Abstract
Since low-dose antibiotic-loaded bone cement (ALBC) was approved by the FDA for second-stage reimplantation after infected arthroplasties in 2003, commercially premixed low-dose ALBC has become available in the United States. However, surgeons continue to mix ALBC by hand. We presumed hand-mixed ALBC was not as homogeneous as commercially premixed ALBC. We assessed homogeneity by determining the variation in antibiotic elution by location in a batch, from premixed and hand-mixed formulations of low-dose ALBC. Four hand-mixed methodologies were used: (1) suspension—antibiotic powder in the liquid monomer; (2) no-mix—antibiotic powder added but not mixed with the polymer powder before adding monomer; (3) hand-stirred—antibiotic powder stirred into the polymer powder before the monomer was added; and (4) bowl-mix—antibiotic powder mixed into polymer powder using a commercial mixing bowl before the monomer was added. Antibiotic elution was measured using the Kirby-Bauer bioassay. None of the mixing methods had consistently dissimilar homogeneity of antibiotic distribution from the others. Based upon our data we conclude hand-mixed low-dose ALBC is not less homogeneous than commercially premixed formulations.
Introduction
In the United States, the primary use of antibiotic-loaded bone cement (ALBC) has been for treatment of established infections (high dose) and revision arthroplasty (low dose), while only a small minority of primary cemented arthroplasties are fixed using low-dose ALBC [4]. In many locations outside the United States, the majority of primary cemented hip and knee arthroplasties are fixed using low-dose ALBC [1, 6, 8]. Before the FDA approved low-dose ALBC in 2003, hand mixing was the only method available to use ALBC in the United States. Despite considerable clinical use, guidelines for antibiotic dose and mixing methodology have not been established.
Commercially premixed low-dose ALBC is now available in the United States; however, surgeons continue to mix ALBC by hand. Vendor marketing information [2] claims commercial premixing saves operative time and that the antibiotic is more homogenously mixed in the ALBC, leading to better drug delivery. Furthermore, gentamicin in powder form is not available in the United States, making the commercially premixed formulation the only way to clinically utilize gentamicin in ALBC. These advantages come at an increased cost of $250 or more per batch. The primary drawback to the use of commercially premixed ALBC is that the antibiotic used is dictated by the chosen bone cement. Surgeons are unable to tailor the antibiotic to a specific organism or use multiple antibiotics in combination.
Studies comparing the elution of antibiotics from different hand-mixing methods and commercially premixed ALBC [4, 5, 7], report both similarity and differences of elution from different formulations of ALBC. Lewis et al. [5] reported inferior gentamicin release from hand-stirred and commercial powder-mixer formulations compared to commercially pre-mixed samples of cement. Neut et al. [7] reported similar elution with manual mixing in a commercial vacuum mixing cartridge with and without vacuum applied. None of the studies evaluated the homogeneity of antibiotic throughout the ALBC for any mixing method. These inconclusive data comparing mixing methods led to the question of whether hand-mixed ALBC is mixed homogeneously.
We therefore hypothesized hand-mixed ALBC and commercially premixed ALBC provide different antibiotic release in an in vitro setting. We further hypothesized that different mixing techniques result in different homogeneity of the cement.
Materials and Methods
We studied five commercially premixed ALBC formulations: Cemex® G with 1 g gentamicin (Exactech, Gainesville, Fla.); Cobalt™ G-HV with 500 mg gentamicin (Biomet, Warsaw, Ind.); Palacos® G with 500 mg gentamicin (Biomet); Simplex® P with 1 g tobramycin (Stryker, Kalamazoo, Mich.); and Smart Set® G HV with 1 g gentamicin (DePuy, Warsaw, Ind.). Each commercially premixed ALBC formulation was reproduced by hand using four different hand-mixing methodologies representing a spectrum of clinically possible mixing methods: (1) suspension—antibiotic powder suspended in the liquid monomer was considered the best possible mixing method; n = 5 × 10 = 50; (2) no-mix—antibiotic powder added to but not mixed with the polymer powder before adding monomer was considered the worst possible method; n = 5 × 10 = 50; (3) hand-stirred—antibiotic powder added and stirred into the polymer powder by hand using a spatula; one circle per second, five right alternating with five left, for 30 seconds, before the monomer was added, was a standardized methodology for a commonly used technique; n = 5 × 10 = 50; and (4) bowl-mix—antibiotic powder mixed into the polymer powder using a commercial mixing bowl; n = 5 × 10 = 50; one handle turn per second, five right alternating with five left for 30 seconds, before the monomer was added, was a standardized methodology considered the closest available method to commercial premixing (Table 1).
Table 1.
Structure of the study: 30 batches of 56 cylinders, with 10 randomly selected cylinders eluted from each batch
| Cement | Pre-mix | Suspension | Hand-stirred | No-mix | Bowl-mix | Machine-mix |
|---|---|---|---|---|---|---|
| Cemex® | Cylinders = 10 | Cylinders = 10 | Cylinders = 10 | Cylinders = 10 | Cylinders = 10 | Cylinders = 10 |
| Cobalt™ | Cylinders = 10 | Cylinders = 10 | Cylinders = 10 | Cylinders = 10 | Cylinders = 10 | N/A |
| Palacos® S | N/A | Cylinders = 10 | Cylinders = 10 | Cylinders = 10 | Cylinders = 10 | N/A |
| Simplex® | Cylinders = 10 | Cylinders = 10 | Cylinders = 10 | Cylinders = 10 | Cylinders = 10 | N/A |
| Smart Set® | Cylinders = 10 | Cylinders = 10 | Cylinders = 10 | Cylinders = 10 | Cylinders = 10 | N/A |
| Palacos® C | Cylinders = 10 | Cylinders = 10 | Cylinders = 10 | Cylinders = 10 | Cylinders = 10 | N/A |
When available the hand-mixed formulations used the same antibiotic as that used in the commercially premixed formulations. For Palacos® G and Cobalt™ G-HV, the commercial premixed formulation is made with a crystallized form of gentamicin, the hand-mixed samples in this study were formulated with the crystallized gentamicin, provided by Biomet. The gentamicin used for hand-mixing formulations of Smart Set® HV was provided by the manufacturer (DePuy, Warsaw IN). The gentamicin used for hand-mixed formulations of Cemex® was USP grade gentamicin (New Chemic, Montvale NJ). All gentamicin doses were weight-adjusted to have the same activity per batch of cement as the premixed formulation. The tobramycin (X-Gen, Northport NY) used for hand-mixed formulations of Simplex® P was generic, weight-adjusted to have the same activity per batch of cement. Thirty combinations of ALBC formulation and mixing method were studied: five mix methods for each of the five brands of low dose ALBC cement, one commercially premixed and four hand-mixed methods. Additionally, for only the Cemex® cement, the premixed formulation was polymerized using two methods: hand stirring and using an automated vibrator/mixer designed to for use with Cemex®. For Palacos cement an additional formulation with USP grade gentamicin was used to compare crystalline gentamicin with standard gentamicin. Commercially premixed Palacos® G was compared to hand-mixed formulations made with both crystallized gentamicin (proprietary gentamicin formulation provided by Biomet, Warsaw IN) in this study termed “Palacos C,” and with standard USP grade gentamicin (New Chemic, Montvale NJ) in this study termed “Palacos S.”
For all 30 formulation/mixing combinations, the ALBC was polymerized by hand combining the polymer with the monomer in a bowl without vacuum. ALBC test specimens were made using a Teflon mold with the cement in the dough phase. Excess ALBC was machined off the ends of the mold using low cutting speed to prevent smearing. Fifty-six identical test specimen cylinders, 12-mm long by 6-mm diameter (ASTM 451-99) were created for every mixing method and for all five cement types for a total of 30 × 56 = 1680 cylinders. Full batches of cement were mixed for each test condition to maintain consistency with clinical usage.
Ten of the 56 cylinders from each formulation/mixing group were randomly selected for a total of 30 × 10 = 300 test specimens. These 300 specimens were eluted individually in 5 mL of deionized water using 15 mL scintillation vials. Total volume of eluate was removed on days 1, 3, 7, 15, and 30 and replaced with fresh deionized water. The antibiotic concentration was measured in each eluate sample using the Kirby-Bauer bioassay [3]. Briefly, agar plates were prepared using plastic bioassay Petri dishes, dimensions 245 × 245 × 18 mm (BD Biosciences, Bedford, MD), and Difco™ Antibiotic Medium 11 (agar) (BD, Sparks, MD) inoculated with Kocuria rhizophilia ATCC® 9341™ in Difco™ nutrient broth (BD, Sparks, MD). Filter paper test discs 6 mm in diameter (Whatman, Sanford, ME) were arranged in a 6 × 6 grid on the agar plates. Each plate had six discs with standard solutions of known concentrations spanning the experimental antibiotic concentration range so that each plate had its own calibration curve. Thirty μL of each eluate sample was pipetted onto a test disc. The agar plates were placed in an incubator at 37°C for 30 hours. Digital photographs were then taken of each plate. The radius of each inhibition zone was measured using Image J (NIH, Bethesda MD). Antibiotic concentrations were determined by comparing the radius of the inhibition zone from the eluate samples to the standard curve calculated for that plate. Each eluate sample was assayed in triplicate. The mass of recovered antibiotic was calculated by multiplying the antibiotic concentration by the eluate volume of 5 mL and cumulatively summed over the duration of the study.
To determine whether or not hand-mixed and commercially premixed ALBC had different release performance, we first performed an ANOVA using mixing method and cement manufacturer as factors, with the mass of released antibiotic as the response variable. This analysis was replicated on Day 1 and on Day 30 to account for changes in the relationship of the factors over time. Subsequently, differences between the cement manufacturer/mixing method groups were determined using Tukey’s multiple comparison test as a post-hoc test. Finally, to determine whether or not hand-mixed and commercially premixed ALBC had different homogeneity, the coefficient of variance (CoV; standard deviation divided by the mean) for the cumulative recovered antibiotic on Day 1 and Day 30 from each of the 10 individual test cylinders in each mixing group was used as the response variable in an ANOVA with post hoc Tukey’s multiple comparison test. The CoV was tabulated because it provides a normalized estimate of the deviation in the data between groups. Without normalization, samples that release more antibiotic are likely to have a larger absolute standard deviation. Data for individual formulations are assumed to be normally distributed in accordance with small sample statistics.
Results
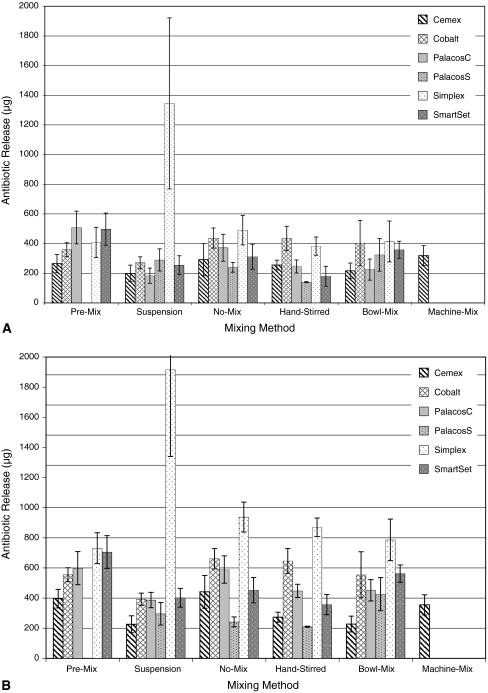
On Day 1 the antibiotic release either increased or decreased depending upon the manufacturer (p < 0.001) and mixing method (p < 0.001) (Fig. 1A). However, no mixing method had consistently greater or lower antibiotic release than any other mixing method for all cements. Similarly for Day 30 data, no method produced consistently greater or lower antibiotic release than any other mixing method (Fig. 1B). For commercially premixed Palacos® G (formulated with crystallized gentamicin), the average recovered gentamicin on Day 1 was two times greater than the average recovered gentamicin from the hand-mixed formulations of both gentamicin types. On Day 1 the gentamicin recovered from Cemex® G polymerized using the proprietary vibrator/mixer was 1.2 times greater than the average recovered gentamicin from the premixed Cemex® polymerized by hand.
Fig. 1A–B.
(A) The total mass of antibiotic released from the various ALBC formulations by mixing method at Day 1 is shown. (B) Also shown is the comparative total mass of antibiotic released from the various ALBC formulations by mixing method at Day 30. The values shown for Cemex are for the premixed samples, and do not show the vibrational mixer.
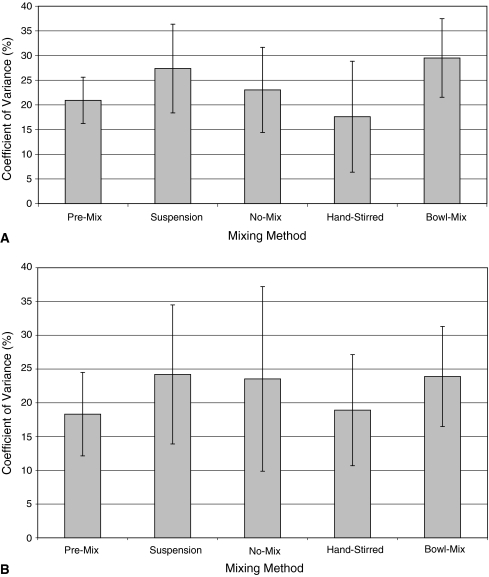
On Day 1, mixing technique did not influence (p = 0.14) the homogeneity of the cement mix (Fig. 2A). The mixing method also did not influence (p = 0.705) the homogeneity of the mix on Day 30 (Fig. 2B). The CoV among the premixed cements on Day 30 was similar: Cobalt, 12.7; Palacos C, 19.5; Cemex, 15.9; Smart Set, 14.9; and Simplex, 28.4, suggesting they were all mixed equally.
Fig. 2A–B.
(A) The average coefficient of variance across cement brands for each mixing method at Day 1 is shown. Coefficient of variance normalizes the standard deviation in relationship to the mean, and provides an indication of the homogeneity of release amongst individual test cylinders between formulations. (B) The average coefficient of variance across cement brand for each mixing method at Day 30 is shown.
Discussion
While commercial preparations of ALBC are available, many surgeons continue to hand-mix cement with antibiotics. There are, however, inconclusive data comparing mixing techniques and we therefore questioned whether hand-mixed ALBC is mixed homogeneously. We specifically hypothesized hand-mixed ALBC and commercially premixed ALBC would provide different antibiotic elution and the mixing technique would influence the homogeneity of the cement as reflected by the coefficient of variance.
Because there are formulation differences with different commercial cements, we studied five products from four companies. The formulations tested differed in antibiotic used, gentamicin (crystallized and standard) and tobramycin, all with variations in activity per gram of drug, and the dose used (500 mg and 1 g). These factors affect antibiotic release to the extent that comparisons of average release among the brands studied are meaningless; however, these factors do not affect the comparison of homogeneity among the mixing methods. While ACM mixed three of the batches of cement studied in this manuscript, the other batches were mixed by HK. Multiple people mixing the cement could confound the effect of mixing. Our data do not show a resulting bias as the batches mixed by ACM were similar to other batches. Another limitation of the study design is that only one batch of each cement/antibiotic/mixing method formulation was performed due to the large number of assays that would be required to replicate the batches of each formulation. Therefore we did not directly replicate the batch-to-batch variability that may be seen in mixing many batches of the same formulation. However, considering the replication present in the multiple cements that we studied, and considering that the batch-to-batch variation for hand mixing in the operating room is likely similar to the batch-to-batch variation we encountered for hand mixing in the lab, the experimental design has sufficient replication to compare hand mixing with commercially premixed ALBC. Based on the similarity we saw in the ranges of recovered antibiotic at Day 30, we consider this a reasonable estimation.
Initially, we hypothesized that there would be differing amounts of antibiotic eluted from our samples based on mix technique. Our data suggest none of the mixing methods consistently outperformed or underperformed another for average cumulative released antibiotic. While our data analysis indicates that mixing type was a determinant in antibiotic elution between some of the cement manufacturer/mixing method groups, the groups that were different were entirely random; no mixing technique consistently outperformed the other techniques. This indicates that any effect of mixing technique on elution is likely small relative to batch-to-batch variation. Only premixed Palacos® G formulated with crystallized gentamicin was more than one standard deviation greater than the average of its respective hand-mixed methods on Day 1. The average release from Simplex® using the suspension mixing method was more than double all the other formulations including its respective premixed formulation. Since the suspension mixing method involved suspending insoluble antibiotic particles in the liquid monomer, we question whether the polymer powder acted like a filter, holding the antibiotic particles in one region of the ALBC at the onset of polymerization. This supposition is supported by the large standard deviation in cumulative recovered antibiotic for this mixing method. Opposite to what we had thought at the outset, the suspension mixing method did not provide better antibiotic distribution than no mixing. Interestingly, when no attempt was made to mix the antibiotic powder in the polymer, the homogeneity of the ALBC was similar to the other mixing methods, presumably due to the stirring that occurs when the monomer is combined with the polymer during polymerization. The coefficient of variance measurements indicate that homogeneity of ALBC is not meaningfully affected by the mixing method. Lewis et al. [5] report differences as high as 36% in elution between mixing methods. Our findings differ from those of Lewis and colleagues in that we did not see the difference between hand-stirred or bowl-mixed methods (similar to commercial powder mixer) and commercial pre-mixed that Lewis reported [5]. Neut et al. [7] found that using vacuum during mixing had a minor effect on elution using a mechanical mixing method similar to the bowl-mix method in this study. Lewis et al. [5] and Neut et al. [7] both analyzed their data with Student t tests across the single factor of mixing within single batches whereas we analyzed using both cement type and mixing method with ANOVA and Tukey’s allowing estimates of batch to batch variation.
We conclude that within the sensitivity of our study, hand-mixed ALBC and commercially premixed ALBC do not provide different antibiotic release and that none of the studied mixing methods produced different homogeneity in the ALBC.
Acknowledgments
We thank Biomet, DePuy, Exactech, and Stryker for the generous donation of materials used in this investigation.
Footnotes
This work was supported by a grant from OREF and funds from Banner Health. Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
This work was performed at Arizona State University, Phoenix, AZ.
References
- 1.Australian Orthopaedic Association National Joint Replacement Registry Annual Report. Available at:http://www.dmac.adelaide.edu.au/aoanjrr/publications.jsp. Accessed September 3, 2008.
- 2.Buyers Guide for Medical Professionals, Bone Cement Now Comes Pre-mixed with Antibiotic. Available at: http://www.medcompare.com/spotlight.asp?spotlightid=70. Accessed March 12, 2009.
- 3.Fernandes PB, Ramer N, Rode RA, Freiberg L. Bioassay for A-56268 (TE-031) and identification of its major metabolite, 14-hydroxy-6-methyl erythromycin. Eur J Clin Microbiol Infect Dis. 1988;7:73–76. [DOI] [PubMed]
- 4.Jiranek WA, Hanssen AD, Greenwald AS. Antibiotic-loaded bone cement in aseptic total joint replacement: whys, wherefores, and caveats. Available at: www.orl-inc.net/aaos_publications/2003/ALBC2003.PDF. Accessed Sept 3, 2008.
- 5.Lewis G, Janna S, Bhattaram A. Influence of the method of blending an antibiotic powder with an acrylic bone cement powder on physical, mechanical, and thermal properties of the cured cement. Biomaterials. 2005;26:4317-4325. [DOI] [PubMed]
- 6.Norwegian Arthroplasty Register Report. Available at: http://www.haukeland.no/nrl/. Accessed September 3, 2008.
- 7.Neut D, van de Belt H, van Horn JR, van der Mei HC, Busscher HJ. The effect of mixing on gentamicin release from polymethylmethacrylate bone cements. Acta Orthop Scand. 2003;74:670–676. [DOI] [PubMed]
- 8.Swedish Hip Arthroplasty Register Annual Report. Available at: http://www.jru.orthop.gu.se/. Accessed September 3, 2008.