Abstract
Aseptic loosening attributable to wear-related osteolysis historically has been the predominant cause of failure in THA. Advances in low-wear bearing couples show great promise to substantially reduce this long-standing problem. However, there always has been striking variability in wear rate in any given cohort of patients who are similarly implanted, with some individuals typically experiencing near order-of-magnitude elevations above group mean. Third-body wear is likely a major contributor to many of these most osteolysis-prone outliers. For the patients affected, third-body effects may obviate many of the gains otherwise achieved by contemporary bearing surface improvements. Toward heightening visibility in terms of consequences for patients, this review paper summarizes an interrelated series of investigations quantifying construct level manifestations of third-body wear. Long-term followup of a unique group of patients with elevated third-body challenge shows statistically significant and clinically important wear-rate increases. A series of finite element models, validated physically, shows the linkage of location of third-body damage with variability of volumetric wear-rate acceleration and shows the effects of various implant factors, surgeon factors, and patient factors in the presence of third-body challenge. Finally, a mechanism for third-body debris access to wear-critical locations on the bearing surface is identified analytically and corroborated in laboratory experiments and implant retrievals.
Introduction
Contemporary alternative bearing surfaces have changed the landscape regarding wear-induced osteolysis being the primary factor limiting longevity of THA. The short- and intermediate-term clinical wear rates [5] for highly cross-linked polyethylenes (PE) are largely consistent with the excellent performance exhibited by these materials in laboratory wear simulators. Also, current generation metal-metal and ceramic-ceramic bearing couples unquestionably produce very low rates of volumetric wear [12]. These alternative bearings also bring new issues of concern, such as brittle fracture owing to reduced toughness of cross-linked PE cups [46], ion release from metal-metal [39], and squeaking/stripe wear for ceramic-ceramic [47]. Historical experience has taught these (and other) new areas of concern merit careful proactive scrutiny in clinical and laboratory settings. At least currently, however, none of these potential new complications appear to represent fatal flaws likely to result in widespread abandonment of these bearing surface advances, given the spectacular wear-rate dividends.
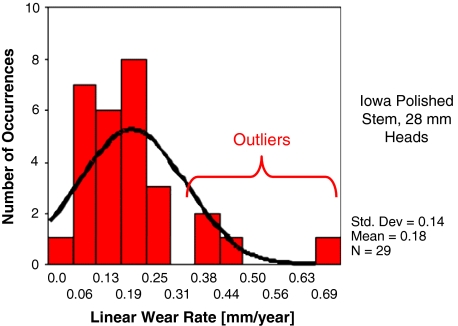
There is no discernible down side to aggregate wear-rate reduction. However, what is less well recognized is not all patients will benefit commensurately. Wear rates in any given cohort of consecutive patients having THA (ie, same surgeon, same technique, same implant/construct, etc) exhibit non-Gaussian distributions [38], characteristically with a subset of outliers whose wear rates approach order-of-magnitude elevation above cohort mean (Fig. 1). The now-well-established linkage between wear particle burden and osteolysis propensity [35] suggests these outlier patients are highly disproportionately at risk. Reduction of cohort mean wear rates per se therefore offers an incomplete answer to the osteolysis problem in the absence of substantial progress against outlier wear.
Fig. 1.
A graph shows the linear wear rate variations among patients in a representative THA cohort. All patients were consecutively implanted by the same surgeon using the same technique. In this particular instance, the construct involved a cemented titanium metal-backed acetabular component (TiBac; Zimmer, Inc, Warsaw, IN) and PE that had been gamma-irradiated in air.
Why, then, do some patients experience wear greatly in excess of cohort average? Seemingly, functional demand (body weight and activity level) is a contributing factor, although attempts to document objective causality have thus far met with mixed success [41], with side-to-side wear variability approaching 40% in patients who are identically bilaterally implanted. Although sufficiently powered series may manage to establish a consensus quantitative relationship in the future, the small fraction of wear-rate variance accounted for by studies performed to date calls into question whether even large meta-series will be able to convincingly establish functional demand as the predominant cause of outliers.
An alternative explanation, although admittedly one even more elusive to directly document, is third-body effects (Fig. 2). Few if any observers would disagree, in principle, that third-body challenge can accelerate wear. Nevertheless, acknowledgement of third-body effects in the orthopaedic community has to date been largely pro forma. Numerous factors responsible for third bodies have been identified (Table 1), but attempts to eliminate these third-body sources have been concerted only for the most egregious situations [13]. This is because many of these same factors (eg, modularity) have compelling attractions from other perspectives. The modest prioritization accorded third-body minimization therefore is understandable, given that virtually no information has been available regarding effect potency at the clinical/construct level.
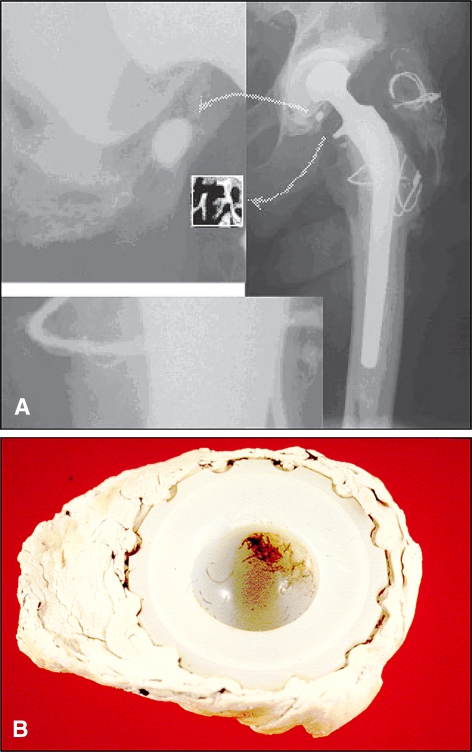
Fig. 2A–B.
(A) Third-body debris from fragmentation of trochanteric reattachment cables is shown. (B) A photograph shows embedded third-body particles in a retrieved (failed) acetabular cup.
Table 1.
Sources of third-body wear
| Source |
| Bone cement |
| Radiopacifier particles |
| Bone particles |
| Trochanteric reattachment wires |
| Burnishing from loose stems |
| Hydroxyapatite particles |
| Fixation screw fretting |
| Neck impingement |
| Matte/precoat stem abrasion |
| Instrument scratching |
| Modular connection fretting |
| Cutting guide abrasion |
| Porous coating particles |
| Locking mechanism breakage |
| Microseparation impact |
| Assembly/impaction chipping |
The goal of this summarized body of translational research is to elevate the visibility of third-body wear as a clinical problem by means of establishing quantitative mechanistic linkages between influence parameters and construct level wear-rate acceleration. Specifically, the role of site and severity of localized femoral head damage, the role of the site of cup-embedded debris causing that femoral head damage, and mechanisms by which third-body debris could gain access to wear-critical bearing surface sites are systematically investigated. Also, the work includes retrospective long-term followups of otherwise similar patient groups having substantially different severity of third-body challenge. Our hope is that the enhanced tangibility afforded by quantitative data will help stimulate enhanced surgeon awareness of the importance of this wild card and commensurate steps to reduce its impact.
Proximate Rationale for the Work
Two pieces of evidence provided immediate impetus for this series of studies. The first evidence [17] arose from observations in a cohort of 330 patients having THA, originally operated on by Dr. Richard C. Johnston of Des Moines, IA, and being followed by the clinical coauthor (JJC). At the time of surgery, newly available braided cable had been adopted instead of solid wire for trochanteric fixation. Within 30 months, it became apparent the cable was highly prone to fretting and strand breakage when used in this application, dictating its immediate discontinuance in favor of returning to solid wire. This cohort of patients who had cable-implantation therefore constituted an unintended opportunity to observe the effects of a substantial third-body challenge, relative to 379 consecutive patients with lesser-challenges (before and after) with otherwise identical constructs except for solid wire trochanteric fixation. A (then) newly developed technique in our laboratory for digital edge detection of radiographically apparent wear [42] showed the patients with third-body-challenge with implanted cable on average had substantially increased wear at a minimum of 10 years compared with the patients with wire-implantation. (A recently completed 20-year followup of these same patients, summarized below [1], has shown this higher third-body challenge has now led to a substantially higher failure rate.)
The second piece of prelude evidence arose from laboratory work with sliding-distance-coupled finite element analysis (FEA) of THA wear [30]. This computational formulation [27, 28] initially was developed as an expedited means to complement laboratory physical wear simulators for parametric studies of design parameters such as head size and bearing surface conformity [29]. Close agreement of the FEA model with collaborative physical experiments [30] had lent confidence that the model might be useful for studying a wider range of influence factors, including alternative modalities for acetabular component fixation. Unexpectedly, however, for otherwise identical input loadings and articulation kinematics, the data showed indistinguishable wear-rate differences for cemented monobloc PE versus cemented metal-backed versus noncemented (porous ingrowth) metal-backed components, despite ample clinical experience [9, 19, 45] of substantially higher wear rates for metal-backed components. This intriguing finding suggested some other very potent factor, apart from construct/design, must be responsible for the clinical wear-rate differential. Third-body effects stood out as a plausible suspect.
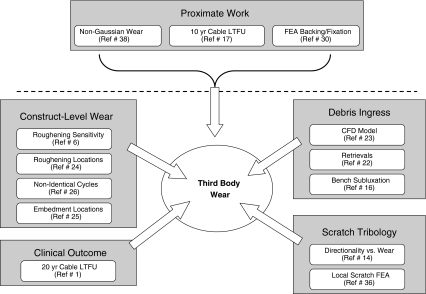
Previous work addressing third-body effects mostly has been confined to two types of studies: (1) idealized bench measurements of wear-rate acceleration from discrete scratches [10, 33] or (2) laboratory hip simulator studies where challenges had been imposed either from heads prescratched/preroughened directly or from abrasive particles added to the simulator lubricant fluid [8, 48]. The idealized scratch measurements initiated generally high but extremely variable wear-rate accelerations on a per-scratch basis. Similarly, the laboratory simulator work showed a wide range of wear-rate sensitivities, depending on the specifics of the imposed challenge. Consistent quantitative relationships were not evident, apparently because of the idiosyncratic and/or anecdotal nature of the various protocols used. To help develop a unifying framework, a research program strategy (Fig. 3) was formulated to compile a body of evidence systematically quantifying dose-response relationships influencing the potency of third-body wear rate acceleration.
Fig. 3.
A flowchart illustrates the interrelationship among the 10 original investigations of third-body wear acceleration summarized in this review. LTFU = long-term followup.
Quantitative Studies of Third-body Wear Acceleration
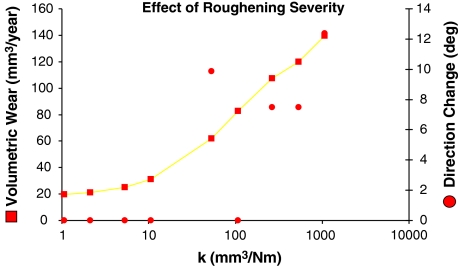
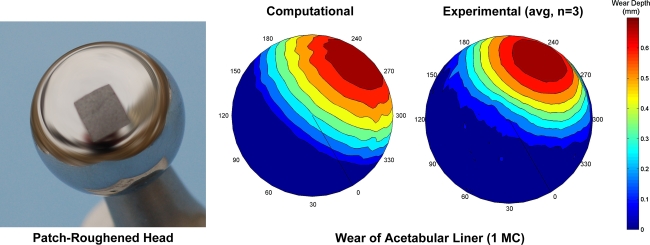
The first step [6] was to use the sliding distance computational model of PE wear to test the hypothesis that, other factors being equal, clinically typical variability in regions of localized femoral head roughening could account for much of the variation observed clinically in wear magnitude and wear direction. The computational formulation was based on the Archard/Lancaster abrasive/adhesive wear relationship, by means of which local wear rate was computed as the duty cycle product of local contact stress, local sliding distance, and a wear coefficient, k, that depended on bearing surface tribology (materials, surface finish, lubrication, etc). However, unlike previous wear computations for undamaged bearing surfaces [27–30], for which the wear coefficient had been spatially uniform at all sites on the femoral head surface, the formulation was augmented to incorporate local roughening of the femoral head surface, implemented by means of local elevations of the wear coefficient. This augmentation added algorithmic complexity because it required identifying the specific femoral head site (surface finite element node) apposing each specific acetabular site (contact finite element face centroid) at each instant of the duty cycle. Systematic parametric trials were done to explore the influences of head roughening severity, roughened area size, and roughened area location. The results showed head roughening variability (conservatively) typical of retrieval specimens led to approximately a 30° variation in wear direction and approximately a sevenfold variation in volumetric wear rate (Fig. 4). A series of purpose-conducted laboratory wear simulator runs with patch-roughened femoral heads provided direct validation of these computational results (Fig. 5). Also, unlike the spherical-front test tube material removal distributions characteristic of wear of nondamaged THA bearing surfaces [20], the computed wear distributions for local head roughening were much more irregular, consistent with the distinctly nonspherical wear fronts typical of highly worn cups retrieved at revision [49]. These results supported the hypothesis that randomness in head scratching can account for otherwise-difficult-to-explain variations in wear direction and wear rate, reinforcing the premise that third-body debris may be a key factor causing excessive wear in the most problematic subset of the THA population.
Fig. 4.
A graph shows the changes in finite element-computed volumetric wear rate (square symbols) and in wear direction (circular symbols) accompanying progressive roughening of a contiguous patch encompassing 5% of the femoral component head surface area.
Fig. 5.
Validation trials for patch-roughening (by grit blasting) of otherwise undamaged femoral component heads showed close agreement of UHMWPE removal in a laboratory wear simulator (Leeds ProSim; Prosim Ltd, Leeds, UK) versus for identical motion and loading inputs programmed in the finite element model. Peak wear depth experimentally averaged 0.783 mm (standard deviation = 0.09 mm) versus 0.76 mm computationally. Directionally, experimental (average) versus computational wear directions differed by approximately 9º.
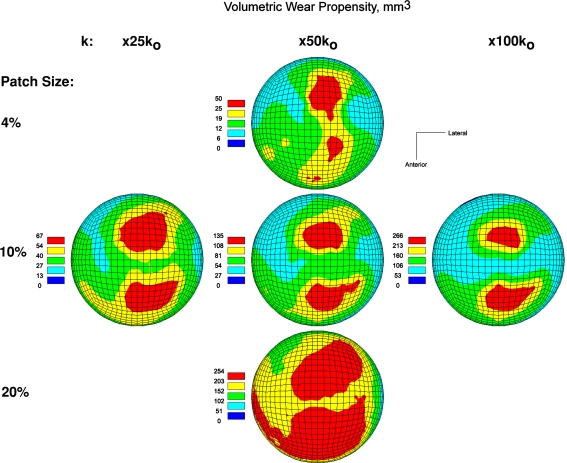
A next logical step [24] was to systematically explore which regions of femoral head roughening would be most kinetically critical, other factors being equal, in terms of the resulting acetabular component wear. Again using the local-roughening computational wear model, this task was approached by postulating otherwise similar levels of femoral head damage severity (size of roughened area and severity of k-factor elevation) located at systematically varied locations across the femoral head. This began with a coarse sampling strategy, followed by a stage of finer spatial refinement in the near vicinity of sensitivity sites identified at the coarse stage of sampling. Two maximally wear-inducing femoral head damage sites, of nominally comparable kinetic criticality, were consistently evident across a wide range of roughening severities (Fig. 6). These two critical sites were located quasisuperiorly near the sagittal midline of the head, one slightly anterior and one slightly posterior of the coronal midline. This systematic mapping series also identified the full range of third-body-attributable wear direction variability (Fig. 7), which spanned approximately 60º of arc, nearly twice the range observed in the earlier exploratory/sensitivity study.
Fig. 6.
The maps show relative volumetric wear rate acceleration as a function of location of otherwise-similar degrees of femoral head roughening. Across a wide range of roughening patch sizes and wear factor (k) increases, two similarly located sites of maximum kinetic criticality emerged. (Reprinted with permission and copyright 2005 by Lippincott Williams and Wilkins from Lundberg HJ, Stewart KJ, Callaghan JJ, Brown TD. Kinetically critical sites of femoral head roughening for wear rate acceleration in total hip arthroplasty. Clin Orthop Relat Res. 2005;430:89–93.)
Fig. 7.
The maps show variability of the direction of polyethylene wear attributable to variability in site and severity of femoral head roughening. (Reprinted with permission and copyright 2005 by Lippincott Williams and Wilkins from Lundberg HJ, Stewart KJ, Callaghan JJ, Brown TD. Kinetically critical sites of femoral head roughening for wear rate acceleration in total hip arthroplasty. Clin Orthop Relat Res. 2005;430:89–93.)
Early in the development of the local-roughening computational wear formulation, it became apparent the morphology of the (nonspherical-front) wear scar evolved asymptotically with advancing numbers of duty cycles, with the most rapid rate of material removal occurring initially. Each time the adaptive remeshing algorithm reached a point at which geometric updating of the acetabular surface finite element nodes was performed (typically, at intervals in the range of every 20,000 to 50,000 cycles, depending on model parameters), the remeshed acetabular surface experienced a fresh burst of wear, with a different spatial distribution from that of the previously existing wear front. Effectively, before mesh updating, regions of local head roughening had progressively abraded a trough in the acetabular surface, an asymptotically self-limiting effect so long as the relative motion between the roughened patch and the acetabular surface remained unchanged. Use of identical duty cycles has been commonplace in laboratory simulator testing, whereas THA implants in vivo experience various loading/motion inputs. This suggested a (previously unexplored) potential contributing explanation regarding why laboratory simulators have tended to substantially underestimate in vivo wear rates.
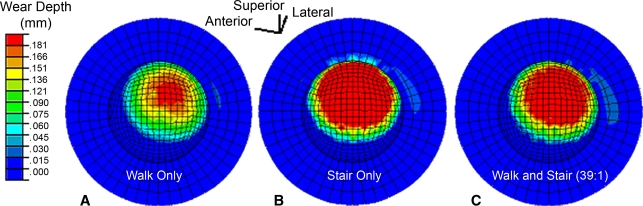
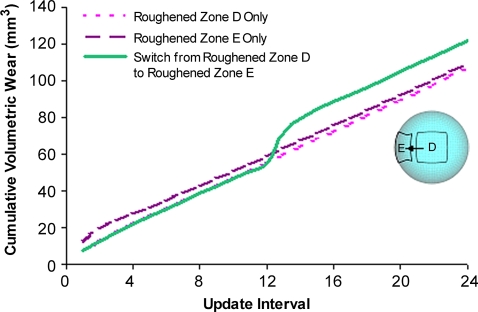
To systematically study this phenomenon [26], the sliding-distance-coupled contact finite element wear formulation was used to test the hypothesis that nonidentical duty cycles (from differing activities) and/or changes of femoral head roughening (from fresh third-body challenge) would cause accelerations in PE wear. In the absence of femoral head roughening, nonidentical duty cycles involving a 39:1 combination of walking and stair climbing [3] failed to result in appreciably higher volumetric wear than a walk-only simulation. However, when a roughened zone was included, walk/stair climbing volumetric wear increased by approximately 57% above that for a similarly roughened walk-only simulation (Fig. 8). To investigate time-variant femoral head roughening, wear simulations were begun with femoral head roughening at one location on the femoral head, switching to another location halfway through the simulation. The magnitude of the resulting volumetric wear increases varied depending on the specific roughening sites involved, but model runs in which a substantial increase in wear occurred characteristically showed an abrupt transient jump in wear rate (Fig. 9) shortly after the change of head roughening location. Apart from the wear-rate decreases presumably occurring owing to polishing of previously roughened surfaces [44], these results indicated wear-rate increases in laboratory physical third-body wear simulations using identical duty cycles and static head damage patterns would tend to be much more self-limited (owing to the trough-cutting effect) than otherwise would occur in vivo.
Fig. 8A–C.
The maps show the changes in the distribution of linear wear attributable to nonidentical duty cycles, ie, (A) walking only, (B) stair climbing only, and (C) walking and stair climbing, for one-million simulated wear cycles. Even very occasional instances (1:39) of alternative kinematic/kinetic input suffice to markedly change wear in the presence of local femoral head roughening. (Reprinted with permission and copyright 2007 by Wiley Interscience from Lundberg HJ, Stewart KJ, Pedersen DR, Callaghan JJ, Brown TD. Nonidentical and outlier duty cycles as factors accelerating UHMWPE wear in THA: a finite element exploration. J Orthop Res. 2007;25:30–43.)
Fig. 9.
A graph shows the transient increase in volumetric wear rate attributable to a simulated zone of fresh femoral head roughening. (Reprinted with permission and copyright 2007 by Wiley Interscience from Lundberg HJ, Stewart KJ, Pedersen DR, Callaghan JJ, Brown TD. Nonidentical and outlier duty cycles as factors accelerating UHMWPE wear in THA: a finite element exploration. J Orthop Res. 2007;25:30–43.)
Except for unusual circumstances such as instances of intraoperative scratching by surgical instrumentation, most third-body damage to the femoral head bearing surface occurs owing to scratching by discrete particulate debris [31]. Creating a scratch requires that the offending particle move relative to the femoral head surface while being pressed against that surface by confinement from the opposing acetabular surface. Particles embedded in the acetabular PE would undergo the greatest motion relative to the femoral head, but depending on the site of embedment, their propensity for creating scratches in wear-critical locations of the femoral head seemingly would vary appreciably. (As an extreme illustration, a particle embedded at an acetabular site that never makes contact with the femoral head bearing surface would make absolutely no contribution to third-body wear acceleration, regardless of that particle’s degree of abrasiveness.) To identify the site(s) of most wear-critical particle embedment [25], a novel computational formulation was developed to build on the above-described femoral head damage criticality mapping study. By means of inverse rotational transforms of the level-walking duty cycle [37], the locus of femoral head sites overpassing each acetabular finite element centroid was identified. From these overpassage locus data, it was possible to map the relative wear criticality of the head damage of otherwise-identical third-body particles similarly embedded at alternative sites spanning the entire acetabular bearing surface. Parameterized in terms of a damage propensity index, the data showed debris embedded superior-laterally in the cup surface (Fig. 10) had highly disproportionately elevated wear acceleration propensity. These findings served to accentuate that even for patients with nominally comparable third-body particle challenge, the consequences in terms of wear-rate acceleration would be markedly different, depending on where particulate debris happen to become embedded in the cup.
Fig. 10.
A graph shows damage propensity index (DPI) mapping of the relative wear potency of a given third-body particle if embedded at alternative sites on the acetabular bearing surface. (Reprinted with permission and copyright 2006 by Elsevier from Lundberg HJ, Stewart KJ, Pedersen DR, Callaghan JJ, Brown TD. Problematic sites of third body embedment in polyethylene for total hip wear acceleration. J Biomech. 2006;39:1208–1216.)
Factors governing debris access to critical sites on the acetabular bearing surface therefore emerge as a major consideration in third-body wear acceleration. Although contemporary manufacturing tolerances for metal-on-PE implants typically involve head-cup conformity differentials in the range of 0.1 to 0.2 mm [40], initial cold flow (bedding-in) and run-in wear rapidly reduce this conformity differential into the micrometer or submicrometer range [18]. However, the third-body particles typically embedded in retrieval cups are frequently up to hundreds of micrometers in equivalent circular diameter [22], ie, one to two orders of magnitude greater in size than could plausibly gain access deep into the bearing space. How such large third-body particles manage to enter the very closely conforming bearing space of a metal-on-PE articulation couple is not well understood.
We hypothesized one such mechanism is from convective fluid transport during impingement/subluxation of the THA. To test this hypothesis [23], a three-dimensional computational fluid dynamics (CFD) model was developed and physically validated to quantify fluid ingress into the bearing space during subluxation events. The physical validation (error < 15%) was performed using particle image velocimetry (PIV), a technique by which motions of reflective, neutrally buoyant micromarkers dispersed in the fluid are tracked and their velocities measured. The CFD results (Fig. 11) suggested periarticular joint fluid could be vigorously drawn nearly to the pole of the cup with even very small (subclinical) impingement-induced subluxations of the femoral head (< 0.60 mm), as occur for example during leg-crossing maneuvers [34]. Debris initially suspended just outside the equator of the cup at the site of maximum fluid velocity just before the subluxation began could be transported to within 11º from the cup pole. Larger head diameters resulted in increased fluid velocity at all sites around the entrance to the gap compared with smaller head sizes, with fluid velocity being greatest along the anterosuperolateral cup edge for all head sizes. However, fluid pathlines indicated suspended debris would reach approximately similar angular positions in the bearing space regardless of head size. Increased inset of the femoral head into the acetabular cup resulted in higher fluid velocity and in transport of third-body debris further into the bearing space, whereas fluid/debris ingress patterns were relatively insensitive to variations of cup bevel or of head/trunion seating geometry. Nevertheless, all simulated cases of impingement/subluxation forcefully drew joint fluid (and by implication, suspended debris) well into wear-critical areas of the bearing space. This novel finding indicates the deleterious effects of impingement are not confined to dislocation propensity or even to rim indentation damage but also extend to wear-rate acceleration if third-body particles are present in the periarticular joint fluid.
Fig. 11.
A diagram illustrates the fluid velocity distribution (CFD model) during a subluxation event accompanying femoral neck impingement on the acetabular rim. Fluid ingress patterns are such that suspended third-body debris would reach nearly to the pole of the cup. (Reprinted with permission and copyright 2007 by Elsevier from Lundberg HJ, Pedersen DR, Baer TE, Muste M, Callaghan JJ, Brown TD. Effects of implant design parameters on fluid convection, potentiating third body debris ingress into the bearing surface during THA impingement/subluxation. J Biomech. 2007;40:1676–1685.)
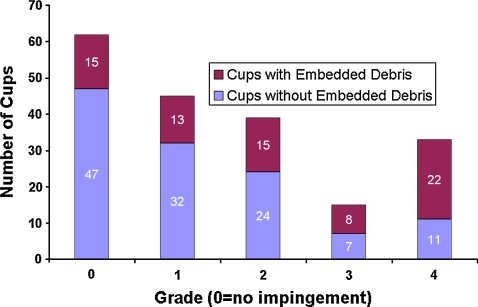
Despite the novel information it provided and despite its laboratory validation by PIV, the simplifications necessary for implementing the CFD convection model nevertheless involved numerous substantial idealizations of in vivo reality. To further substantiate the clinical credibility of the CFD model’s premise of subluxation-mediated particle ingress, the association between severity of liner rim indentation damage (indicative of impingement frequency/vigor) and the presence of embedded third-body debris was catalogued in 194 acetabular components retrieved at revision [22]. Rim damage was graded using the 5-point Hospital for Special Surgery scale [43]. Particle embedment was assessed manually and with a purpose-written image analysis computer program that detected each particle’s composition, size, and location. Approximately 68% of the cups had rim indentation damage. There was a strong association between severity of rim damage and presence of embedded debris (Fig. 12). There was substantial nonuniformity of the spatial distribution of the embedded debris, with the predominance of particles being at intermediate latitudes. These findings corroborate the premise of convection of debris-laden joint fluid during lever-out subluxation as a mechanism for wear-consequential third-body particles to gain access to highly loaded regions of the bearing surface, thus potentiating increased wear.
Fig. 12.
A graph shows the association between acetabular rim impingement damage severity (Hospital for Special Surgery scale) and the presence of embedded debris in a group of 194 cup retrievals.
Numerous confounding factors potentially interact to influence the numbers and patterns of embedded third-body particles evident on the bearing surface of retrieved acetabular cups. The association between liner rim damage and embedded debris presence constitutes strong circumstantial corroboration, but it does not directly establish mechanism. To go to this next level, a laboratory study [16] was designed to directly test the hypothesis that occasional events of femoral head subluxation would increase the number of third-body particles that enter the bearing space and become embedded in the acetabular liner, as compared with level-walking cycles alone. Ten new metal-on-PE hip head-liner pairs were tested in a multiaxis joint motion simulator, with clinically representative [2] CoCrMo third-body particles added to the synovial fluid analog. All component pairs were tested for 2 hours in level walking, with ½ also being subjected to 20 intermittent subluxation events. The number and location of embedded particles on the acetabular liners then were determined. Subluxation during hip simulator testing dramatically increased the number of third-body particles that became embedded in the bearing surface (Fig. 13) and also considerably increased the amount of scratching on the femoral heads. As subluxation events and third-body challenge habitually occur in vivo, this potent interaction needs to be borne prominently in mind when considering the mechanistic basis for outlier wear.
Fig. 13A–B.
The number and sites of particles embedded are shown in a laboratory third-body challenge bench experiment for simulated normal-level walking (A) with versus (B) without impingement. (Reprinted with permission and copyright 2008 of Elsevier from Heiner AD, Lundberg HJ, Baer TE, Pedersen DR, Callaghan JJ, Brown TD. Effects of episodic subluxation events on third body ingress and embedment in the THA bearing surface. J Biomech. 2008;41:2090–2096.)
The influences dominating the rate of PE debris liberation by counterface scratches are not fully understood, but one seemingly contributory factor is the scratch orientation relative to the direction of instantaneous local bearing surface sliding. To mechanistically study this directionality influence [14], arrays of 550 straight parallel scratches, each representative of the severe end of the clinical range [32], were diamond stylus-ruled onto the surface of polished stainless steel plates. These ruled plates then were worn reciprocally against 25-mm-diameter cylindrical PE faces (conventional and highly cross-linked) at traverse angles varied parametrically relative to the scratch direction. Wear was measured gravimetrically, and particulate debris was harvested and morphologically characterized. Both PE variants tested showed pronounced wear rate peaks at acute scratch traverse angles (15º for conventional, 5º for cross-linked) and had nominally comparable absolute wear-rate magnitudes. These data suggest counterface damage regions with preferential scratch directionality can liberate large amounts of PE debris, apparently by a slicing/shearing mechanism, at critical (acute) attack angles.
A complementary local three-dimensional finite element model was developed of orientation-specific PE articulation with a scratched metal counterface [36] to explore continuum level stress/strain parameters potentially correlating with the orientation dependence of scratch wear in the above scratch-directionality physical experiment. Computed maximum stress values exceeded the yield strength of UHMWPE for all scratch orientations but did not vary appreciably among scratch orientations. Two continuum level parameters that showed most consistent overall correlation with the direction dependence of experimental wear were (1) cumulative compressive total normal strain in the direction of loading and (2) maximum instantaneous compressive total normal strain transverse to the sliding direction. These stress/strain metrics are attractive for use in global computational models of scratch-induced wear acceleration as surrogates to account for anisotropy of local metal surface roughening.
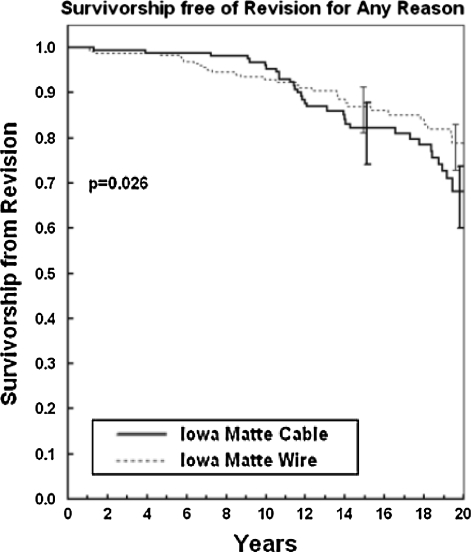
Coming full circle clinically, a review was completed recently [1] of the 20-year outcomes for the cohort of patients with third-body-challenge (n = 196 then still living) who had cable trochanteric reattachment versus consecutive control (lesser third-body-challenge) patients (n = 304 then still living) with wire reattachment. The data showed the patients who had cable-implantation have fared dramatically worse by virtually all measures associated with wear/osteolysis/loosening. The prevalence of proximal femoral osteolysis was 1.66 times as high (47.4% versus 28.6%; chi square p < 0.0001), the prevalence of acetabular osteolysis was 2.25 times as high (21.4% versus 9.5%; chi square p < 0.0003), the mean linear radiographic wear rate was 23.2% higher (0.101 mm/year versus 0.082 mm/year; Wilcoxon rank-sum p < 0.039), the prevalence of acetabular radiographic loosening was 1.46 times as high (20.1% versus 13.8%; Cox regression for Kaplan-Meier survivorship p < 0.059), the fraction of cases with revision for acetabular loosening was 1.94 times as high (15.3% versus 7.9%; Cox regression for Kaplan-Meier survivorship p < 0.030), and the fraction of hips having aseptic revision of one or both components was 1.80 times as high (15.3% versus 8.5%; Cox regression for Kaplan-Meier survivorship p < 0.034). Most importantly, overall implant survivorship for the patients with third-body-challenge was worse (p < 0.026) and becoming increasingly more so (Fig. 14). These data provide compelling evidence that construct level quantitative study of third-body wear mechanisms translates directly to the clinical setting.
Fig. 14.
A graph shows 20-year implant survivorship for otherwise identical constructs, differing in terms of elevated third-body burden attributable to to fretting/breakage of trochanteric fixation cable.
Discussion
Toward the goal of developing a unifying framework for quantitative study of third-body wear in THA, a series of complementary laboratory and clinical studies was performed to parametrically evaluate the influence of site and severity of localized femoral head roughening and of the relative wear acceleration potency of third-body debris as a function of site of embedment in the acetabular liner. Additionally, a mechanism for debris ingress to wear-critical liner locations was studied by computational modeling, by bench physical testing, and in implant retrieval specimens. Long-term followup of patients with third-body challenge provided confirmation of adverse effects in terms of elevated wear rates, increased osteolysis, and reduced implant longevity.
Much of the work summarized here has involved novel parametric computational simulations. Although physically validated where possible in terms of key biomechanical aspects of model performance, these computational models necessarily invoke simplifications of full physiologic complexity, in the interest of studying individual variables of interest. A particular specific limitation has been postulation of discrete focal regions of homogeneous femoral head damage or of liner debris embedment, clearly an idealization of the complexity and heterogeneity of third-body challenge occurring in vivo. It would be desirable to develop a framework to characterize actual global patterns of clinically occurring third-body challenge in a manner lending itself to similarly systematic study. A second major limitation is that this family of wear acceleration simulations implemented head damage in terms of isotropic (ie, nondirectional) homogeneous roughening, rather than in terms of the directional scratches physically present on the head surface. Additional work along these lines ideally would involve models that simulate the wear effects of individual scratches or groups of scratches, whose directionality would interact with the directionality of relative motion at the bearing surface. Finally, in the clinical series, although the patients with cable fixation clearly had a much more severe third-body challenge than those with wire fixation, there was no basis by which to quantify the relative severity of those respective challenges. Techniques to quantify third-body challenge in vivo would be of enormous value for identifying at-risk patients, but unfortunately these remain to be developed.
Now that wear-resistant highly cross-linked PEs have widespread use in THA, going forward, there is good reason to anticipate a substantial reduction of the incidence of osteolysis. At the same time, however, new bearing surface technologies involve a range of potential third-body generators not previously encountered, so heightened attention to the challenge of reducing outlier wear is warranted. Moreover, substantially increasing absolute numbers of THAs are projected in coming decades [21], and those THA constructs will confront ever-higher lifetime functional demand owing to indications being extended to ever-younger patients [7] and to the societal problem of increasing overweight/obesity [11]. When present, third-body debris unfortunately can corrupt even the best of bearing surface materials and the most advanced of implant designs [33] and can serve to undermine otherwise excellent surgical technique. Although discussed above largely in the context of THA, third-body challenge is of comparable if not greater concern in the context of TKA, where the absolute numbers of procedures now and for the future are even higher [21], and where various technical aspects of the surgery (eg, saw blade abrasion against cutting guides [15]) are even more problematic in terms of debris production. Yet another consideration is that the acceptance of minimally invasive surgical technique in a substantial segment of the arthroplasty community unfortunately brings the increased possibility of debris production from instrument use accompanying the correspondingly reduced surgical field visibility/accessibility [4].
Given the many surgical and engineering complexities involved, it is unrealistic to expect emergence of simple panacea solutions to third-body wear. Nevertheless, this is an area that historically has been the province of nebulous subjective conjecture and where quantitative cause/effect mechanistic relationships largely have been lacking. Translationally, building an improved base of scientific understanding regarding third-body wear phenomena is a key element toward heightening visibility of the problem in the orthopaedic surgical community and in turn to stimulating industry’s response to surgeon demand for implants, instruments, and technologies that will reduce third-body burden and its adverse consequences for patients having arthroplasties. This reviewed body of translational research has endeavored to quantitatively link regional head roughening with regional and construct level wear acceleration and to link periarticular third-body particle burden with debris ingress to the bearing surface and subsequent head roughening, along with documenting increased osteolysis and loosening in long-term followup of a cohort of patients with elevated third-body challenge.
Acknowledgments
We gratefully acknowledge the contributions of many colleagues and students in terms of formal collaboration in one or more of the studies summarized and/or behind-the-scenes technical assistance. These include Dr. Aaron Altenburg, Thomas Baer, Dr. John Clohisy, Kevin Dahl, Dr. John Fisher, Dr. Alison Galvin, Liam Glennon, Dr. Devon Goetz, Dr. Anneliese Heiner, Dr. Richard Johnston, Dr. William Lack, Jessica Leinen, Dr. Steve Liu, Dr. James Martin, Julie Mock, Dr. Marion Muste, John Neiman, Dr. Michael O’Rourke, Matthew Paul, Dr. M. James Rudert, Kristofer Stewart, Dr. David Vittetoe, and Tameem Yehyawi. Implants for physical testing and implant CAE files for finite element model development were provided by DePuy Orthopaedics Inc and by Smith and Nephew, Inc.
Footnotes
References
- 1.Altenburg AJ, Callaghan JJ, Yehyawi TM, Pedersen DR, Leinen JA, Dahl KA, Goetz DD, Brown TD, Johnston RC. The effect of cable debris on acetabular construct durability in cemented total hip arthroplasty at 19 to 20 year followup. J Bone Joint Surg Am, in press. [DOI] [PMC free article] [PubMed]
- 2.ASTM F75-01, 2001; ASTM F75-01, 2001. Standard specification for cobalt-28 chromium-6 molybdenum alloy castings and casting alloy for surgical implants. West Conshohocken, PA: ASTM International; 2001.
- 3.Benson LC, DesJardins JD, Harman MK, LaBerge M. Effect of stair descent loading on ultra-high molecular weight polyethylene wear in a force-controlled knee simulator. Proc Inst Mech Eng H. 2002;216:409–418. [DOI] [PubMed]
- 4.Berry DJ, Berger RA, Callaghan JJ, Dorr LD, Duwelius PJ, Hartzband MA, Lieberman JR, Mears DC. Minimally invasive total hip arthroplasty: development, early results, and a critical analysis. J Bone Joint Surg Am. 2003;85:2235–2246. [PubMed]
- 5.Bragdon CR, Greene ME, Freiberg AA, Harris WH, Malchau H. Radiostereometric analysis comparison of wear of highly cross-linked polyethylene against 36- vs 28-mm femoral heads. J Arthroplasty. 2007;22(6 suppl 2):125–129. [DOI] [PubMed]
- 6.Brown TD, Stewart KJ, Neiman JC, Pedersen DR, Callaghan JJ. Local head roughening as a factor contributing to variability of total hip wear: a finite element analysis. J Biomech Eng. 2002;124:691–698. [DOI] [PubMed]
- 7.Crowninshield RD, Rosenberg AG, Sporer SM. Changing demographics of patients with total joint replacement. Clin Orthop Relat Res. 2006;443:266–272. [DOI] [PubMed]
- 8.Davidson JA, Poggie RA, Mishra AK. Abrasive wear of ceramic, metal, and UHMWPE bearing surfaces from third-body bone, PMMA bone cement, and titanium debris. Biomed Mater Eng. 1994;4:213–229. [PubMed]
- 9.Devane PA, Robinson EJ, Bourne RB, Rorabeck CH, Nayak NN, Horne JG. Measurement of polyethylene wear in acetabular components inserted with and without cement: a randomized trial. J Bone Joint Surg Am. 1997;79:682–689. [DOI] [PubMed]
- 10.Dowson D, Taheri S, Wallbridge NC. The role of counterface imperfections in the wear of polyethylene. Wear. 1987;119:277–293. [DOI]
- 11.Fehring TK, Odum SM, Griffin WL, Mason JB, McCoy TH. The obesity epidemic: its effect on total joint arthroplasty. J Arthroplasty. 2007:22(6 suppl 2):71–76. [DOI] [PubMed]
- 12.Fisher J, Jin Z, Tipper J, Stone M, Ingham E. Tribology of alternative bearings. Clin Orthop Relat Res. 2006;453:25–34. [DOI] [PubMed]
- 13.Gilbert JL, Buckley CA, Jacobs JJ. In vivo corrosion of modular hip prosthesis components in mixed and similar metal combinations: the effect of crevice, stress, motion, and alloy coupling. J Biomed Mater Res. 1993;27:1533–1544. [DOI] [PubMed]
- 14.Glennon LP, Baer TE, Martin JA, Lack WD, Brown TD. Sliding direction dependence of polyethylene wear for metal counterface traverse of severe scratches. J Biomech Eng. 2008;130:051006. [DOI] [PMC free article] [PubMed]
- 15.Gotterson PR, Nusem J, Pearcy MJ, Crawford RW. Metal debris from bony resection in knee arthroplasty: is it an issue? Acta Orthop. 2005;76:475–480. [DOI] [PubMed]
- 16.Heiner AD, Lundberg HJ, Baer TE, Pedersen DR, Callaghan JJ, Brown TD. Effects of episodic subluxation events on third body ingress and embedment in the THA bearing surface. J Biomech. 2008;41:2090–2096. [DOI] [PMC free article] [PubMed]
- 17.Hop JD, Callaghan JJ, Olejniczak JP, Pedersen DR, Brown TD, Johnston RC. Contribution of cable debris generation to accelerated polyethylene wear. Clin Orthop Relat Res. 1997;344:20–32. [DOI] [PubMed]
- 18.Jalali-Vahid D, Jagatia M, Jin ZM, Dowson D. Prediction of lubricating film thickness in UHMWPE hip joint replacements. J Biomech. 2001;34:261–266. [DOI] [PubMed]
- 19.Jasty M, Goetz DD, Bragdon CR, Lee KR, Hanson AE, Elder JR, Harris WH. Wear of polyethylene acetabular components in total hip arthroplasty: an analysis of one hundred and twenty-eight components retrieved at autopsy of revision operations. J Bone Joint Surg Am. 1997;79:349–358. [DOI] [PubMed]
- 20.Kabo JM, Gebhard JS, Loren G, Amstutz HC. In vivo wear of polyethylene acetabular components. J Bone Joint Surg Br. 1993;75:254–258. [DOI] [PubMed]
- 21.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. [DOI] [PubMed]
- 22.Lundberg HJ, Liu SS, Callaghan JJ, Pedersen DR, O’Rourke MR, Goetz DD, Vittetoe DA, Clohisy JC, Brown TD. Association of third body embedment with rim damage in retrieved acetabular liners. Clin Orthop Relat Res. 2007;465:133–139. [DOI] [PubMed]
- 23.Lundberg HJ, Pedersen DR, Baer TE, Muste M, Callaghan JJ, Brown TD. Effects of implant design parameters on fluid convection, potentiating third body debris ingress into the bearing surface during THA impingement/subluxation. J Biomech. 2007;40:1676–1685. [DOI] [PMC free article] [PubMed]
- 24.Lundberg HJ, Stewart KJ, Callaghan JJ, Brown TD. Kinetically critical sites of femoral head roughening for wear rate acceleration in total hip arthroplasty. Clin Orthop Relat Res. 2005;430:89–93. [DOI] [PubMed]
- 25.Lundberg HJ, Stewart KJ, Pedersen DR, Callaghan JJ, Brown TD. Problematic sites of third body embedment in polyethylene for total hip wear acceleration. J Biomech. 2006;39:1208–1216. [DOI] [PubMed]
- 26.Lundberg HJ, Stewart KJ, Pedersen DR, Callaghan JJ, Brown TD. Nonidentical and outlier duty cycles as factors accelerating UHMWPE wear in THA: a finite element exploration. J Orthop Res. 2007;25:30–43. [DOI] [PubMed]
- 27.Maxian TA, Brown TD, Pedersen DR, Callaghan JJ. A sliding-distance-coupled finite element formulation for polyethylene wear in total hip arthroplasty. J Biomech. 1996;29:687–692. [DOI] [PubMed]
- 28.Maxian TA, Brown TD, Pedersen DR, Callaghan JJ. Adaptive finite element modeling of long-term polyethylene wear in total hip arthroplasty. J Orthop Res. 1996;14:668–675. [DOI] [PubMed]
- 29.Maxian TA, Brown TD, Pedersen DR, Callaghan JJ. Three-dimensional sliding/contact computational simulation of total hip wear. Clin Orthop Relat Res. 1996;333:41–50. [DOI] [PubMed]
- 30.Maxian TA, Brown TD, Pedersen DR, McKellop HA, Lu B, Callaghan JJ. Finite element analysis of acetabular wear: validation, and backing and fixation effects. Clin Orthop Relat Res. 1997;344:111–117. [DOI] [PubMed]
- 31.McKellop HA. The lexicon of polyethylene wear in artificial joints. Biomaterials. 2007;28:5049–5057. [DOI] [PubMed]
- 32.McNie CM, Barton DC, Ingham E, Tipper JL, Fisher J, Stone MH. The prediction of polyethylene wear rate and debris morphology produced by microscopic asperities on femoral heads. J Mater Sci Mater Med. 2000;11:163–174. [DOI] [PubMed]
- 33.Minakawa H, Stone MH, Wroblewski BM, Lancaster JG, Ingham E, Fisher J. Quantification of third-body damage and its effect on UHMWPE wear with different types of femoral head. J Bone Joint Surg Br. 1998;80:894–899. [DOI] [PubMed]
- 34.Nadzadi ME, Pedersen DR, Yack HJ, Callaghan JJ, Brown TD. Kinematics, kinetics, and finite element analysis of commonplace maneuvers at risk for total hip dislocation. J Biomech. 2003;36:577–591. [DOI] [PubMed]
- 35.Orishimo KE, Claus AM, Sychterz CJ, Engh CA. Relationship between polyethylene wear and osteolysis in hips with a second-generation porous-coated cementless cup after seven years of follow-up. J Bone Joint Surg Am. 2003;85:1095–1099. [DOI] [PubMed]
- 36.Paul MC, Glennon LP, Baer TE, Brown TD. Field variable associations with scratch orientation dependence of UHMWPE wear: a finite element analysis. J Biomech Eng. 2008;130:061019. [DOI] [PMC free article] [PubMed]
- 37.Pedersen DR. Brown TD, Maxian TA, Callaghan JJ. Temporal and spatial distributions of directional counterface motion at the acetabular bearing surface in total hip arthroplasty. Iowa Orthop J. 1998;18:43–53. [PMC free article] [PubMed]
- 38.Pedersen DR, Callaghan JJ, Johnston TL, Fetzer GB. Johnston RC. Comparison of femoral head penetration rates between cementless acetabular components with 22-mm and 28-mm heads. J Arthroplasty. 2001;16(suppl 1):111–115. [DOI] [PubMed]
- 39.Rasquinha VJ, Ranawat CS, Weiskopf J, Rodrigues JA, Skipor AK, Jacobs JJ. Serum metal levels and bearing surfaces in total hip arthroplasty. J Arthroplasty. 2006;21(6 suppl 2):47–52. [DOI] [PubMed]
- 40.Ritter MA, Meding JB, Faris PM, Keating EM. Femoral head size: variation among manufacturers. Orthopedics. 1996;19:877–878. [DOI] [PubMed]
- 41.Schmalzried TP, Huk OL. Patient factors and wear in total hip arthroplasty. Clin Orthop Relat Res. 2004;418:94–97. [DOI] [PubMed]
- 42.Shaver SM, Brown TD, Hillis SL, Callaghan JJ. Digital edge detection measurement of polyethylene wear in total hip arthroplasty. J Bone Joint Surg Am. 1997;79:690–700. [DOI] [PubMed]
- 43.Shon WY, Baldini T, Petersen MG, Wright TM, Salvati EA. Impingement in total hip arthroplasty: a study of retrieved acetabular components. J Arthroplasty. 2005;20:427–435. [DOI] [PubMed]
- 44.Sychterz CJ, Engh CA Jr, Swope SW, McNulty DE, Engh CA. Analysis of prosthetic femoral heads retrieved at autopsy. Clin Orthop Relat Res. 1999;358:223–234. [DOI] [PubMed]
- 45.Sychterz CJ, Moon KH, Hashimoto Y, Terefinko KM, Engh CA, Bauer TW. Wear of polyethylene cups in total hip arthroplasty: a study of specimens retrieved post mortem. J Bone Joint Surg Am. 1996;78:1193–1200. [DOI] [PubMed]
- 46.Tower SS, Currier JH, Currier BH, Lyford KA, VanCitters DW, Mayor MB. Rim cracking of the cross-linked longevity polyethylene acetabular liner after total hip arthroplasty. J Bone Joint Surg Am. 2007;89:2212–2217. [DOI] [PubMed]
- 47.Walter WL, O’Toole GC, Walter WK, Ellis A, Zicat BA. Squeaking in ceramic-on-ceramic cups: the importance of component orientation. J Arthroplasty. 2007;22:496–503. [DOI] [PubMed]
- 48.Wang A, Polineni VK, Stark C, Dumbleton JH. Effect of femoral head surface roughness on the wear of ultrahigh molecular weight polyethylene acetabular cups. J Arthroplasty. 1998;13:615–620. [DOI] [PubMed]
- 49.Yamaguchi M, Bauer TW, Hashimoto Y. Three-dimensional analysis of multiple wear vectors in retrieved acetabular cups. J Bone Joint Surg Am. 1997;79:1539–1544. [DOI] [PubMed]