Abstract
Accurate evaluation of programmed cell death, or apoptosis, in chondrocytes is essential to studying cartilage injury. We evaluated four methods of detecting chondrocyte-programmed cell death in formalin-fixed, paraffin-embedded cartilage after experimental osteochondral fracture. Human osteochondral explants were subjected to experimental fracture in a manner known to induce high levels of chondrocyte-programmed cell death. After 4 days in culture, specimens were fixed and analyzed for programmed cell death using: (1) terminal deoxynucleotidyl transferase end labeling; (2) DNA denaturation analysis using an antibody specific for single-stranded DNA; (3) immunohistochemistry using antisera specific for active caspase-3; and (4) in situ oligonucleotide ligation. Quantitative analysis of programmed cell death levels for each technique was performed comparing injured and uninjured areas of cartilage. We observed differences between injured and uninjured areas of cartilage using the four methods. Human cartilage fixed in zinc-formalin and embedded in paraffin is amenable to programmed cell death analysis using any of four independent methods, each of which ostensibly has some advantages in terms of assaying different steps along the apoptotic pathway. Using the protocols described in this article, investigators may have additional tools to identify and quantify chondrocytes undergoing programmed cell death after experimental cartilage injury.
Introduction
Chondrocyte-programmed cell death (PCD) is a critical event during normal embryologic development and bone growth. PCD has been implicated in the pathogenesis of osteoarthritis, rheumatoid arthritis, and posttraumatic arthritis, all increasingly common diagnoses in our active and aging population [3, 11]. A thorough investigation of chondrocyte PCD is critical to our understanding of degenerative joint diseases and for developing new therapeutic approaches. One of the greatest technical challenges facing investigators in this field of study is the requirement for accurate, consistent, and convenient methods for identifying apoptotic chondrocytes in cartilage. The need for multiple complementary techniques is well accepted as a result of recognized shortcomings of relying on any single method for PCD analysis. Chondrocyte PCD analysis is particularly difficult in paraffin-embedded specimens in which loss of antigenicity can render antibody-based techniques useless.
Classically, cells undergoing apoptosis were identified based on morphologic criteria with characteristic features further delineated by electron microscopy [10]. Studies have described the use of standard bright field light microscopy and hematoxylin and eosin staining to identify and quantify chondrocyte apoptosis in samples of arthritic cartilage [1, 11]. However, efforts using this methodology to assess chondrocyte PCD after experimental cartilage injury have been inconsistent [16, 17]. One contributing factor may be that chondrocytes undergoing PCD may not exhibit classic apoptotic morphologic features for most other sorts of cells [18].
There are, however, four readily available methods to detect PCD in fixed, paraffin-embedded cartilage samples, including terminal deoxynucleotidyl transferase end labeling (TUNEL), DNA denaturation analysis using anti-single-stranded DNA (ssDNA) antibody, anti-active caspase-3, and in situ oligonucleotide ligation (ISOL). TUNEL identifies apoptotic cells based on DNA fragmentation, which is one hallmark of apoptosis. The DNA fragmentation results in large numbers of free 3′-OH termini. In the TUNEL technique, these free ends are enzymatically labeled. Although TUNEL is by far the most commonly used method for analyzing chondrocyte PCD in cartilage, substantial controversy exists concerning its ability to distinguish between apoptotic and necrotic cell death [8]. When used to study apoptosis in osteoarthritic cartilage, TUNEL is believed to overestimate apoptosis [1]. In hepatocytes, TUNEL is nonspecific for PCD because other methods of cell death involve DNA fragmentation [8].
DNA denaturation analysis using anti-ssDNA antibody is based on the selective denaturing of DNA in apoptotic cells with heat treatment. The increased sensitivity of DNA to heat treatment observed in apoptotic cells is believed to be the result of disruption of DNA-histone interactions and is independent of DNA strand breaks [4]. This technique may identify cells at earlier stages of apoptosis than TUNEL [23]. Most importantly, this technique is highly specific for cells undergoing PCD and does not label cells undergoing necrotic cell death [7].
Caspase-3 is a critical enzymatic mediator of PCD and appears to play key roles in the late initiation and early execution phases of apoptosis. Detection of activated caspase-3 using antibodies specific for the active enzyme has been used successfully in a wide variety of tissues [13, 15, 22]. Investigators studying chondrocyte PCD in osteoarthritic cartilage have reported good correlation between anti-active caspase-3 staining and TUNEL analysis [14].
ISOL, like TUNEL, detects DNA fragmentation that occurs in apoptotic cells. However, ISOL is more specific than TUNEL because it labels only double-stranded DNA fragments with blunt ends or single 3′ base overhangs [5, 9]. These types of free ends are much more common in cells undergoing PCD than in cells undergoing necrosis [5]. Of these four techniques, only TUNEL has been widely used for analysis of PCD in cartilage [3].
To compare these methods, we used two cartilage sources with known apoptotic cells. First, we mechanically induced PCD with a simulated intraarticular fracture. Second, we used human growth plate cartilage in which PCD occurs during the process of endochondral ossification.
Materials and Methods
We obtained human tibial plateau osteochondral specimens from a skeletally mature male at the end of his second decade of life undergoing limb salvage for a distal femoral tumor not involving the regions of the specimens. After surgical excision, the specimen was stored in sterile lactated Ringer’s solution at room temperature until the experimental cartilage injury was created (approximately 4 hours after excision). To create the injury, we used a curette to make a single sagittal groove beneath the central portion of the lateral plateau penetrating through the cancellous bone into the subchondral bone. The specimen then was subjected to a three-point bending force across the weakened area until the cartilage and subchondral bone fractured. The osteochondral specimens then were placed in standard cell culture conditions with DMEM for 4 days at 37°C in the incubator with 5% CO2. The specimens then were fixed in zinc-buffered formalin (Z-Fix; Anatech Ltd, Battle Creek, MI) at 4°C overnight. Specimens were dehydrated, embedded in paraffin, sectioned at 7-μm thickness, and mounted on Superfrost® Plus slides (Fisher Scientific, Pittsburgh, PA). Sections were deparaffinized in xylene and rehydrated before subsequent analysis. A minimum of four near-adjacent sections were analyzed by each of the methods described subsequently.
Optimization of the signal-to-noise ratio involved adjusting the duration and dose of proteinase K digestion for all techniques, whereas TUNEL staining required dilution of TdT concentrations. Excessive proteinase K digestion created excess background noise. The absence of positive staining in areas of the growth plate where no apoptosis is believed to occur shows these techniques have sufficient specificity. The growth plate cartilage was obtained from one surgical specimen taken at the time of epiphyseodesis, washed in saline, and immediately fixed in Z-fix. After decalcification of these specimens, using 0.45 mol/L EDTA and 0.1 mol/L Tris buffer, the growth plates were dehydrated, embedded in paraffin, and sectioned at 7-μm thickness. A minimum of four sections then was analyzed using each of the same protocols.
Detection of double-stranded DNA breaks by TUNEL was performed using direct incorporation of fluorescein-labeled nucleotides with modification of the protocol provided with the ApopTag® Fluorescein Direct (Millipore Corp, Bedford, MA). After deparaffinization and rehydration, the samples were treated with 20 μg/mL proteinase K (Roche, Indianapolis, IN) for 15 minutes. After washing, the samples were incubated in Equilibrium Buffer for 30 seconds. Fifty-five microliters of TUNEL reaction mixture consisting of 12.4 μL terminal deoxynucleotidyl transferase enzyme, 28.9 μL reaction buffer, and 68.8 μL sterile phosphate-buffered saline (PBS) was added to the sections and incubated at 37°C in a humidified chamber for 1 hour. We found this dilution of the TdT reaction mixture was useful in reducing background noise from prior experiments. The reaction was stopped with stop/wash buffer and incubated for 10 minutes. Slides then were washed in PBS, counterstained with DAPI, 4′,6-diamidino-2-phenylindole (Vector Labs, Burlingame, CA), and observed under fluorescent microscopy.
Apoptosis detection using DNA denaturation analysis was performed following a protocol provided by Chemicon (Temecula, CA). After deparaffinization and rehydration, sections were permeabilized in 0.1 mg/mL saponin for 20 minutes and washed in PBS. Sections then were digested with 10 μg/mL proteinase K for 10 minutes and washed in distilled H2O. Slides were incubated in 50% formamide w/v in distilled H2O for 20 minutes at 56° to 60°C and then washed in ice-cold PBS for 5 minutes. Endogenous peroxide was quenched. Nonspecific binding was blocked with 3% nonfat dry milk. The primary antibody, anti-mouse F7-26 anti-ssDNA (Millipore) at 1:10 dilution, was applied for 30 minutes at room temperature in a humidified chamber and then washed in PBS. The secondary antibody, biotinylated anti-mouse IgM (Vector Labs) at 1:200 dilution, was applied for 30 minutes and washed in PBS. Streptavidin-peroxidase (Millipore) incubation for 30 minutes was followed by development in 3,3′-diaminobenzidine (DAB) (Millipore), washed in PBS and distilled H2O, and counterstained with DAPI (Vector Labs). Negative controls were run without primary antibody.
For the anti-activated caspase-3 method, sections were deparaffinized, rehydrated, and then incubated in 1 mg/mL hyaluronidase (Sigma-Aldrich Corp, St Louis, MO) in PBS for 60 minutes at 37°C and washed in PBS. Endogenous peroxide then was quenched. Nonspecific binding was blocked with 5% normal goat serum. Proteinase K at 10 μg/mL was applied for 10 minutes and sections were washed in PBS. Sections then were incubated in the primary antibody, 1:100 anti-active caspase-3 pAb rabbit IgG (Promega Corp, Madison, WI) overnight at 4°C, followed by anti-rabbit IgG biotinylated antibody (Vector Labs) at 1:100 dilution in PBS, and then streptavidin-peroxidase. Slides were developed in DAB, washed in PBS and distilled H2O, and counterstained with DAPI. Negative controls were run without primary antibody incubation.
Single-base 3′ overhangs and blunt ends in double-stranded DNA were detected using ISOL following the protocol provided in the ApopTag® ISOL kit (Millipore). After deparaffinization and rehydration, endogenous peroxide was quenched with 3% hydrogen, and the sections then were incubated in 10 μg/mL proteinase K for 10 minutes. Sixty microliters working-strength DNA ligase solution, 54 μL biotin-labeled oligonucleotide, and 6 μL T4 DNA ligase were placed on the sections, and the sections were incubated in a humidified chamber for 14 hours at 20°C. Oligonucleotides specific for single-base 3′ ends (Oligo A) and blunt ends (Oligo B) were used with single-base 3′ detection being more specific for apoptosis and blunt end detection being more sensitive. After incubation in T4 DNA ligase, the sections were washed in PBS, and streptavidin-peroxidase was applied for 30 minutes. The signal was developed in DAB, the reaction was stopped with PBS followed by distilled H2O washes, and the slides were counterstained in DAPI.
For histologic analysis, near-adjacent sections were stained with hematoxylin and eosin (Fisher Scientific, Fairlawn, NJ) and safranin O (Fisher Scientific) using standard methods to assess proteoglycan loss.
For the injured cartilage specimens, the injury zone was defined as the area of cartilage starting from the edge of the fractured cartilage and extending up to 1.3 mm away from the injury site. The control zone was defined as an area of cartilage approximately 10 mm from the cartilage fracture site. We intentionally chose areas that should be positive and negative. The deep layer provides a better control for true-negatives in testing detection methods as a result of processing and culture conditions that can affect the superficial layer of cartilage. This study was not designed to look at the model system of the injury, but rather the ability of these detection methods to distinguish between positive and negative cells. With superficial cartilage, there may be a baseline level of apoptosis resulting from storage. The deep cartilage should not be expected to have cells undergoing PCD.
Each zone of analysis measured 1.3 mm × 1.3 mm with the depth of visualization determined by the depth of field of the microscope objective. Images were captured digitally at one-megapixel resolution with a Zeiss AxioCam mounted on an Axioskop 2 microscope (Carl Zeiss, Inc, Thornwood, NY). Appropriate filters were used for fluorescence-based techniques. We (AD) performed semiautomated cell counting using Photoshop® (Adobe Systems Inc, San Jose, CA) and Scion Image (Scion Corp, Frederick, MD) software packages optimized to detect cells based on nuclear and/or cytoplasmic staining. The apoptotic index for each area was calculated by dividing the number of positive cells by the total number of cells in the area of analysis.
Results are presented as the apoptotic index in percentage ± standard error of the mean. We compared the apoptotic indices from the injury zone with those of the internal control of the zone of chondrocytes in the central area and also between detection methods in the injury zone, using unpaired two-tailed t tests. We performed all analyses using GraphPad Prism® (GraphPad Software, Inc, La Jolla, CA).
Results
All four methods when used for chondrocyte PCD showed differences when comparing the injury zone with the control (Table 1). In addition, similar spatial patterns of staining were observed using all techniques (Fig. 1). The magnitude of positive signal also was similar in all samples (Fig. 2).
Table 1.
Summary of findings
| Apoptotic marker | Apoptotic index* | p Value | |
|---|---|---|---|
| Zone of injury | Zone away from injury | ||
| TUNEL | 46.9 ± 5.2 | 4.9 ± 0.9 | < 0.05 |
| ssDNA | 40.4 ± 5.9 | 2.8 ± 0.7 | < 0.05 |
| Anti-caspase-3 | 54.1 ± 6.5 | 6.5 ± 2.2 | < 0.05 |
| ISOL A | 45.3 ± 5.9 | 4.0 ± 1.5 | < 0.05 |
| ISOL B | 39.5 ± 5.2 | 7.0 ± 1.7 | < 0.05 |
* Values expressed as percentage ± standard error of the mean; TUNEL = terminal deoxynucleotidyl transferase end labeling; ssDNA = DNA denaturation analysis using anti single-stranded DNA antibody; ISOL = in situ oligonucleotide ligation.
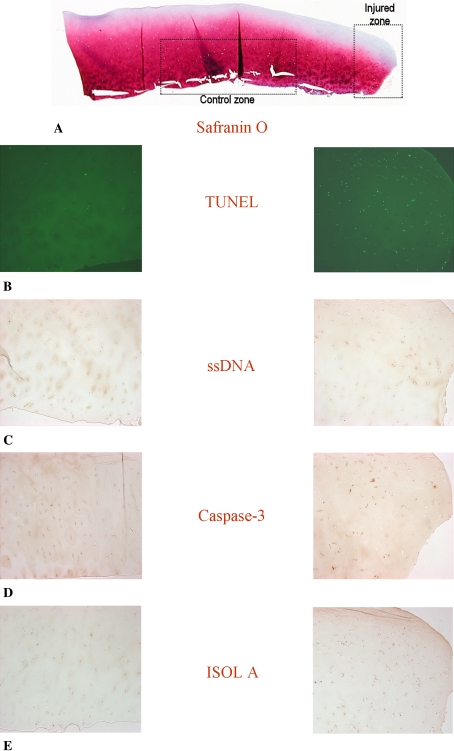
Fig. 1A–E.
Photomicrographs (Stain, Safranin O (A) and DAB primary antibody staining with Fast Green counter stain (B–E); original magnification, ×10) compare (A) the injured zone and the control zone in (B) TUNEL, (C) ssDNA, (D) anti-active caspase-3, and (E) ISOL. The break in the cartilage is to the right of the injured zone images. TUNEL = terminal deoxynucleotidyl transferase end labeling; ssDNA = DNA denaturation analysis using anti single-stranded DNA antibody; ISOL = in situ oligonucleotide ligation.
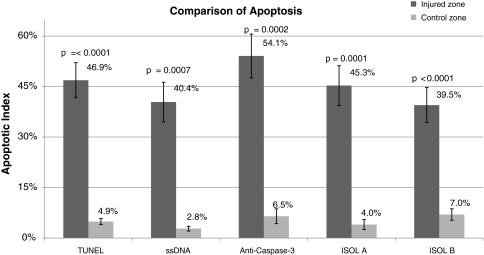
Fig. 2.
A graph compares the apoptotic indices in the injured zone and control zone using TUNEL, ssDNA, anti-active caspase-3, ISOL A (single 3′ base overhang), and ISOL B (blunt DNA ends). Error bars represent standard errors of the mean. The apoptotic indices in the control versus the injured zones were statistically different in all test groups; the representative p values are above each paired control and injured group. TUNEL = terminal deoxynucleotidyl transferase end labeling; ssDNA = DNA denaturation analysis using anti single-stranded DNA antibody; ISOL = in situ oligonucleotide ligation.
Many TUNEL-positive cells were observed in the zone of injury with an apoptotic index of 46.9% ± 5.2%. The fluorescence was restricted primarily to the nucleus in positive cells; only a handful of positive cells had signal in the cytoplasm. Very few positive TUNEL cells were seen in the control zone with an apoptotic index of 4.9% ± 0.9%, which was smaller (p < 0.0001) than the apoptotic index observed in the injury zone (Fig. 1B).
Immunohistochemistry using an anti-ssDNA antibody after heat treatment of sections in formamide identified positive cells in a similar distribution. In these positive cells, the DAB signal was restricted to nuclear staining. No cytoplasmic staining and very little background staining were observed. A larger (p = 0.0007) apoptotic index was observed for the zone of injury (40.4% ± 5.9%) than that observed for the control zone (4.9% ± 0.9%) (Fig. 1C).
Immunohistochemistry using anti-active caspase-3 antisera identified positive cells with the DAB signal in the cytoplasm of affected cells. In the zone of injury, chondrocytes with active caspase-3 were clearly identified, with a higher (p = 0.0002) apoptotic index in the injured compared with the control zone (54.1% ± 6.5% versus 6.5% ± 2.2%, respectively) (Fig. 1D).
ISOL-labeled cells had staining primarily in the nucleus of chondrocytes. Background matrix staining by DAB was slightly higher using this technique compared with the other techniques. Similar results were obtained using either the oligonucleotide specific for single-base overhangs (Oligo A) or the oligonucleotide specific for blunt ends (Oligo B). With Oligo A, the zone of injury had a larger (p = 0.0001) apoptotic index (45.3% ± 5.9%) than the control zone (4.0% ± 1.5%) (Fig. 1E). With Oligo B, the zone of injury also had a larger (p < 0.0001) apoptotic index (39.5% ± 5.2%) than the zone away from injury (7.0% ± 1.7%).
Safranin O staining showed diminished staining in the area adjacent to the injury edge, suggesting disruption of cartilage matrix. This correlated with areas where PCD was detected by the previously described methods. In the control zone, there was strong safranin red staining. Hematoxylin and eosin staining revealed slightly reduced cell numbers just adjacent to the injury edge. A few cells with irregular, pyknotic nuclei occasionally were observed in the injured area, whereas none were seen in the control zone.
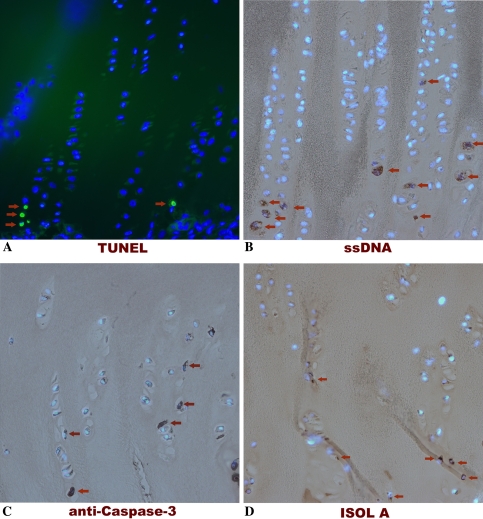
Qualitative examination of the growth plate cartilage using the previously described protocols revealed apoptotic cells only localized to the hypertrophic zone. The hypertrophic zone was identified by visually scanning through the entire slide section. Overall incidence of positive staining cells was very low and variable across the section, even in the hypertrophic zones. Scanning of the entire slides revealed all positive cells were in the hypertrophic zone. No positively staining chondrocytes were seen in any other area (Fig. 3). These data are presented qualitatively given the heterogeneity in the growth plate hypertrophic zone.
Fig. 3A–D.
Photomicrographs (Stain, A fluorescein with DAPI counterstain, B–D DAB for primary antibody with DAPI counterstain; original magnification, ×20) show growth plate cartilage stained with (A) TUNEL, (B) ssDNA, (C) anti-active caspase-3, and (D) ISOL A. In all techniques, positive cells were observed only in the hypertrophic zone of the growth plate cartilage, showing good spatial distribution for these techniques. TUNEL = terminal deoxynucleotidyl transferase end labeling; ISOL = in situ oligonucleotide ligation; ssDNA = DNA denaturation analysis using anti-single-stranded DNA antibody.
When comparing the different PCD detection methods with each other in a pair-wise manner, there were no statistical differences in which p < 0.05.
Discussion
Traditionally, cells undergoing apoptosis were identified based on morphologic criteria by light microscopy with characteristic features further delineated by electron microscopy [10]. This method is limited as a result of the inability to assay a large area of cartilage, making it highly dependent on sample selection. In addition, chondrocytes undergoing PCD may not exhibit the apoptotic appearances of other cells [18]. Therefore, we compared four methods of PCD detection, which target different characteristics of PCD—DNA fragmentation (TUNEL and ISOL), DNA instability (ssDNA), and upregulation of biochemical intermediates (anti-caspase3)—and studied each method in injured and growth plate cartilage.
One limitation of our study is the use of a single source specimen for our intraarticular fracture model. Other cartilage specimens could show a different response to the same injury given the age of the donor patient and initial quality of the cartilage. However, one advantage of using a single specimen is that it reduces the variability between different samples. In addition, the slides chosen for analysis for the different assays were taken from alternating slides of adjacent sections, so each technique was evaluating a similar spatial area. The use of a geographically distant area as a control is analogous to using a segment of cartilage that had not been subjected to the three-point break. It is well known that different areas of articular cartilage have different thickness, material properties, and likely cellular environments, and by using a geographic control on the same section, we hoped to minimize this variability.
We did not intend to show a rate of PCD after this type of injury model, but rather to look at the consistency of different methods of PCD detection. Other methods of cartilage injury may produce different results attributable to a difference in proportion of apoptosis to necrosis and/or the timing of apoptotic events. We did not perform a time course of analysis to see if each method would have different peaks of PCD, which would be plausible given that they detect different stages of PCD. Our study was not specifically designed to prove each method is equivalent or interchangeable in finding rates of apoptosis, but rather that all these methods can be used to detect apoptotic versus nonapoptotic control chondrocytes.
As research in this field has progressed, specific markers based on biologic processes unique to PCD have been identified and exploited in assays designed to identify apoptotic cells. However, many of these assays can be performed only on living cells and often are optimized for cells grown in tissue culture [20]. Because chondrocyte biology is intimately tied to the surrounding cartilage matrix, the ability to study chondrocyte PCD in cartilage after fixation and sectioning is often desirable and/or necessary.
To allow for multiple types of analyses of near-adjacent sections from one specimen, a method of cartilage fixation amenable to all of the proposed techniques was necessary. Unfortunately, relatively few PCD markers are preserved after traditional formalin fixation and paraffin embedding. Consequently, one critical factor was the use of a zinc-formalin fixative such as Z-Fix instead of standard buffered formalin. Zinc ions are believed to preserve immunoreactivity by maintaining the tertiary structure of proteins [4]. We noted markedly improved sensitivity and specificity using zinc-formalin fixation compared with 10% neutral-buffered formalin fixation with several antibodies, including anti-active caspase-3. Detection of active caspase-3 after fixation in neutral-buffered formalin required antigen retrieval in citrate and postlabeling signal enhancement using Tyramide Signal Amplification™ (Perkin-Elmer, Boston, MA) (data not shown). These additional steps not only resulted in higher nonspecific background staining, but also tended to cause smaller cartilage sections to detach from the slides.
One potential limitation of using active caspase-3 as a marker for apoptosis is evidence that PCD, under certain conditions, can be caspase-independent [21]. However, all existing data indicate chondrocyte PCD requires caspase-3 activation [12, 14, 15]. Regardless, active caspase-3 is only one of several potential PCD markers amenable to detection using immunohistochemistry.
Alternative PCD markers for which commercially available antibodies are available include the 85-kD PARP cleavage product (anti-PARP p85; Promega) and a neoepitope produced by cleavage of cytokeratin 18 (M30 cytodeath; Roche). Neither was tested in the current study; however, we have successfully used the PARP p85 antibody in other experiments using zinc formalin fixatives (data not shown). To our knowledge, the M30 cytodeath reagent has not been used successfully for detecting apoptosis in cartilage.
The use of zinc-buffered formalin did not appear to have any detrimental effects on TUNEL, DNA denaturing analysis, or ISOL despite anecdotal reports that zinc could interfere with some types of DNA analyses such as in situ hybridization [2, 19]. However, each technique required optimization to yield the best results. TUNEL, in particular, was quite sensitive to duration of fixation, matrix digestion conditions, terminal deoxynucleotidyl transferase enzyme concentration, and substrate concentration. When performed following the protocol described in this article, TUNEL yielded results directly comparable to DNA denaturing analysis and ISOL, two techniques reportedly much more specific for apoptotic cells [5, 6]. One factor that may contribute to the observed consistency is a relatively low rate of necrotic cell death in the model of cartilage injury used in this study. Therefore, situations in which necrosis plays a greater role may lead to a relatively higher percentage of TUNEL-positive cells compared with DNA denaturing analysis and ISOL.
The images from the growth plate cartilage analysis suggest these methods are able to detect chondrocytes undergoing PCD in the hypertrophic zone. Our low background level of staining also suggests the fixation and specimen preparation steps did not induce artifacts that would lead to false-positive staining.
We found no differences in the apoptotic indices with these four techniques. However, our primary objective was not to compare these techniques with each other, but rather to determine whether they are complementary in the detection of apoptosis. Increasing our sample size may have given us sufficient power to detect differences among these methods. However, the differences would likely not be large enough to change our basic conclusions.
These results should be used in the evaluation of detection of apoptosis in paraffin embedded sections. A limitation is that we did not look for total cell viability with live/dead staining. Preparing these samples for live/dead staining, for vibratome sectioning, would involve removing the cartilage layer from the calcified cartilage or subchondral bone, adding an additional injury to the cartilage, which would not be present on the fixed samples.
Based on our data, we conclude TUNEL, ssDNA, anti-active caspase-3, and ISOL are each able to distinguish between apoptotic and nonapototic chondrocytes under the described experimental conditions. All four techniques appeared well suited for detecting chondrocyte PCD in paraffin-embedded cartilage after zinc formalin fixation, precluding the need for multiple fixation methods. The observation that independent markers for PCD identified the same populations of chondrocytes increases our confidence that these cells are undergoing PCD and not necrosis. However, limitations with each technique make reliance on any single detection method unwise, and we advocate using at least two of these complementary techniques in any analysis of cartilage PCD. With the described techniques added to their arsenals, researchers should be able to investigate the mechanisms and pathways of chondrocyte PCD with increased confidence in the validity of their findings.
Acknowledgments
We thank UCSF School of Medicine, UCSF Department of Orthopaedic Surgery, and San Francisco VA Medical Center.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
One or more of the authors (HK) have received funding from UCSF School of Medicine, UCSF Department of Orthopaedic Surgery, and San Francisco VA Medical Center.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Aigner T, Hemmel M, Neureiter D, Gebhard PM, Zeiler G, Kirchner T, McKenna L. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritis human articular knee cartilage: a study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis Rheum. 2001;44:1304–1312. [DOI] [PubMed]
- 2.Arnold MM, Srivastava S, Fredenburgh J, Stockard CR, Myers RB, Grizzle WE. Effects of fixation and tissue processing on immunohistochemical demonstration of specific antigens. Biotech Histochem. 1996;71:224–230. [DOI] [PubMed]
- 3.Blanco FJ, Guitian R, Vazquez-Martul E, de Toro FJ, Galdo F. Osteoarthritis chondrocytes die by apoptosis: a possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998;41:284–289. [DOI] [PubMed]
- 4.Dapson RW. Fixation for the 1990’s: a review of needs and accomplishments. Biotech Histochem. 1993;68:75–82. [DOI] [PubMed]
- 5.Didenko VV, Hornsby PJ. Presence of double-strand breaks with single-base 3’ overhangs in cells undergoing apoptosis but not necrosis. J Cell Biol. 1996;135:1369–1376. [DOI] [PMC free article] [PubMed]
- 6.Frankfurt OS, Krishan A. Identification of apoptotic cells by formamide-induced dna denaturation in condensed chromatin. J Histochem Cytochem. 2001;49:369–378. [DOI] [PubMed]
- 7.Frankfurt OS, Robb JA, Sugarbaker EV, Villa L. Monoclonal antibody to single-stranded DNA is a specific and sensitive cellular marker of apoptosis. Exp Cell Res. 1996;226:387–397. [DOI] [PubMed]
- 8.Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R. In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology. 1995;21:1465–1468. [DOI] [PubMed]
- 9.Hornsby PJ, Didenko VV. In situ DNA ligation as a method for labeling apoptotic cells in tissue sections: an overview. Methods Mol Biol. 2002;203:133–141. [DOI] [PubMed]
- 10.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. [DOI] [PMC free article] [PubMed]
- 11.Kim HA, Lee YJ, Seong SC, Choe KW, Song YW. Apoptotic chondrocyte death in human osteoarthritis. J Rheumatol. 2000;27:455–462. [PubMed]
- 12.Kim HT, Teng MS, Dang AC. Chondrocyte apoptosis: implications for osteochondral allograft transplantation. Clin Orthop Relat Res. 2008;466:1819–1825. [DOI] [PMC free article] [PubMed]
- 13.Marshman E, Ottewell PD, Potten CS, Watson AJ. Caspase activation during spontaneous and radiation-induced apoptosis in the murine intestine. J Pathol. 2001;195:285–292. [DOI] [PubMed]
- 14.Matsuo M, Nishida K, Yoshida A, Murakami T, Inoue H. Expression of caspase-3 and -9 relevant to cartilage destruction and chondrocyte apoptosis in human osteoarthritic cartilage. Acta Med Okayama. 2001;55:333–340. [DOI] [PubMed]
- 15.Nuttall ME, Nadeau DP, Fisher PW, Wang F, Keller PM, DeWolf WE, Jr., Goldring MB, Badger AM, Lee D, Levy MA, Gowen M, Lark MW. Inhibition of caspase-3-like activity prevents apoptosis while retaining functionality of human chondrocytes in vitro. J Orthop Res. 2000;18:356–363. [DOI] [PubMed]
- 16.Perez HE, Luna MJ, Rojas ML, Kouri JB. Chondroptosis: an immunohistochemical study of apoptosis and Golgi complex in chondrocytes from human osteoarthritic cartilage. Apoptosis. 2005;10:1105–1110. [DOI] [PubMed]
- 17.Roach HI, Aigner T, Kouri JB. Chondroptosis: a variant of apoptotic cell death in chondrocytes? Apoptosis. 2004;9:265–277. [DOI] [PubMed]
- 18.Roach HI, Clarke NM. Physiological cell death of chondrocytes in vivo is not confined to apoptosis: new observations on the mammalian growth plate. J Bone Joint Surg Br. 2000;82:601–613. [DOI] [PubMed]
- 19.Tbakhi A, Totos G, Hauser-Kronberger C, Pettay J, Baunoch D, Hacker GW, Tubbs RR. Fixation conditions for DNA and RNA in situ hybridization: a reassessment of molecular morphology dogma. Am J Pathol. 1998;152:35–41. [PMC free article] [PubMed]
- 20.Willingham MC. Cytochemical methods for the detection of apoptosis. J Histochem Cytochem. 1999;47:1101–1110. [DOI] [PubMed]
- 21.Zamzami N, Kroemer, G. Apoptosis: condensed matter in cell death. Nature. 1999;401:127–128. [DOI] [PubMed]
- 22.Zhu C, Wang X, Hagberg H, Blomgren K. Correlation between caspase-3 activation and three different markers of DNA damage in neonatal cerebral hypoxia-ischemia. J Neurochem. 2000;75:819–829. [DOI] [PubMed]
- 23.Zunino SJ, Singh MK, Bass J, Picker LJ. Immunodetection of histone epitopes correlates with early stages of apoptosis in activated human peripheral T lymphocytes. Am J Pathol. 1996;149:653–663. [PMC free article] [PubMed]