradiction in reported theoretical advantages of MIS THA for patients. Sculco et al. reported the results of the miniposterior to standard THA and found a decrease in operative times and a difference in function (limp) at 6 weeks in the mini-incision group [26, 27]. Hartzband and Berry et al. reported earlier return to function using an MIS technique for primary THA [8, 15]. Likewise, Berger et al. reported decreased blood loss, decreased length of stay, and more rapid return to function [2–7]. However, in a randomized, controlled trial, Ogonda et al. reported no difference in the theoretical advantages previously reported for MIS techniques [25]. Moreover, no difference in their length of hospital stay, noted complications involving wound healing, and acetabular component malposition contradict the theoretical short-term results. Also, some reports turn to objective variable measurements with equally conflicting results [10, 29, 30]. Woolson et al. reported the results of a study comparing a miniposterior THA with a standard posterior approach for THA [30]. No major differences were measured regarding operative time, estimated intraoperative blood loss, or time to discharge. Wound complications, acetabular component malposition, and femoral component fit and fill undersizing were greater in the mini-incision group. However, in a separate study, Goldstein et al. reported major decreases in estimated intraoperative blood loss for the same surgical approach comparison [13]. We attempted to identify objective, systemic measurements for surgical procedures for use in primary THA. Creatine kinase is a naturally occurring enzyme with the greatest concentrations found in skeletal muscle, cardiac muscle, and the brain [9, 19]. Myoglobin also is found in cardiac and skeletal muscle and is released into the circulation when muscle is damaged through disease, exertion, or trauma and is excreted in the urine [9, 16, 19, 20, 22]. The use of serum markers, including CK, CPK, and serum myoglobin, may allow for an objective evaluation of the potential advantages among various MIS techniques. The use of minimally invasive techniques theoretically disrupts less of the soft tissue envelope surrounding the hip and thus may have less of an effect on these serum markers. We found early increased releases of CK, CPK, and myoglobin into the circulation after primary THA using all three of these incision types, but without statistical differences. To rule out any contributory effect of cardiac muscle, we measured troponin I serum levels [17], a serum enzyme marker specific to cardiac muscle. We found there was only one incident of elevated troponin I, which occurred 72 hours after surgery. Therefore, all serum markers measured were a result of the muscle damage resulting from surgery.
We found CK, CPK, and serum myoglobin all had peak values 8 hours postoperatively. Feinfeld et al. reported CK values used to predict renal failure can reach 20,000 ng/mL [11]. However, in our study, there were CK levels of 10 ng/mL, which is considerably less than any levels related to systemic failure or traumatic injury. The assays used were sensitive enough to show specific time-related systemic changes resulting from each surgical technique. However, all three procedures followed parallel trends and any separation between surgical procedures was not noteworthy. This may be the result of various confounding factors, including the sensitivity of the assay, patient body mass index, and total surgical time rather than surgical approach or incision length. Moreover, the number of patients enrolled was too small to reach any level of statistical power and additional study will require 65 patients per group to achieve 90% power.
We observed measurable trends in serum enzyme levels consistent with skeletal muscle damage resulting from THA. However, in the three surgical approaches compared, no serum enzyme level approached levels reported with severe trauma or myocardial disease, and troponin I remained normal in all but one patient throughout the entire study indicating no myocardial contribution. Although these trends may have specificity with approach type, they were not statistically different across the three groups at any one time. This is contradictory to any previous subjective claims of decreased muscular trauma with a single MIS incision versus a standard incision or any increased muscular trauma with a MIS-II incision THA versus a miniposterior THA [2, 8, 13, 25, 27]. Moreover, the sensitivity of the tests used may not be able to differentiate small differences in the contributory effects of surgical incision length, muscle groups affected, and operative time. However, we suggest all three MIS procedures for primary THA affect systemic enzyme markers in a similar fashion. Additional study would determine the values of a more traditional surgical approach such as the Southern approach or a standard Hardinge-type approach to the MIS procedures described. However, in our practice, patients commonly request less invasive surgical options and thus it may be difficult to accrue the numbers needed for additional study.
Acknowledgments
We thank Andrea H. Robinson, CCRC, for assistance in collecting and organizing data presented in this article and Amy Parks for assistance in preparation of this manuscript.
Footnotes
One or more of the authors (RGC, JAK) have received funding from Zimmer, Inc for this study.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Alzeer AH, el-Hazmi MA, Warsy AS, Ansari ZA, Yrkendi MS. Serum enzymes in heat stroke: prognostic implication. Clin Chem. 1997;43:1182–1187. [PubMed]
- 2.Berger RA. Total hip arthroplasty using the minimally invasive two-incision approach. Clin Orthop Relat Res. 2003;429:232–241. [DOI] [PubMed]
- 3.Berger RA. Mini-incision total hip replacement using an anterolateral approach: technique and results. Orthop Clin North Am. 2004;35:143–151. [DOI] [PubMed]
- 4.Berger RA. Minimally invasive THR using two incisions. Orthopedics. 2004;27:382–383. [DOI] [PubMed]
- 5.Berger RA, Duwelius PJ. The two-incision minimally invasive total hip arthroplasty: technique and results. Orthop Clin North Am. 2004;35:163–172. [DOI] [PubMed]
- 6.Berger RA, Jacobs JJ, Meneghini RM, Della VC, Paprosky W, Rosenberg AG. Rapid rehabilitation and recovery with minimally invasive total hip arthroplasty. Clin Orthop Relat Res. 2004;429:239–247. [DOI] [PubMed]
- 7.Berger RA, Sanders S, D’Ambrogio E, Buchheit K, Deirmengian C, Paprosky W, la Valle CJ, Rosenberg AG. Minimally invasive quadriceps-sparing TKA: results of a comprehensive pathway for outpatient TKA. J Knee Surg. 2006;19:145–148. [DOI] [PubMed]
- 8.Berry DJ, Berger RA, Callaghan JJ, Dorr LD, Duwelius PJ, Hartzband MA, Lieberman JR, Mears DC. Minimally invasive total hip arthroplasty: development, early results, and a critical analysis. J Bone Joint Surg Am. 2003;85:2235–2246. [PubMed]
- 9.de Meijer AR, Fikkers BG, de Keijzer MH, van Engelen BG, Drenth JP. Serum creatine kinase as predictor of clinical course in rhabdomyolysis: a 5-year intensive care survey. Intensive Care Med. 2003;29:1121–1125. [DOI] [PubMed]
- 10.Fehring TK, Mason JB. Catastrophic complications of minimally invasive hip surgery: a series of three cases. J Bone Joint Surg Am. 2005;87:711–714. [DOI] [PubMed]
- 11.Feinfeld DA, Cheng JT, Beysolow TD, Briscoe AM. A prospective study of urine and serum myoglobin levels in patients with acute rhabdomyolysis. Clin Nephrol. 1992;38:193–195. [PubMed]
- 12.Fransen EJ, Diris JH, Maessen JG, Hermens WT, van Dieijen-Visser MP. Evaluation of ‘new’ cardiac markers for ruling out myocardial infarction after coronary artery bypass grafting. Chest. 2002;122:1316–1321. [DOI] [PubMed]
- 13.Goldstein WM, Branson JJ, Berland KA, Gordon AC. Minimal-incision total hip arthroplasty. J Bone Joint Surg Am. 2003;85(suppl 4):33–38. [DOI] [PubMed]
- 14.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty: an end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed]
- 15.Hartzband MA. Posterolateral minimal incision for total hip replacement: technique and early results. Orthop Clin North Am. 2004;35:119–129. [DOI] [PubMed]
- 16.Healey JH, Kagen LJ, Velis KP, Levine DB. Creatine kinase MB in skeletal muscle and serum of spine-fusion patients. Clin Orthop Relat Res. 1985;195:282–288. [PubMed]
- 17.Jules-Elysee K, Urban MK, Urquhart B, Milman S. Troponin I as a diagnostic marker of a perioperative myocardial infarction in the orthopedic population. J Clin Anesth. 2001;13:556–560. [DOI] [PubMed]
- 18.Kawaguchi Y, Matsui H, Gejo R, Tsuji H. Preventive measures of back muscle injury after posterior lumbar spine surgery in rats. Spine. 1998;23:2282–2287. [DOI] [PubMed]
- 19.Kodatsch I, Finsterer J, Stollberger C. Serum creatine kinase elevation in a medical department. Acta Med Austriaca. 2001;28:11–15. [DOI] [PubMed]
- 20.Laurence AS. Serum myoglobin and creatine kinase following surgery. Br J Anaesth. 2000;84:763–766. [DOI] [PubMed]
- 21.Lenke LG, Bridwell KH, Jaffe AS. Increase in creatine kinase MB isoenzyme levels after spinal surgery. J Spinal Disord. 1994;7:70–76. [DOI] [PubMed]
- 22.Malinoski DJ, Slater MS, Mullins RJ. Crush injury and rhabdomyolysis. Crit Care Clin. 2004;20:171–192. [DOI] [PubMed]
- 23.McCord J, Nowak RM, Hudson MP, McCullough PA, Tomlanovich MC, Jacobsoen G, Tokarski G, Khoury N, Weaver WD. The prognostic significance of serial myoglobin, troponin I, and creatine kinase-MB measurements in patients evaluated in the emergency department for acute coronary syndrome. Ann Emerg Med. 2006;42:343–350. [DOI] [PubMed]
- 24.Neill F, Sear JW, French G, Lam H, Kemp M, Hooper RJ, Foex P. Increases in serum concentrations of cardiac proteins and the prediction of early postoperative cardiovascular complications in noncardiac surgery patients. Anaesthesia. 2000;55:641–647. [DOI] [PubMed]
- 25.Ogonda L, Wilson R, Archbold P, Lawlor M, Humphreys P, O’Brien S, Beverland D. A minimal-incision technique in total hip arthroplasty does not improve early postoperative outcomes: a prospective, randomized, controlled trial. J Bone Joint Surg Am. 2005;87:701–710. [DOI] [PubMed]
- 26.Sculco TP. Less invasive total hip arthroplasty. Am J Orthop. 2006;35:217. [PubMed]
- 27.Sculco TP, Jordan LC, Walter WL. Minimally invasive total hip arthroplasty: the Hospital for Special Surgery experience. Orthop Clin North Am. 2004;35:137–142. [DOI] [PubMed]
- 28.Ward MM. Factors predictive of acute renal failure in rhabdomyolysis. Arch Intern Med. 1988;148:1553–1557. [DOI] [PubMed]
- 29.Wenz JF, Gurkan I, Jibodh SR. Mini-incision total hip arthroplasty: a comparative assessment of perioperative outcomes. Orthopedics. 2002;25:1031–1043. [DOI] [PubMed]
- 30.Woolson ST, Mow CS, Syquia JF, Lannin JV, Schurman DJ. Comparison of primary total hip replacements performed with a standard incision or a mini-incision. J Bone Joint Surg Am. 2004;86:1353–1358. [DOI] [PubMed]