Abstract
Although there has been some enthusiasm over the early clinical results obtained using the Graf ligament, associated mid- to long-term results are controversial. We retrospectively reviewed 43 patients (67 segments) treated with the Graf ligament for degenerative lumbar stenosis. The minimum followup was 8 years (mean, 10 years; range, 8–14 years). At last followup, we observed angular instability in 19 of the 67 segments (28%) and translational instability in five (7%). The disc height decreased from postoperatively (mean 93% of the preoperative disc) to the final followup (mean 82%). Of the 43 patients, 18 (42%) had adjacent segmental instability at the upper segment, including angular instability in 11 patients, translational instability in four patients, and both in three patients. The adjacent segment instability at the lower segment revealed 13 patients (30%) with angular instability. The data suggest the anticipated mechanical effects of the Graf ligament can be altered by degeneration of the disc and facet joints at instrumented segments and the adjacent segment can be affected, perhaps as a result of abnormal load transmission.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Decompression and fusion are the most important principles in surgically treating degenerative lumbar diseases. Achieving fusion is one of the most important factors influencing clinical outcome [4, 10, 34]. With the improvements in fusion techniques, the rate of successful fusion reportedly ranges from 60% to 100% [3, 27]. However, this has not been reflected by a corresponding increase in the rate of successful clinical outcomes [3, 14]. Several studies suggest 9% to 44% of patients fail to have improvement of back pain [1, 2, 33, 34]. This failure sometimes is associated with pseudarthrosis [23] or adjacent segment disease to fusions after long-term followup [6, 8, 9, 19, 22, 24].
Options other than fusion include dynamic stabilization [7, 18, 30, 31]. The Graf ligament (Neoligaments, Leeds, UK), described by Henry Graf [15], is the most commonly used device in this group. Given lumbar segmental instability is defined by abnormal movement, the Graf ligament was introduced to correct abnormal motion to a spinal motion segment. The system consists of a pair of Dacron® ligaments applied with pedicle screws with a predetermined force. The system immobilizes the spine in lordosis and locks the facet joints in full extension. One independent biomechanical study suggests it increases load over the posterior part of the disc and the annulus [25]. On reviewing the results of the Graf ligament at a mean 8.9 years’ followup, Onda et al. [26] suggested the Graf ligament does not always prevent adjacent segment disease and typically leads to progressively reduced motion of the treated segment. However, Kanayama et al. [17], at a mean 75 months’ followup, suggested the Graf ligament maintains lumbar mobility and decreased the risk of adjacent segment deterioration compared with posterolateral fusion with instrumentation. Although the Graf ligament was developed to diminish degenerative changes in the adjacent segments, compared with those encountered with rigid instrumentation, this theoretical advantage has not been clinically confirmed. Long-term followup results are necessary to determine the efficacy of the Graf ligament to maintain stability at the instrumented segment and prevent adjacent segment disease.
We therefore asked whether (1) the Graf ligament maintained lumbar stability as indicated by changes in disc height and angular and translational motions at the instrumented segment after a minimum 8 years followup, (2) one-segment and two-segment instrumentation differed in the likelihood of maintaining stability and preventing degeneration at the adjacent segment, and (3) improvement of back pain correlated with lumbar stability and adjacent disc degeneration.
Materials and Methods
We retrospectively reviewed all 47 patients (74 segments) who had dynamic stabilization with the Graf ligament for lumbar spinal stenosis from January 1992 to December 1996. We excluded four patients: one died of causes unrelated to the spinal disorder and three were lost to followup, leaving 43 patients (67 segments) for study. During the same time, we operated on 25 patients with lumbar spinal stenosis using posterior decompression and posterolateral fusion. Of the 43 included patients, there were 18 males and 25 females with an average age of 51.1 years (range, 28–73 years) at the time of surgery. The indication for surgery was symptomatic degenerative lumbar spinal stenosis. All patients had chronic back pain, radicular leg pain, and claudication with acute exacerbation with increasing severity and frequency. The mean period of the disability associated with the latter symptoms was 13 months (range, 10–48 months). In these patients, the back pain was worse than the leg pain and claudication. The radiographic assessment consisted of plain radiographs, flexion and extension stress radiographs, and computed tomography (CT) or MRI scans. We defined unstable segments as those showing angular movement greater than 10° or translational displacement greater than 3 mm on active flexion and extension lateral radiographs. Fifteen of the 43 patients had MRI and 28 patients had CT. MRI and CT revealed disc protrusion in 37 of the 67 segments and lateral recess stenosis with facet arthropathy in 45 segments. Five patients with disc protrusion of the adjacent segment underwent additional discography or selective nerve root block to determine the painful segments. The Graf ligament was used to reconstruct the unstable segments that had been assessed before surgery when we judged preservation of the facet joint was possible from adequate decompression. We considered spondylolysis, isthmic spondylolisthesis, and facet joint failure after previous decompression as contraindications to the Graf ligament, and therefore these 25 patients were treated with posterior decompression and posterolateral fusion. The minimum followup was 8 years (average, 10 years; range, 8–14 years).
All patients underwent laminectomy. The decompression procedure was extended laterally to identify each nerve root using an undercutting facetectomy to preserve the joint [12, 28]. In the presence of a disc protrusion, there is a risk of producing nerve root compression after Graf instrumentation. We therefore performed discectomy in 35 patients (43 of the 67 segments) who had a protruded disc. Stabilization was performed at one, two, and three levels in 20, 22, and one patient, respectively.
Postoperatively, the patients were mobilized 3 to 4 days after surgery and wore a brace for 6 weeks. This was followed by a rehabilitation program consisting of isometric training of the lower back muscles. We allowed the patients to return to work 2 to 6 months after the operation depending on the physical demands of their occupation. We recommended an annual visit when possible.
Back pain was assessed by applying a 10-point visual analog scale (VAS) preoperatively and at the last followup. We recorded any treatments to control persistent back pain at the last followup.
One of the authors (KS), who did not participate in the treatment, radiographically measured disc height on the lumbar lateral radiograph and instability at the instrumented segment and adjacent segment on a flexion-extension stress lateral radiograph of the lumbar spine. The disc height was defined as the distance from a line drawn from the superior end plate of the vertebra to the inferior end plate of the above adjacent vertebra and was presented as a ratio based on the anterior height of the upper adjacent vertebra to eliminate magnification errors of radiographic measurement. On the lateral stress views of flexion and extension, the displacement and angular movement of the vertebra before surgery and at the final followup were measured. Segmental instability was defined as a segment with angular movement greater than 10° or translational displacement greater than 3 mm. The adjacent segment degeneration was evaluated radiographically by comparing the preoperative and final followup radiographs.
The data were expressed as mean ± standard deviation. We determined the differences in the disc height between the postoperative and final followup radiographs and the differences in angulation and translation motions between the preoperative and final followup radiographs using the paired t test and Wilcoxon signed-ranks test, respectively. We used the chi square test and Fisher’s exact test to compare the presence or absence of adjacent segment instability between one-segment and two-segment instrumentation. We determined changes in back pain (VAS score) preoperatively and at final followup using the paired t test. Finally, we analyzed the correlation between back pain and loss of disc height and angular or translational motion at the instrumented level and at the adjacent segment. Statistical analyses were performed using SPSS® (Version 12.0; SPSS Inc, Chicago, IL).
Results
There was a decrease in the disc height from postoperatively (93% ± 27%) to final followup (82% ± 22%) (p = 0.001), especially at the L4–L5 segment with the discectomy (p = 0.004) (Table 1). However, there was no difference (p = 0.193) in the decrease in disc height at final followup between the discectomy group (79.0% ± 21.6%) and the nondiscectomy group (86.5% ± 21.6%). The angular and translational motions were improved at the L4–L5 segment in the nondiscectomy group (angulation: preoperative 12.0° ± 5.2° versus 5.5° ± 4.0° at final followup; p = 0.002; translation: preoperative 1.4 ± 1.5 mm versus 0.5 ± 0.6 mm at final followup; p = 0.053). However, the discectomy group showed less angulation (p = 0.090) and translation (p = 0.059) at the L5–S1 segment than at the L3–L4 and L4–L5 segments. Of the 67 segments, 19 (28%) had angular instability at the final followup, 13 (19%) of which had discectomy and six (9%) of which had not. Five (7%) segments had translational instability (Fig. 1), three (4%) of which had discectomy and two (3%) of which had not.
Table 1.
Radiographic changes in the instrumented segments
| Level | Change in disc height (%) | Instability | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Postoperative | Followup | p Value* | Angular (degrees) | p Value* | Translational (mm) | p Value* | |||
| Preoperative | Followup | Preoperative | Followup | ||||||
| Discectomy segment | |||||||||
| L1–L2 (n = 1) | 88 | 75 | 2 | 8 | 1 | 2 | |||
| L2–L3 (n = 0) | |||||||||
| L3–L4 (n = 7) | 88.35 ± 11.86 | 72.25 ± 11.76 | 0.056 | 9.57 ± 3.45 | 6.85 ± 2.41 | 0.159 | 0.71 ± 0.95 | 0.85 ± 0.69 | 0.736 |
| L4–L5 (n = 26) | 92.67 ± 39.63 | 77.75 ± 22.41 | 0.004* | 9.26 ± 5.45 | 7.38 ± 4.87 | 0.189 | 0.65 ± 1.23 | 0.96 ± 1.28 | 0.457† |
| L5–S1 (n = 9) | 93.57 ± 17.17 | 88.68 ± 25.33 | 0.461 | 12.22 ± 7.84 | 7.66 ± 6.02 | 0.090 | 0.66 ± 0.70 | 0.11 ± 0.33 | 0.059† |
| Subtotal (n = 43) | 92.05 ± 31.85 | 79.08 ± 21.68 | 0.001 | 9.77 ± 5.93 | 7.37 ± 4.68 | 0.025 | 0.67 ± 1.06 | 0.79 ± 1.10 | 0.585 |
| Nondiscectomy segment | |||||||||
| L1–L2 (n = 0) | |||||||||
| L2–L3 (n = 1) | 92.6 | 84.7 | 5 | 5 | 2 | 3 | |||
| L3–L4 (n = 5) | 99.20 ± 7.40 | 89.64 ± 23.60 | 0.397 | 12.40 ± 7.56 | 7.80 ± 4.14 | 0.299 | 0.20 ± 0.44 | 1.20 ± 1.95 | 0.178 |
| L4–L5 (n = 13) | 92.65 ± 15.60 | 84.90 ± 26.30 | 0.232 | 12.08 ± 5.27 | 5.53 ± 4.09 | 0.002 | 1.46 ± 1.50 | 0.53 ± 0.66 | 0.053 |
| L5–S1 (n = 5) | 87.20 ± 11.89 | 88.38 ± 4.77 | 0.839 | 4.00 ± 3.46 | 6.60 ± 4.66 | 0.320 | 0.20 ± 0.44 | 0.40 ± 0.89 | 0.374 |
| Subtotal (n = 24) | 92.88 ± 13.30 | 86.58 ± 21.60 | 0.132 | 10.17 ± 6.25 | 6.20 ± 4.05 | 0.012 | 0.95 ± 1.30 | 0.75 ± 1.11 | 0.640† |
| Total (n = 67) | 92.35 ± 26.60 | 81.78 ± 21.79 | 0.001 | 9.91 ± 6.00 | 6.96 ± 4.47 | 0.001 | 0.78 ± 1.15 | 0.79 ± 1.12 | 0.946† |
Values are expressed as mean ± standard deviation; *paired t test; †Wilcoxon signed-ranks test.
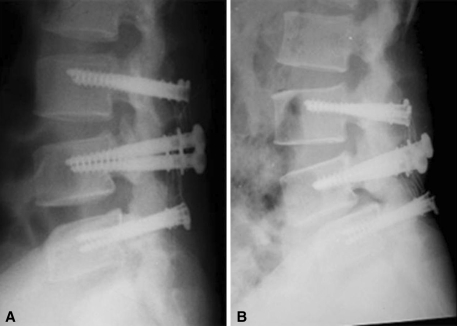
Fig. 1A–B.
In a comparison of (A) a postoperative radiograph with (B) one taken 10 years 7 months after the index operation, a loss of disc height and spondylolisthesis of L4 on L5 can be seen.
Of the 43 patients, 18 (41.8%) had instability at the adjacent superior segment: angular instability in 11 patients, translational instability in four patients, and both in three patients (Fig. 2). The adjacent segment instability at the lower segment revealed 13 patients with angular instability. We observed no difference in adjacent segment instability between one-segment and two-segment instrumentation (Table 2).
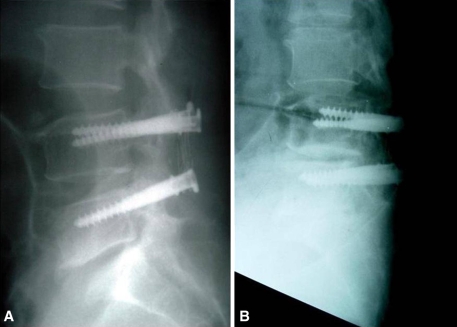
Fig. 2A–B.
In a comparison of (A) a postoperative radiograph with (B) a flexion stress radiograph taken 10 years 3 months after the index operation, translational instability is evident at the adjacent segment to L4.
Table 2.
Adjacent segment instability between one-segment and two-segment instrumentation
| Location | Angular instability (degrees) | p Value | Translational instability (mm) | p Value | ||
|---|---|---|---|---|---|---|
| One segment | Two segment | One segment | Two segment | |||
| Above segment | 7/20 | 7/22 | 0.827* | 3/20 | 4/22 | 1.000† |
| Below segment | 9/17 | 4/12 | 0.296* | 0/17 | 0/12 | |
Values are expressed as the number of instability segments over the total number of segments for the specific location and instrumentation; *chi square test; †Fisher’s exact test.
Low back pain (VAS score) decreased (p = 0.001) from 9.3 ± 0.8 preoperatively to 4.4 ± 1.9 at final followup. Back pain did not correlate with loss of disc height (p = 0.831), angular difference (p = 0.800), or translational difference (p = 0.301) at instrumented segments and did not correlate with adjacent segment angular (p = 0.503) or translational instability (p = 0.698). At the final followup, 10 patients (23%) had no pain, 20 (46%) had occasional mild pain, eight (18%) had moderate pain that could be ameliorated with nonnarcotic NSAIDs, four (9%) had pain affecting daily routines and requiring analgesics stronger than nonnarcotic NSAIDs, and one (2%) had severe pain substantially limiting her daily life and requiring constant analgesics.
There were no instrumentation failures (eg, Graf ligament dislodgement or rupture or screw loosening). In two patients, we converted the reconstruction to fusion. One patient had spinal stenosis and segmental instability at instrumented segments 14 years after the index operation (Fig. 3), and another patient had adjacent segment degeneration and kyphosis 11 years 5 months after the index operation.
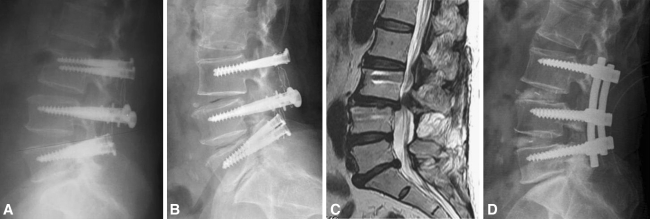
Fig. 3A–D.
For a 59-year-old female patient, a comparison of (A) a postoperative radiograph with (B) a lateral radiograph taken 14 years after the index operation shows retrolisthesis at L3–L4 and loss of disc height at L3–L5. (C) MRI reveals canal stenosis at L3–L4. (D) The patient was treated with additional decompression and conversion to fusion.
Discussion
The Graf ligament was developed as a nonfusion alternative to spinal arthrodesis based on the concept of flexible stabilization. However, the long-term outcomes of this device are controversial [17, 26]. We therefore asked whether (1) the Graf ligament maintained lumbar stability as indicated by loss of disc height and angular and translational motions at the instrumented segment after a minimum 8 years’ followup, (2) one-segment and two-segment instrumentation differed in the likelihood of maintaining stability and preventing degeneration at the adjacent segment, and (3) improvement of back pain correlated with lumbar stability and adjacent disc degeneration.
This study had limitations. First, as we used differing indications for the Graf ligament and spinal fusion, we cannot directly compare the patients. Second, we had no control group of patients treated by other methods for comparison to our patient cohort. Third, we did not preoperatively assess the condition of the adjacent segment. Therefore we have no comparison to the final condition.
The Graf ligament stabilizes the unstable segment through coaptation of bilateral facet joints. One biomechanical study [32] reported the Graf ligament reduces range of motion and flexibility values in some loading conditions. However, this experiment assessed only the immediate stabilization characteristics of this implant system in cadaveric material under limited loading conditions. Further research should address the fatigue characteristics of the ligament and whether they could influence the ability of the device to stabilize the joints. Kanayama et al. [17] suggested the Graf ligament has the potential to control flexion instability. However, we found 19 of the 67 instrumented segments (28%) had angular instability at the instrumented segments, although angular stability was improved at a minimum of 8 years’ followup in comparison to the preoperative status.
It is unclear if adjacent segment degeneration is a continuing degenerative process or a late complication of fusion. Solid fusion alters the biomechanics at the adjacent segment, resulting in increased mechanical demands. Increased biomechanical forces, mobility, and intradiscal pressure in the adjacent segments after fusion have been suggested to accelerate pathologic changes [5, 13, 19–21] (Table 3). Lehmann et al. [21] reported spinal stenosis was detected in the segment immediately cephalad to the fused segment in 30% of patients, instability of the adjacent segments was detected in 45%, and surgical treatment was required in 4.5%. The Graf ligament originally was developed to decrease the degenerative changes in the adjacent segments compared with rigid instrumentation. Kanayama et al. [17] reported adjacent segment morbidity in the Graf ligament compared with lumbar posterolateral fusion at minimum 5-year followup. There was a tendency with posterolateral fusion for a higher rate of adjacent disc deterioration compared with the Graf ligament. Onda et al. [26] reported a modest and gradual degeneration of adjacent segments after implantation of the Graf ligament. They could not exclude the possibility that the involved procedure may not prevent adjacent segment-related disease after 10 years because the Graf ligament is believed eventually to create rigid fixation. With a minimum 8-year followup we found adjacent upper segment degeneration in 14 segments (32%) with angular instability and seven segments (16%) with translational instability and adjacent lower segment degeneration in 13 segments (30%) with angular instability. The findings suggest the Graf ligament system also can accelerate the degenerative changes in the adjacent segments. We speculate this is because adjacent segments would alter load transmission during mid- to long-term followup after the Graf ligament.
Table 3.
Summary of articles reporting incidence data for adjacent segment disease
| Study | Number of patients | Incidence (%) | Average followup (years) | Type of surgery |
|---|---|---|---|---|
| Lehmann et al. [21] (1987) | 33 | 45% segmental instability, 30% stenosis | 33 | PF |
| Kumar et al. [19] (2001) | 83 | 36.1% listhesis/stenosis/loss of disc height | 5 | PLF + screw-rod + PLIF in 30 patients |
| Ghiselli et al. [13] (2004) | 215 | 27.4% symptomatic | 6.7 | PLF; 51% instrumented |
| Lai et al. [20] (2004) | 101 | 22.7% instability | 6.5 | PLF−screw-rod |
| Cheh et al. [5] (2007) | 188 | 42.6% listhesis/segmental kyphosis/disc collapse | 7.8 | PF + screw-rod |
| Kanayama et al. [17] (2001) | 18 | 18% loss of disc height/listhesis/spur vacuum | 6.2 | Graf ligament |
| Schaeren et al. [29] (2008) | 19 | 47% instability/loss of disc height | 4.3 | Dynesys instrument |
| Current study | 43 | 41.8% instability | 10.3 | Graf ligament |
PF = posterior fusion; PLF = posterolateral fusion, PLIF = posterior lumbar interbody fusion.
Although there was some enthusiasm over the early clinical results obtained using the Graf ligament, the associated mid- to long-term results are controversial. In one study of 83 patients who had Graf ligament or posterolateral lumbar fusion, the Graf ligament was associated with a worse outcome and a higher revision rate than the posterolateral lumbar fusion [16]. Seven-year followup results [11] showed the mean Oswestry Disability Score had improved from a mean of 59% preoperatively to 38% after 7 years. In 31 of 40 patients, 23% were not using analgesics, 54% occasionally used analgesics, and 13% used analgesics daily. Gardner and Pande suggested beneficial effects of the Graf ligament were sustained at a mean of 7 years despite the presence of an established degenerative process [11]. We found a mean improvement of pain (VAS score) from 9 ± 0.8 preoperatively to 4 ± 2 at a minimum 8 years’ followup. However, four patients’ (9%) daily routines were impeded and they required analgesics stronger than nonnarcotic NSAIDs; one patient (2%) had severe pain that limited her daily life substantially and analgesics were needed constantly. Two patients were treated with additional decompression and conversion to fusion.
Our data suggest the mechanical effects of the Graf ligament could be altered by degeneration of the disc and facet joints at instrumented segments, and adjacent segment disease could occur owing to abnormal load transmission.
Acknowledgments
We thank Hee-Young Shin, MD, PhD, from the Department of Biomedical Science, Chonnam National University Medical School, for statistical analyses of the data.
Footnotes
Each author certifies that he has no commercial association (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Agazzi S, Reverdin A, May D. Posterior lumbar interbody fusion with cages: an independent review of 71 cases. J Neurosurg. 1999;91:186–192. [DOI] [PubMed]
- 2.Ariga K, Miyamoto S, Nakase T, Okuda S, Meng W, Yonenobu K, Yoshikawa H. The relationship between apoptosis of endplate chondrocytes and aging and degeneration of the intervertebral disc. Spine. 2001;26:2414–2420. [DOI] [PubMed]
- 3.Boos N, Webb JK. Pedicle screw fixation in spinal disorders: a European view. Eur Spine J. 1997;6:2–18. [DOI] [PMC free article] [PubMed]
- 4.Bridwell KH, Sedgewick TA, O’Brien MF, Lenke LG, Baldus C. The role of fusion and instrumentation in the treatment of degenerative spondylolisthesis with spinal stenosis. J Spinal Disord. 1993;6:461–472. [DOI] [PubMed]
- 5.Cheh G, Bridwell KH, Lenke LG, Buchowski JM, Daubs MD, Kim Y, Baldus C. Adjacent segment disease following lumbar/thoracolumbar fusion with pedicle screw instrumentation: a minimum 5-year follow-up. Spine. 2007;32:2253–2257. [DOI] [PubMed]
- 6.Chou WY, Hsu CJ, Chang WN, Wong CY. Adjacent segment degeneration after lumbar spinal posterolateral fusion with instrumentation in elderly patients. Arch Orthop Trauma Surg. 2002;122:39–43. [DOI] [PubMed]
- 7.Christie SD, Song JK, Fessler RG. Dynamic interspinous process technology. Spine. 2005;30:S73–S78. [DOI] [PubMed]
- 8.Eck JC, Humphreys SC, Hodges SD. Adjacent-segment degeneration after lumbar fusion: a review of clinical, biomechanical, and radiologic studies. Am J Orthop. 1999;28:336–340. [PubMed]
- 9.Etebar S, Cahill DW. Risk factors for adjacent-segment failure following lumbar fixation with rigid instrumentation for degenerative instability. J Neurosurg. 1999;90:163–169. [DOI] [PubMed]
- 10.Fritzell P, Hägg O, Wessberg P, Nordwall A. 2001 Volvo Award Winner in Clinical Studies. Lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine. 2001;26:2521–2534. [DOI] [PubMed]
- 11.Gardner A, Pande KC. Graf ligamentoplasty: a 7-year follow-up. Eur Spine J. 2002;11:S157–S163. [DOI] [PMC free article] [PubMed]
- 12.Getty CJ, Johnson JR, Kirwan EO, Sullivan MF. Partial undercutting facetectomy for bony entrapment of the lumbar nerve root. J Bone Joint Surg Br. 1981;63:330–335. [DOI] [PubMed]
- 13.Ghiselli G, Wang JC, Bhatia NN, Hsu WK, Dawson EG. Adjacent segment degeneration in the lumbar spine. J Bone Joint Surg Am. 2004;86:1497–1503. [DOI] [PubMed]
- 14.Gibson JN, Grant IC, Waddell G. The Cochrane review of surgery for lumbar disc prolapse and degenerative lumbar spondylosis. Spine. 1999;24:1820–1832. [DOI] [PubMed]
- 15.Graf H. Lumbar instability: surgical treatment without fusion. Rachis. 1992;412:123–137.
- 16.Hadlow SV, Fragan AB, Hiller TM, Fraser RD. The Graf ligamentoplasty procedure: comparison with posterolateral fusion in the management of low back pain. Spine. 1998;23:1172–1179. [DOI] [PubMed]
- 17.Kanayama M, Hashimoto T, Shigenobu K, Harada M, Oha F, Ohkoshi Y, Tada H, Yamamoto K, Yamane S. Adjacent-segment morbidity after Graf ligamentoplasty compared with posterolateral lumbar fusion. J Neurosurg. 2001;95:5–10. [DOI] [PubMed]
- 18.Kumar A, Beastall J, Hughes J, Karadimas EJ, Nicol M, Smith F, Wardlaw D. Disc changes in the bridged and adjacent segments after Dynesys dynamic stabilization system after two years. Spine. 2008;33:2909–2914. [DOI] [PubMed]
- 19.Kumar MN, Baklanov A, Chopin D. Correlation between sagittal plane changes and adjacent segment degeneration following lumbar spine fusion. Eur Spine J. 2001;10:314–319. [DOI] [PMC free article] [PubMed]
- 20.Lai PL, Chen LH, Niu CC, Fu TS, Chen WJ. Relation between laminectomy and development of adjacent segment instability after lumbar fusion with pedicle fixation. Spine. 2004;29:2527–2532; discussion 2532. [DOI] [PubMed]
- 21.Lehmann TR, Spratt KF, Tozzi JE, Weinstein JN, Reinarz SJ, El-Khoury GY, Colby H. Long-term follow-up of lower lumbar fusion patients. Spine. 1987;12:97–104. [DOI] [PubMed]
- 22.Markwalder TM, Wenger M. Adjacent-segment morbidity. J Neurosurg. 2002;96:139–140. [DOI] [PubMed]
- 23.McAfee PC. Interbody fusion cages in reconstructive operations on the spine. J Bone Joint Surg Am. 1999;81:859–880. [DOI] [PubMed]
- 24.Minns RJ, Walsh WK. Preliminary design and experimental studies of a novel soft implant for correcting sagittal plane instability in the lumbar spine. Spine. 1997;22:1819–1825. [DOI] [PubMed]
- 25.Mulholland RC, Sengupta DK. Rationale, principles and experimental evaluation of the concept of soft stabilization. Eur Spine J. 2002;11:S198–S205. [DOI] [PMC free article] [PubMed]
- 26.Onda A, Otani K, Konno S, Kikuchi S. Mid-term and long-term follow-up data after placement of the Graf stabilization system for lumbar degenerative disorders. J Neurosurg Spine. 2006;5:26–32. [DOI] [PubMed]
- 27.Papakostidis C, Kontakis G, Bhandari M, Giannoudis PV. Efficacy of autologous iliac crest bone graft and bone morphogenetic proteins for posterolateral fusion of lumbar spine: a meta-analysis of the results. Spine. 2008;33:E680–E692. [DOI] [PubMed]
- 28.Sanderson PL, Getty CJ. Long-term results of partial undercutting facetectomy for lumbar lateral recess stenosis. Spine. 1996;21:1352–1356. [DOI] [PubMed]
- 29.Schaeren S, Broger I, Jeanneret B. Minimum four-year follow-up of spinal stenosis with degenerative spondylolisthesis treated with decompression and dynamic stabilization. Spine. 2008;33:E636–E642. [DOI] [PubMed]
- 30.Sénégas J, Vital JM, Pointillart V, Mangione P. Long-term actuarial survivorship analysis of an interspinous stabilization system. Eur Spine J. 2007;16:1279–1287. [DOI] [PMC free article] [PubMed]
- 31.Sengupta DK, Mulholland RC. Fulcrum assisted soft stabilization system: a new concept in the surgical treatment of degenerative low back pain. Spine. 2005;30:1019–1029. [DOI] [PubMed]
- 32.Strauss PJ, Novotny JE, Wilder DG, Grobler LJ, Pope MH. Multidirectional stability of the Graf system. Spine. 1994;19:965–972. [DOI] [PubMed]
- 33.Thomsen K, Christensen FB, Eiskjaer SP, Hansen ES, Fruensgaard S, Bünger CE. 1997 Volvo Award Winner in Clinical Studies. The effect of pedicle screw instrumentation on functional outcome and fusion rates in posterolateral lumbar spinal fusion: a prospective, randomized clinical study. Spine. 1997;22:2813–2822. [DOI] [PubMed]
- 34.Zdeblick TA. A prospective, randomized study of lumbar fusion: preliminary results. Spine. 1993;18:983–991. [DOI] [PubMed]