Abstract
Gastroresistant microparticles for oral administration of hesperidin (Hd) were produced by spray-drying using cellulose acetate phthalate (CAP) as enteric polymer in different polymer/Hd weight ratio (1:1, 3:1, and 5:1), and a series of enhancers of the dissolution rate, such as sodium carboxymethylcellulose crosslinked (CMC), sodium dodecylbenzene sulfonate (SDBS), or Tween85. The raw materials and the microparticles were investigated by differential-scanning calorimetry, X-ray diffraction, infrared spectroscopy and imaged using scanning electron and fluorescence microscopy. In vitro dissolution tests were conducted using a pH-change method to investigate the influence of formulative parameters on the dissolution/release properties of the drug. CAP/Hd microparticles showed a good gastro-resistance but incomplete drug dissolution in the simulated intestinal fluid (SIF). The presence of the enhancers in the formulation produced well-formed microparticles with different size and morphology, containing the drug well coated by the polymer. All the enhancers were able to increase the dissolution rate of Hd in the simulated intestinal environment without altering CAP ability to protect Hd in the acidic fluid. The spray-drying technique and process conditions selected were effective in microencapsulating and stabilizing the flavonoid giving satisfactory encapsulation efficiency, product yield, and microparticles morphology, and a complete drug release in the intestine.
Key words: enhancers of the dissolution rate, gastroresistant spray-dried microparticles, hesperidin, morphological and physicochemical characterization
INTRODUCTION
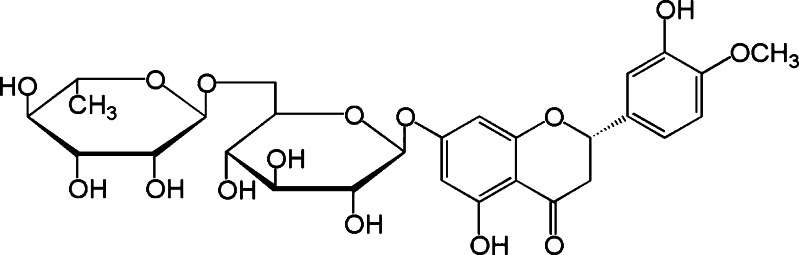
Hesperidin (Hd; Fig. 1) is the major flavanone glycoside in sweet orange and lemon obtained as an abundant by-product of Citrus cultivation (1,2). It has been implicated in the inhibition of enzymes involved in several diseases: phospholipase A2, lipoxygenase, HMG-CoA reductase and cyclooxygenase (3,4). As a result, hesperidin has antioxidant, anti-inflammatory, hypolipidemic, vasoprotective, and cholesterol-lowering properties (5). In the last years, encouraging results on its anticancer activity were observed. A marketed formulation of hesperidin and diosmin, just used for the treatment of chronic venous insufficiency, was shown to be active in inhibiting chemical carcinogenesis of the bladder urinary in mice (6) and neoplastic transformation induced by 3-methylcolanthrene in murine fibroblasts (7). Hesperidin was also found to posses an antimutagenic effect on Salmonella typhimurium against benz-(α)-pyrene-induced mutation (8,9), and on rats against methyl-N-amylnitrosamine-induced tumorigenesis (10). Its metabolite hesperetin as well as naringenin showed in vitro antiproliferative activity on highly metastatic murine B16-F10 melanoma cells. Also, hesperitin or naringenin, orally administered in C57BL6/N mice, induced antimetastatic activity by reduction of cell proliferation (11,12). About the mechanisms involved in flavonoid-mediated antineoplastic activity, the drugs seem to be activators of enzymes such as transglutaminase (TGAse) involved in cell differentiation (13,14,15).
Fig. 1.
Hesperidin chemical structure
The well-documented effects of hesperidin are unfortunately more evident in vitro than in vivo, due to the instability to the gastric pH in which the drug undergoes hydrolysis into aglycone hesperetin and enzymatic degradation. Moreover, probably due to its crystalline state (16), this flavonoid is slightly soluble in water, a characteristic which leads to a very low dissolution rate and an irregular absorption of the drug from oral solid dosage forms in the gastrointestinal tract. In addition, it is recovered in plasma, urine, and bile as glucoronides and sulfoglucoronides and the cumulative recovery indicated low bioavailability (<25%) (2). In contrast to the numerous publications focused on the pharmacological effect of flavonoids, rather no attention has been addressed yet to their formulation in order to have appropriate bioavailability.
Thus, the aim of the present work was to produce Hd gastroresistant microparticles with improved performance for oral administration by spray-drying. Since the design and development of gastroresistant microparticles containing a low-solubility drug such as Hd requires a compromise between the enhancement of the dissolution rate in the intestinal fluid and protection in the gastric environment, a selected coating of gastroresistant polymer, cellulose acetate phthalate (CAP), and a series of enhancers of the dissolution rate were used in the formulation. CAP has just been employed for microencapsulation of other flavonoids (17,18) and drugs such as NSAID (19) and for preventing tablets disintegration and drug release into the stomach (20). Spray-drying is a technique to obtain microparticulate systems by a one-step process transforming liquid feed into a dried particulate form (18), which process conditions strongly affect properties of the resultant dried microparticles such as size, morphology, shape. Moreover, this technique offers other advantages such as a low amount of residual solvent in the final product.
The influence of formulative parameters on drug dissolution/release properties as well as on yield, morphology, and thermal behavior of the produced gastroresistant microparticles were studied.
MATERIALS AND METHODS
Materials
Hesperidin (Hd) was supplied by Sigma Aldrich (Milan, Italy); Cellulose acetate phthalate (CAP) from Eastman® Kodak (Kingsport, Tennessee, United States); Vivasol® Croscarmellose sodium (carboxymethylcellulose crosslinked, CMC) from J. Rettenmaier & Söhne (Rosenberg, Germany). The selected surfactants were sodium dodecylbenzene sulfonate—(SDBS) from Sigma Aldrich (Milan, Italy) and Tween 85 from A.C.E.F. s.p.a. (Piacenza, Italy). All other chemicals used were of reagent grade.
Hesperidin Solubility
The solubility of Hesperidin was previously determined in water (57.0 mg/l) and in simulated biological fluids (prepared according to USP 31 without enzymes), gastric fluid (GF) pH 1.2 (49.1 mg/l) and intestinal fluid (IF) pH 7.5 (68.0 mg/l), as follows: an excess amount of Hd raw was introduced into glass vials containing 50 ml of solvents; the samples were shaken and then were stored at room temperature (25°C). After 3 days, the liquid phases were centrifuged for 15 min at 3,000 rpm, the supernatants were filtered with 0.45 μm filters and the concentration of dissolved Hd was determined spectrophotometrically (UV/Vis spectrometer Lambda 25, Perkin-Elmer Instruments, MA, USA) at a wavelength of 310 nm. The solubility measurements were made in triplicate. Solubility data were confirmed by HPLC using the method reported below (“Drug-Content Evaluation” section).
Microparticles Preparation
Gastroresistant CAP/Hd Microparticles
The composition of the different batches of spray-dried microparticles is reported in Table I. In preliminary experiments, the spray-drying of organic feed solutions (3:1 v/v acetone:ethanol) of CAP (1 and 2%) and Hd (3:1, 1:1, and 5:1 polymer/drug ratio) gave no adequately coated microparticles; encapsulation efficiency values were below 40%, and FM images showed a high amount of Hd uncoated by the polymer (data not shown). For this reason, in the first set of experiments, CAP (1% and 2% w/v) was dissolved in aqueous buffer at pH 7.5 (simulated intestinal fluid, IF, pH 7.5, according to USP 31 without enzymes), then variable amount of Hd (polymer:drug weight ratios 3:1, 1:1, and 5:1) were suspended in the polymeric solution under magnetic stirring. CAP/Hd (batches #1a, #1b, and #1c) microparticles were obtained by spray-drying of these feed solutions.
Table I.
Feed Composition, Drug Concentration, Enhancer Concentration (% w/v), CAP Concentration, % Yield of the Process, Theoretical Drug Content (TDC %), Actual Drug Content (ADC%) and Encapsulation Efficiency (EE %), d 50 (μm ± Standard Deviation) of all Microsystems Prepared
| Batches # | Feed composition | Drug concentration (% w/v) | Enhancer concentration (% w/v) | CAP concentration (% w/v) | Yield % | TDC % | ADC % | EE % | d50 μm (SD) |
|---|---|---|---|---|---|---|---|---|---|
| # 1a | CAP/Hd | 0.67 | – | 2.0 | 94.3 | 25.0 | 18.5 | 74.0 | 12.28 (0.07) |
| # 1b | CAP/Hd | 2.0 | – | 2.0 | 73.0 | 50.0 | 35.0 | 70.0 | 11.50 (0.12) |
| # 1c | CAP/Hd | 0.4 | – | 2.0 | 84.4 | 16.6 | 12.0 | 72.3 | 13.55 (0.10) |
| # 2 | CAP/Hd/CMC | 0.67 | 0.4 | 2.0 | 75.0 | 18.2 | 17.5 | 96.2 | 80.96 (2.40) |
| # 3 | CAP/Hd/SDBS | 0.67 | 0.5 | 2.0 | 93.0 | 21.0 | 17.0 | 80.9 | 43.18 (1.83) |
| # 4 | CAP/Hd/Tween | 0.67 | 0.5 | 2.0 | 76.0 | 21.0 | 18.9 | 90.0 | 42.86 (1.07) |
| # 5 | CAP blank | – | – | 2.0 | 100.0 | – | – | – | 3.90 (0.06) |
| # 6 | CAP/CMC | – | 0.4 | 2.0 | 90.0 | – | – | – | 35.86 (1.21) |
| # 7 | CAP/SDBS | – | 0.5 | 2.0 | 99.1 | – | – | – | 10.12 (0.64) |
| # 8 | CAP/Tween | – | 0.5 | 2.0 | 94.1 | – | – | – | 6.42 (0.51) |
| # 9 | Hd sd | 0.67 | – | – | 58.4 | 100.0 | 100.0 | – | 4.37 (0.12) |
| # 10 | Hd/CMC | 0.67 | 0.4 | – | 41.2 | 28.6 | 25.0 | 87.4 | 28.28 (0.20) |
| # 11 | Hd/SDBS | 0.67 | 0.5 | – | 88.0 | 57.1 | 29.7 | 52.0 | 19.96 (1.03) |
| # 12 | Hd/Tween | 0.67 | 0.5 | – | 65.2 | 57.1 | 28.0 | 49.0 | 21.76 (2.34) |
CAP cellulose acetate phthalate, CMC carboxymethylcellulose crosslinked, SDBS sodium dodecylbenzene sulfonate, Tween Tween 85, Hd Hesperidin, Hd sd, hesperidin spray-dried
Gastroresistant CAP/Hd/Enhancer Microparticles
In the second set of experiments, Hd was suspended in 2% w/v CAP aqueous buffer feed solution with 3:1 polymer/drug ratio in the presence of different enhancers of dissolution rate (CMC 0.4% w/v; SDBS or Tween 85 in 0.5% w/v; Table I) and spray-dried to obtain CAP/Hd/CMC (batch #2), CAP/Hd/SDBS (batch #3), CAP/Hd/Tween (batch #4) gastroresistant microparticles.
For the preparation of batch #2, Hd and CMC were previously blended (Hd/CMC weight ratio 1:2.5), using a Galena Top (Ataena, Tecno-Pro srl, Italy) mixer in the presence of micronizing spheres until uniformity, and the resultant mixture was suspended into the polymeric-CAP liquid feed.
For the preparation of both CAP/Hd/SDBS (batch #3) and CAP/Hd/Tween 85 (batch #4) microparticles, Hd was previously dampened with SDBS (0.5% w/v) or with Tween 85 (0.5% w/v), and then suspended into the polymeric-CAP liquid feed.
Controls
As control, CAP-free microparticles made by Hd and each of the enhancers (Hd/CMC batch #10, Hd/SDBS batch #11, Hd/Tween batch #12) and drug-free blank microparticles made by CAP and the enhancers (CAP/CMC Batch #6, CAP/ SDBS #7 and CAP/ Tween #8) were also prepared in the same experimental conditions by spray-drying.
The solvent, CAP concentration, and the polymer/drug weight ratio were kept constant for each formulation. Each liquid feed was prepared under continuous magnetic stirring and sonicated for 10 min before to be processed by spray-drying using 6–8 g as total amount of raw materials contained in 200 ml of total feed volume sprayed.
The spray-drying conditions were: a Buchi Mini Spray Dryer B-191 (Buchi Laboratoriums—Tecnik, Flawil, Switzerland); inlet temperature 125°C; outlet temperature 78–84°C; spray-flow feed rate 5 ml/min; nozzle diameter 0.5 mm; drying air flow 500 l/h, air pressure 6 atm, aspirator 100%.
Each preparation was carried out in triplicate. All the spray-dried microparticles were collected and stored under vacuum for 48 h at room temperature.
The yield of the process, the percentage of theoretic drug content, the percentage of actual drug content, encapsulation efficiency of gastroresistant and CAP-free microsystems and the mean size (d50) were determined (Table I).
Microparticle Characterization
Particle-Size Analyses
Particle-size analyses of commercially available Hd raw material and of all spray-dried microparticles prepared were carried out with a laser light scattering granulometer (Beckman Counter LS 230, Particle Volume Module Plus, U.K.). The drug and microparticles were suspended in acid water (pH 3.0); a few drops of each sample were poured into the small-volume cell to obtain an obscuration between 8% and 12%. Particle-size distributions were calculated by instrument software, using the Fraunhofer model. The analysis was made in triplicate. The results were expressed as the median diameter of the particles (d50).
Morphology
The morphology of the microsystems was assessed by scanning electron microscopy (SEM): the microparticles were coated with Au/Pd and then observed using a scanning electron microscope (Scanning Microscope JSM 6400 Jeol, Japan) at different extensions.
For the fluorescent microscopy assays (FM), samples were observed with a Zeiss Axiophot fluorescence microscope, with 40, 63, and 100 × 1.4 NA plan Apochromat oil immersion objectives (Carl Zeiss Vision, München-Hallbergmoos, Germany) using standard DAPI (4′,6-diamidino-2-phenylindole) optics that adsorb violet radiation (max 372 nm) and emit a blue fluorescence (max 456 nm).
Drug-Content Evaluation
The drug content in each formulation was assessed by UV and HPLC.
UV Method
Samples (40 mg) of each batch of microparticles were dissolved in aqueous buffer solution at pH 7.5, and the drug content was determined spectrophotometrically (UV/Vis spectrometer Lambda 25, Perkin-Elmer Instruments, MA, USA) at λ 310 nm (1 cm cell; Spectracomp 602, Advanced Products srl, Milan, Italy). Each analysis was made in triplicate and the results expressed as average value. Values were superimposable to those obtained by HPLC analyses.
HPLC Method
HPLC Apparatus (Agilent 1100 series system) equipped with a Model G-pump, a DAD G-1315 A detector set at λmax 284 nm loop 20 μl, and a 150 × 3.9 mm i.d. C-18 μ-Bondapack column. The eluent was MeOH/H2O (1:1 v/v), flow rate of 1.0 mL min−1. Linearity: reference standard solutions were prepared at three concentration levels (0.05–1.0 mg/mL) and were injected (20 μl) three times. The standard curve was analyzed using the linear least-squares regression equation derived from the peak area (regression equation y = 1798.3x − 54.938, R2 = 0.9996, where y is the peak area and x the concentration used). Specificity: the peaks associated with Hd were identified by retention times, UV and MS spectra compared with standard and confirmed by co-injections. Analysis of the microspheres: samples (15 mg) of three batches of microparticles were dissolved in 15 mL MeOH, sonicated for 5 min, centrifuged for 10 min at 300 rpm. Hd concentration was determined in the supernatant solutions using the same chromatographic conditions of standard Hd. Each analysis was made in triplicate and the results (ADC), expressed as average value, are reported in Table I.
The production yields were expressed as the weight percentage of the final product compared to the total amount of polymer and drug sprayed. The encapsulation efficiency of Hd present in all spray-dried powders, was calculated from the ratio of actual to theoretical drug content (Table I) in dry microspheres.
Differential Scanning Calorimetry (DSC)
Differential scanning calorimetry was performed on an indium-calibrated Mettler Toledo DSC 822e (Mettler Toledo, OH, USA) in order to study the thermal behavior on samples of raw materials, drug-free-, CAP-free-, and drug-loaded microparticles. DSC thermograms were recorded by placing accurately weighed quantities (8–10 mg weighed with a microbalance MTS Mettler Toledo, OH, USA) of each sample in a 40-μl aluminum pan which was sealed and pierced. The samples were exposed to two thermal cycles. For the dehydration cycle, the samples were heated up to 130°C with a heating rate of 20°C/min and the temperature was maintained at 130°C for 15 min in order to remove the residual solvent. Afterwards, the samples were cooled at 25°C and heated up to 350°C with a heating rate of 10°/min. From this second thermal cycle, the glass transition temperature (Tg), the melting temperature (Tm), and the heat of fusion (ΔHm) were measured for all samples and compared with each other. The analyses were carried out in triplicate.
Fourier-Transform Infrared (FTIR) Spectroscopy
Fourier-transform infrared spectra were obtained using a Jasco FT-300, (Tokyo, Japan) Fourier-transform IR (FTIR) spectrometer. Samples of each batch of microparticles and the raw materials were analyzed as KBr discs in the spectral region 650–4,000 cm−1.
X-Ray Diffraction
X-ray diffraction patterns on powder were recorded on a Rigaku MiniFlex diffractometer (Tokyo, J) using a CuKα radiation (30 kV, 15 mA) at a scanning speed of 0.05/2 s with a scanning range (2θ°) from 2 to 55.
Long-Term Stability Studies
The stability test was performed according with conventional method (long-term studies—12 months) reported by guide lines ICH (International Conference on Harmonization of Technical Requirements of Pharmaceutical for Human Use). The formulations were stored at 25°C ± 2°C /60%RH ± 5%RH (20) in a climatic chamber (Climatic and Thermostatic Chamber, Mod.CCP37, AMT srl, Milan, Italy); HPLC and DSC analysis of each batch were carried out for 12 months, including four time points (0, 3, 6, and 12 months). Chromatographic peaks were identified on the basis of the retention times and confirmed by co-injections. No peaks corresponding to degradation products of the drug were observed.
In Vitro Dissolution/Release Tests
In vitro dissolution/release tests of Hd from microparticles were carried out under sink conditions using a SOTAX AT Smart Apparatus (Basel, CH) on line with a spectrophotometer (UV/Vis spectrometer Lambda 25, Perkin-Elmer Instruments, MA, USA) and USP 31 dissolution test apparatus n.2: paddle, 100 rpm at 37°C. The pH-change method (USP 31 drug-release test, method A for Enteric-coated Articles) was used, 750 ml of HCl 0.1 N (pH 1.00) from 0 to 2 h, then the addition of 250 ml of 0.2 M tribasic sodium phosphate solution to give a final pH of 6.8 in a total volume of 1,000 ml. All the dissolution/release tests were made in triplicate; only the mean values are reported in graph (standard deviations <5%).
Samples of spray-dried microparticles corresponding to about 15 mg of hesperidin were analyzed spectrophotometrically at λ 310.
RESULTS AND DISCUSSIONS
In a preliminary set of experiments, Hd was spray-dried using CAP (1 and 2% w/v) as coating polymer in different polymer/drug ratios (1:1, 3:1, 5:1) in order to achieve gastroresistant microsystems able to avoid the flavonoid exposure to harsh gastric conditions. Only using 2% polymeric solutions, satisfactory yields of the process and drug encapsulation efficiency were obtained.
Gastroresistant CAP/Hd Microparticles
Microparticles Production
The actual and theoretical drug contents of each batch, production yields, and encapsulation efficiency are reported in Table I. The results showed the encapsulation efficiency ranging from 70 to 74% for all batches #1a, 1b, and 1c. The highest actual drug content and encapsulation efficiency was observed for batch #1a (polymer/drug ratio 3:1). As reported in a previous work (18), very slightly soluble drugs such as flavonoids (2), due to the phase separation, can undergo to deposition in the chamber during the spray-drying process giving a reduction of the encapsulation efficiency. CAP seems be able to limit this loss process giving acceptable results.
The product yields were higher for batch #1a (94.3%) than #1b (73.0%) and #1c (84.4%) and satisfactory in view of the low-quantity materials used for the preparation of each batch, minding the usual loss of the smallest and lightest particles through the exhaust devices of the spray dryer apparatus.
Morphological Characterization
Laser-scattering particle-size analysis showed that CAP/Hd gastroresistant microparticles (batches #1a, #1b, and #1c) obtained spray-drying CAP 2% w/v and Hd in different weight ratios (1:1, 3:1, 5:1) have a very narrow size distribution with a mean diameter in the range 11.50–13.55 μm (Tab. I).
These gastroresistant microsystems as well as raw materials (neat Hd, CAP), spray-dried Hd, and CAP microparticles were analyzed by FM (fluorescence microscopy) and SEM (scanning electron microscopy; Figs. 2, 3, and 4). Some morphological, e.g. diameter, shape of particles and surface characteristics were observed.
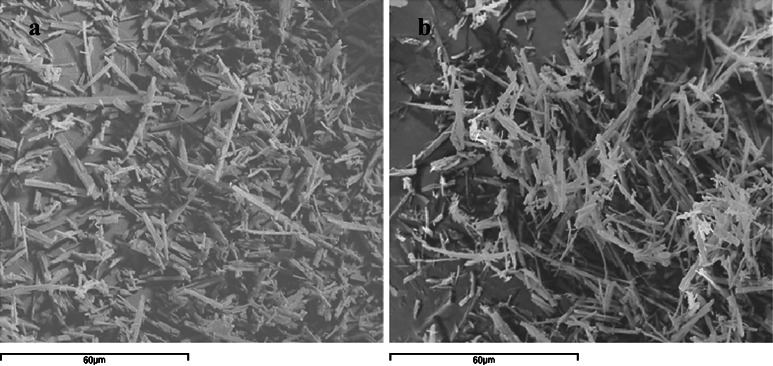
Fig. 2.
Scanning electron microscopy micrographs of Hd raw material (a, ×1,000) and Hd spray-dried (batch #9, b, ×1,000)
Fig. 3.
Scanning electron microscopy micrographs of the CAP raw material (a ×500) and CAP spray-dried (batch #5, b, ×3,000)
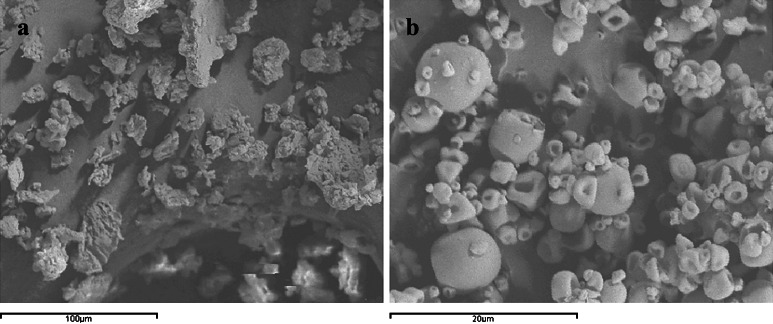
Fig. 4.
Scanning electron microscopy micrograph (×2,000) of batch #1a (25% hesperidin-loaded CAP microparticles)
Raw Materials
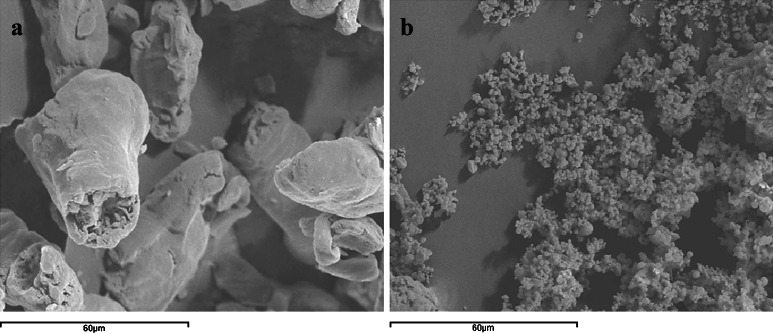
Hd is commercially available in a crystalline state with a needle shape (d50 10.21 ± 1.01) characterized by a very slight water solubility (Fig. 2a). Hd processed by spray-drying (Fig. 2b) and analyzed by SEM does not show changes in its solid crystalline state but only a mean diameter reduction (d50 4.37 ± 0.12) with respect to the raw material was observed (Fig. 2a).
The SEM pictures of CAP raw material show the presence of flakes (Fig. 3a) while spray-dried CAP showed microparticles with spherical trend, some wrinkled and with a variable particle size however lower than 10 μm (Fig. 3b).
Batches #1a, #1b, and 1#c
SEM and FM micrographs of batch #1a showed particles which are not regular in the shape but are almost spherical. Most of the particles are well formed and separated and some are partially collapsed (Fig. 4). Few uncoated flavonoid crystals were observed (Fig. 4). The particle size is about 12 μm, as confirmed by laser-scattering analysis. Similar results were obtained for batches #1b and #1c (data not shown).
Thermal, FTIR, and X-Ray Analyses
Raw Materials
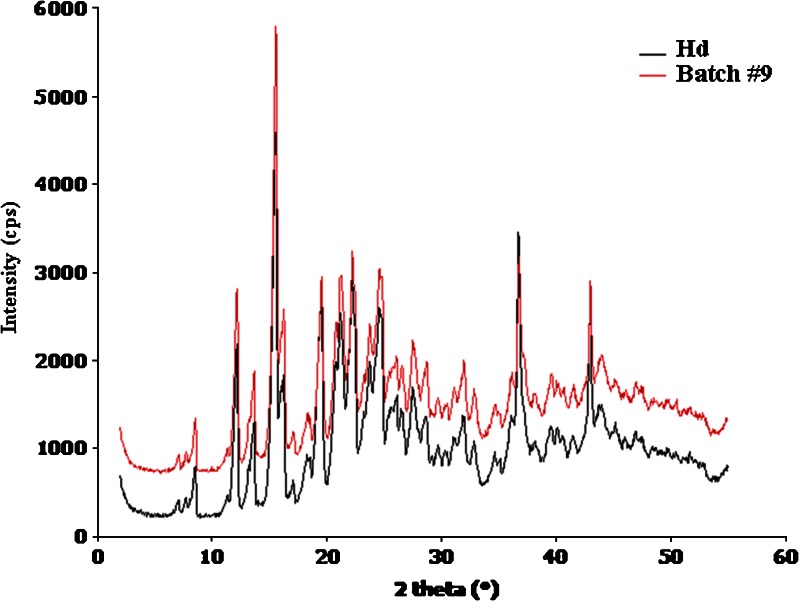
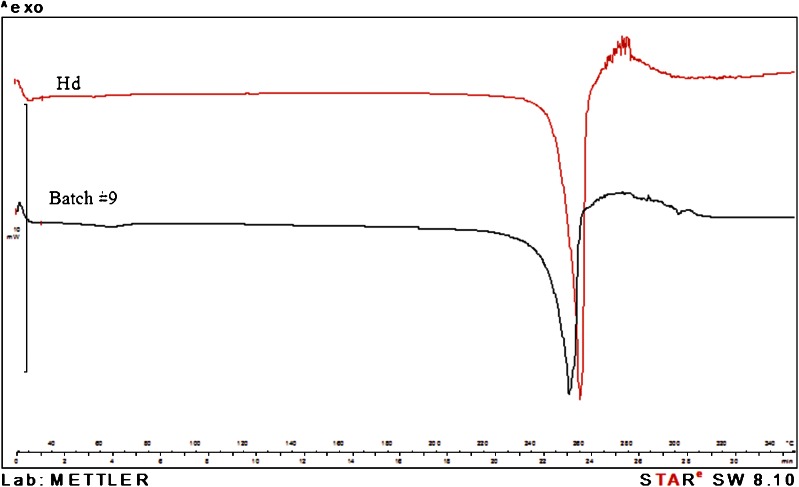
The X-ray diffraction and DSC analyses indicated the same crystallinity grade for both neat commercial Hd and spray-dried Hd showing superimposable diffraction (Fig. 5) and DSC (Fig. 6) profiles. However, in the DSC curves, the melting point of spray-dried Hd showed a small shift to a lower value (Tm 255.29°C) and a half value of heat of fusion (ΔHm) by comparison with neat Hd (Tm 259.17°C). This shift and the lower melting peak intensity (Fig. 6) may be due to the size reduction of Hd crystals obtained by spray-drying
Fig. 5.
X-ray diffraction patterns on hesperidin (Hd) raw material and hesperidin spray-dried (batch #9)
Fig. 6.
Differential scanning calorimetry thermograms of hesperidin raw material (Hd) and hesperidin spray-dried (batch #9)
Batches #1a, #1b, and #1c
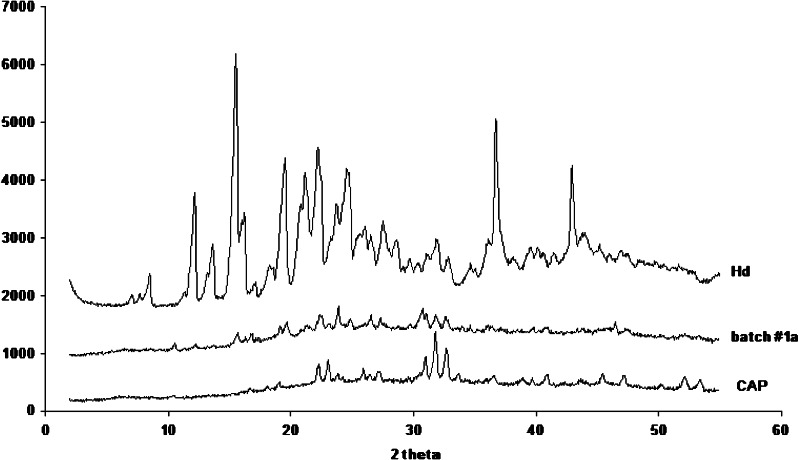
The PXRD analysis (Fig. 7) of batch #1a showed an high reduction in crystalline state of the drug. The diffraction peaks already present, with very low intensity with respect to the Hd raw material, are probably due to the few amount of uncoated drug in crystalline state, as confirmed by FM and SEM analyses. FTIR spectra did not show any new peaks with respect to raw materials. Similar results were obtained for batches #1b and #1c (data not shown).
Fig. 7.
X-ray diffraction patterns on batch #1a (CAP/Hd), Hd raw material and CAP raw material
In conclusion, results from SEM, FM, DSC, and PXRD analyses showed that spray-drying of the feeds containing Hd and CAP (batches #1a, #1b, and #1c) produced a high reduction in drug crystallinity and the encapsulation of the drug in an amorphous state.
Dissolution/Release Studies
The release profile of CAP/Hd gastroresistant microsystems obtained with 3:1, 1:1, and 5:1 polymer/flavonoid weight ratio are reported in Fig. 8 with respect to the profile of neat Hd.
Fig. 8.
Dissolution profile of CAP microparticles loaded with 50 (#1b), 25 (#1a), 16.6% (#1c) of hesperidin prepared by spray-drying buffer feed solutions (IF, pH 7.5) in comparison with the dissolution profile of hesperidin alone (Hd)
CAP seems to be able to protect Hd in the simulated gastric fluid, and the dissolution profiles of all CAP/Hd microparticles (batches #1a, #1b, and #1c), were almost superimposable at pH 1.0 (Fig. 8). The amount of Hd released/dissolved from these three batches in 2 h was about 3.0–4.0% , while about 20.0% of Hd alone was dissolved at the same time.
At pH 6.8, the drug-release profiles from all CAP/Hd microparticles (batches #1a, #1b, and #1c) were almost superimposable with the dissolution profile of neat Hd with slight difference due to microparticles composition. About 23.0% and 25.0% of drug was dissolved/released from batches #1b and #1a, respectively, and about 28.0% from #1c at 45 min after pH change, in comparison with about 25.0% of neat Hd that dissolved at the same time. Thus, CAP was able to protect Hd in gastric environment, but the release of flavonoid was incomplete in the intestinal fluid from all CAP/Hd gastroresistant formulations.
For this reason, considering that the best results in term of yield of the process, actual drug content and encapsulation efficiency (Table I) were obtained for batch #1a (CAP/Hd 3:1 weight ratio), in the second set of experiments, microparticles were formulated in the presence of enhancers of the dissolution rate.
Gastroresistant CAP/Hd/Enhancers Microparticles
The feed liquids were prepared by dispersing Hd in a 2% CAP buffer solution (3:1 polymer/drug weight ratio) and in the presence of two different surfactants such as SDBS or Tween 85 or a superdisintegrant such as CMC. These feeds were spray-dried to give batches #2 (CAP/Hd/CMC), #3 (CAP/Hd/SDBS), and #4 (CAP/Hd/Tween).
SDBS was selected as anionic surfactant, analogue to SLS—sodium lauryl sulfonate—yet used to enhance the dissolution rate of other drugs such as propanolol and theophilline (21); Tween 85 (an esterified and polyethoxylated derivative of sorbitan) as non-ionic surfactant used in pharmaceutical systems for its compatibility, stability, and minimal binding to proteins (22). Carboxymethylcellulose sodium (CMC) is an insoluble, hydrophilic, highly absorbent material reported to possess excellent water-swelling capacities with an enhancement of the drug dissolution rate due to its fibrous nature (23).
As control, CAP-free (batches Hd/CMC #10, Hd/SDBS #11 and Hd/Tween #12) and drug-free (batches CAP/CMC #6, CAP/SDBS #7 and CAP/Tween #8) microparticles were also prepared.
Microparticles Production
The actual and theoretical drug contents of each batch, production yields and encapsulation efficiency are reported in Table I. The production yields were higher for CAP/Hd/SDBS (batch #3, 93.0%) than for CAP/Hd/Tween (batch #4, 76.0%) or CAP/Hd/CMC (batch #2, 75.0%). Satisfactory results were obtained for CAP-free microsystems (batches #10, 11, and 12).
Microparticles Morphology
Laser-scattering particle-size analysis showed that Hd microparticles formulated with CAP in the presence of CMC or SDBS or Tween (batches #2, 3, and 4) had a much higher particle size (80.96, 42.86, and 43.18 μm, respectively) with respect to the microsystems prepared without enhancers (#1a, 12.28 ± 0.07 μm). These results suggest the presence of bigger microparticles or aggregates.
Batch #2
Neat CMC crosslinked showed an irregular and lamellar structure with dimensions of about 50 μm (±1.12; Fig. 9a), whereas spray-dried CMC crosslinked is characterized by spherical microparticles, some typically wrinkled, with a particle size lower than 5 μm (Fig. 9b).
Fig. 9.
Scanning electron microscopy micrographs of neat (a ×1,000) and spray-dried CMC crosslinked (9b, ×1,000)
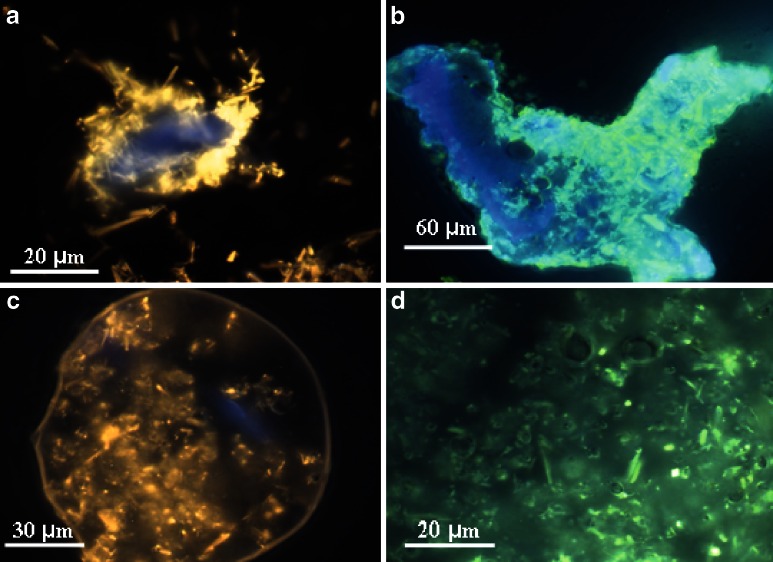
To produce batch #2, Hd was preliminarily loaded on CMC (CMC/Hd weight ratio 2.5:1) by mixing with micronizing spheres until uniformity and then this physical mixture was suspended into the CAP-liquid feed and spray-dried. FM analysis of CMC/Hd mixture showed that Hd is well loaded on CMC, almost keeping its crystalline state (Fig. 10a). As control, the mixture CMC/Hd (batch #10), previously prepared, was also spray-dried and morphologically characterized by FM. The results showed that the spray-drying process is able to reduce the crystal’s dimension and improve the loading of Hd on the polymer, also promoting the formation of some spherical microparticles (Fig. 10b).
Fig. 10.
Fluorescence microscopy images of the physical mixture of Hd/CMC (a, ×63); Hd/CMC spray-dried (batch #10, b, ×40); CAP/Hd/CMC microparticles (batch #2, c, ×40) and their inside (d, ×63)
The micrographs of batch #2 confirmed the presence of bigger microparticles (size about 80 μm) with respect to the microsystems prepared without enhancers, and showed a clear content of Hd crystals well coated by CAP (Fig. 10c and d).
Batches #3 and #4
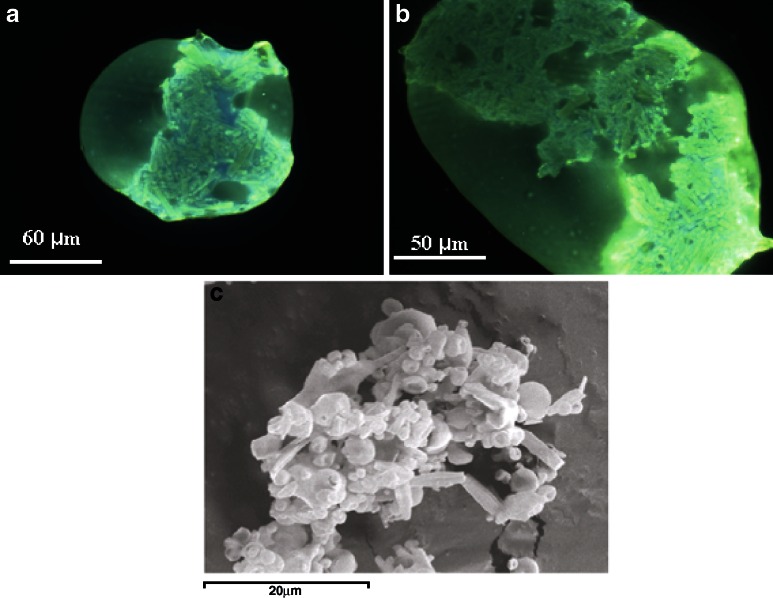
Observing the FM micrographs of batch #3 (CAP/Hd/SDBS), the absence of aggregates is evident; CAP is able to coat drug giving big and well separated microparticles (size about 40 μm) characterized by a spherical or stretched shape with a smooth and flexible surface and crystals of the drug inside (Fig. 11a and b).
Fig. 11.
Fluorescence microscopy images of CAP/Hd/SDBS (batch #3) microparticles at different magnification (a, ×40; b, ×100). Scanning electron microscopy (c, ×2,500) micrographs of CAP/Hd/Tween microsystems (batch #4)
The micrographs of batch #4 (CAP/Hd/Tween85) showed the presence of smaller microparticles (Fig. 11c) with dimensions of about 2 μm but with a dimensional distribution by laser-scattering analysis of 42.86 μm ± 1.07, due to the presence of a few amount of crystals embedded on the surface as well as aggregates. The laser diffraction technique, in fact, interprets the particles as spheres, causing a heterogeneous particle distribution for batches containing crystals. Some microparticles are pierced from the uncovered crystals and partially collapsed.
Thermal, FTIR, and X-Ray Analyses
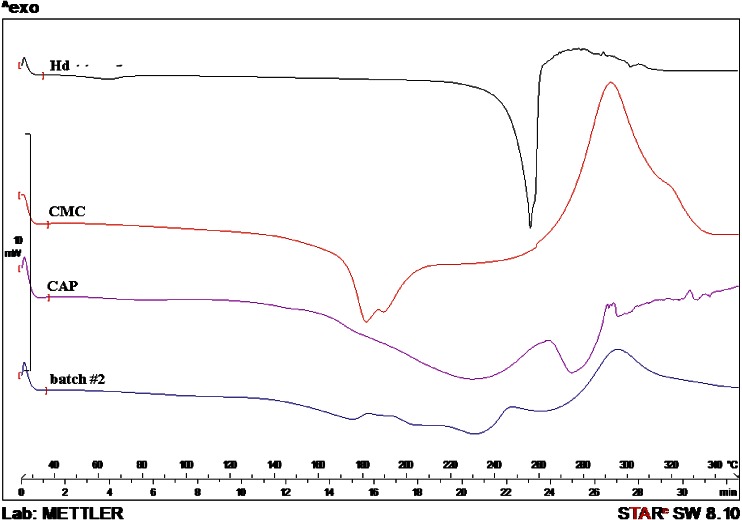
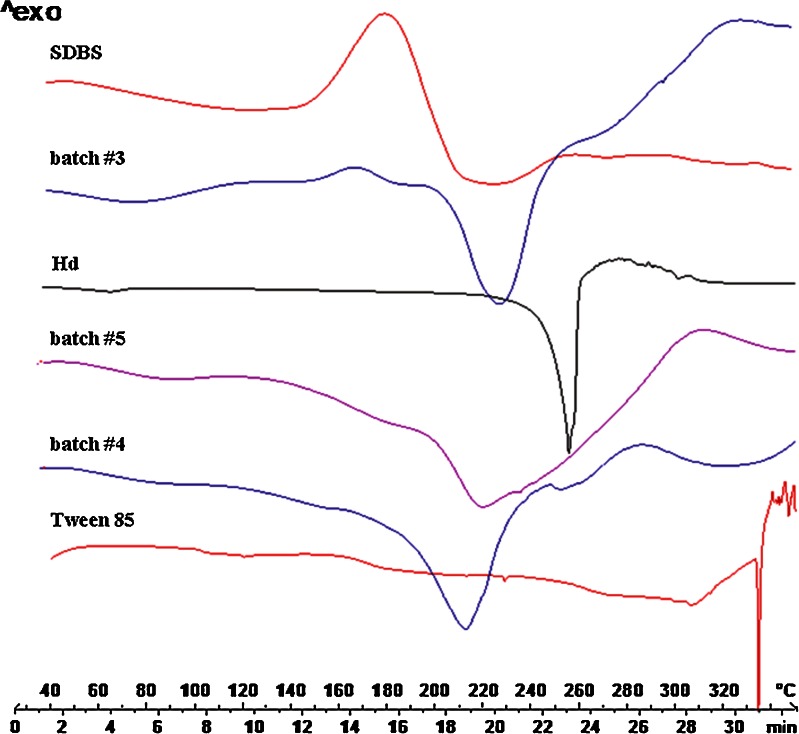
The absence of Hd melting point in the thermal profile of batches #2 and #3 suggested that the flavonoid was well encapsulated as previously shown by FM analysis. Only for batch #4, a small peak in correspondence of the Hd melt value, ascribable to few drug crystals not completely encapsulated, was observed. In addition, the DSC profiles of batches #3 and #4, showed the thermal trend of CAP shifted to lower temperature; the shift may be due to the physical interaction between the polymer and the surfactants (Figs. 12 and 13). These results do not exclude the presence of drug in residual crystalline state, because, sometimes, the DSC technique could not detect any residual crystalline structure, due to the ability of low-melting-point polymers to act as solvent for a drug (16). In fact, by increasing the temperature, CAP which melts at lower temperature than flavonoids starts to melt and dissolve drug crystals. For this reason, XRD analysis was necessary for further investigation.
Fig. 12.
Differential Scanning calorimetry thermograms of raw materials Hd, CMC, and CAP and CAP/Hd/CMC microparticles (batch #2)
Fig. 13.
Differential Scanning calorimetry thermograms of raw materials SDBS, Hd, and Tween 85, CAP blank (batch #5), CAP/Hd/SDBS (batch #3), and CAP/Hd/Tween (batch #4) microparticles
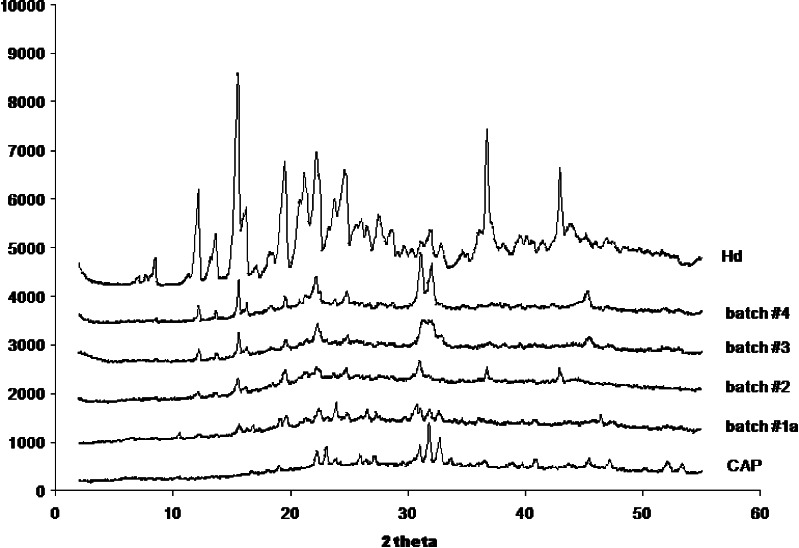
All PXRD profiles of batches #2, #3, and #4 showed an increase in crystallinity of the drug compared to PXRD profile of batch #1a (drug in the amorphous state), but however reduced with respect to crystalline Hd raw material (Fig. 14).
Fig. 14.
X-ray diffraction patterns on Hd raw material, CAP raw material, batches #1a (CAP/Hd), #2 (CAP/Hd/CMC), #3 (CAP/Hd/SDBS), and #4 (CAP/Hd/Tween 85)
Furthermore, PXRD of batch #2 showed the presence of other two peaks (2θ/degree 36.65 and 43) corresponding to part of CAP that probably was not involved in the Hd microspheres formation. This behavior suggests the hypothesis of a competition between CMC and CAP in the formation of Hd microsystems during the spray-drying process.
FTIR spectra of batch #2, Hd and raw polymers (CAP 1,735, 1,587, 1,249, and 1,126 cm−1; CMC 1,587, 1,386, and 1,070 cm−1), did not show any differences in the peak patterns confirming the absence of chemical interaction between the coating materials and the drug. Also, in the case of batches #3 and #4, FTIR analysis showed neither new bands ascribable to any chemical interaction. All these results showed that the major difference between the gastroresistant microsystems formulated with CMC or SDBS or Tween 85 resides on their morphology, particle size and on the solid state of Hd. All the above data indicated that spray-drying technique was not suitable to change solid crystalline state of neat Hd processed alone, but was able to reduce only the crystal size. Spray-drying of gastroresistant polymer (CAP) and Hd (batch #1a) produced small microparticles with the drug and the polymer in amorphous state, some aggregates and very few crystals of the drug not coated by the polymer. Spray-drying CAP and Hd in the presence of the enhancers produced well-formed microparticles with different sizes, bigger in the presence of CMC (80.96 ± 2.40 μm) than in the presence of SDBS (43.18 ± 1.83 μm), or Tween 85 (42.86 ± 1.07 μm; Table I), containing the drug keeping its crystalline state and generally well coated by the polymer. Gastroresistant microsystems formulated with SDBS showed flexible coating and absence of aggregates with respect to those formulated in the presence of Tween 85 in which aggregates and a few amount of crystals not coated and embedded on particles surface were observed. In addition, CMC seems to compete with CAP in the Hd microsystem formation.
Dissolution/Release Studies
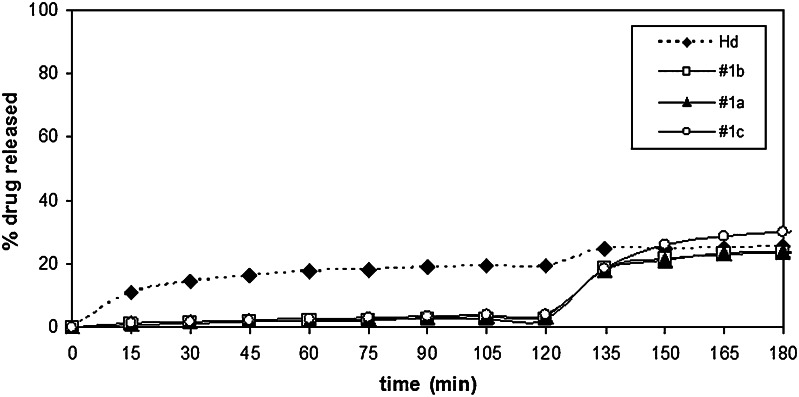
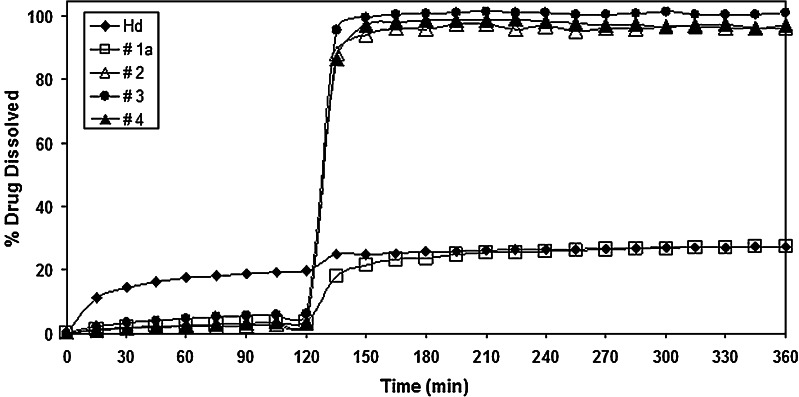
The dissolution/release profile of Hd from batches #2 (CAP/Hd/CMC), #3 (CAP/Hd/SDBS), and #4 (CAP/Hd/Tween) are reported in Fig. 13 in comparison with dissolution/release profiles of neat Hd and batch #1a (CAP/Hd) prepared without the enhancers.
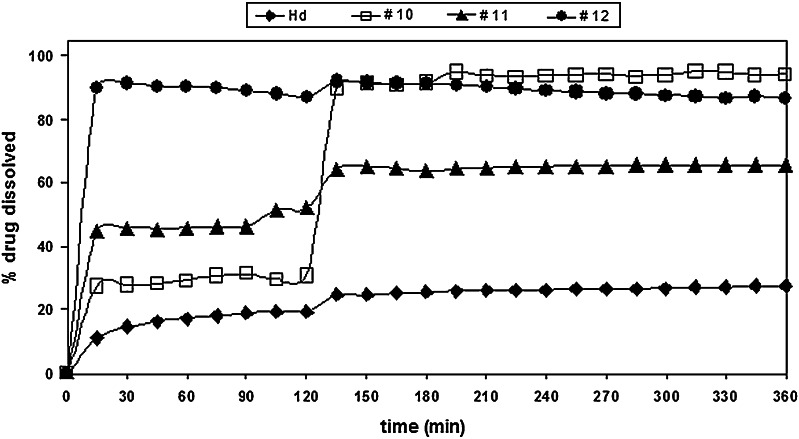
The dissolution profiles of batches #1a, #2, and #4 were almost superimposable at pH 1.0 (Fig. 15); in fact, the amount of Hd released/dissolved from these three batches in 2 h, was about 3.0%. At the same time, about 6.0% of Hd was released/dissolved from CAP/Hd/SDBS (batch #3) microparticles, in comparison with about 20.0% of neat Hd. As expected, a very high amount of flavonoids was released from the non-gastroresistant microparticles (batches #10, #11, and #12) into the gastric fluid (Fig. 16). In particular, the highest Hd release from CAP-free microparticles was observed in the presence of Tween (batch #12, 87.0%) with respect to batches #11 (Hd/SDBS, 46.0%) and #10 (Hd/CMC, 31.0%). Also, after pH change, the release of Hd increased from all these three non-gastroresistant batches with respect to neat Hd, due to the action of enhancers (Fig. 16).
Fig. 15.
Release profile of all CAP/Hd gastroresistant microparticles (batches #1a, #2, #3, #4) in comparison with Hd raw (Hd)
Fig. 16.
Release profile of non-gastroresistant (CAP-free) microparticles (batches #10, #11, #12) in comparison with Hd raw (Hd)
Furthermore, after pH change, an improvement of Hd dissolution rate was observed from gastroresistant microparticles formulated with all different enhancers of the dissolution rate (Fig. 15). At pH 6.8, while dissolution release from batch #1a was incomplete, about 100% of Hd dissolved/released from the microparticles formulated in the presence of CMC (batch #2), Tween 85 (batch #4) and SDBS (batch #3) in 45 min after pH change. This behavior may be explained by an increase of the drug–water interaction due to the presence of the enhancers of the dissolution rate that improved wettability and solubility of Hd. Moreover, the enhancement of the drug dissolution rate in IF was higher for all gastroresistant microparticles than that observed for microsystems prepared without CAP (batches #10, #11, and #12; 61.0–91.0%). This result may be due to the action of the enhancers of the dissolution rate of CAP itself.
These results indicated that CAP was able to protect Hd in gastric environment for all gastroresistant microparticles prepared, with slight differences due to microsystem composition also in the presence of the enhancers. These excipients were able to increase the dissolution rate of Hd in the simulated intestinal environment without altering CAP ability to protect Hd in the acidic fluid.
Long-Term Stability Studies
To examine the effect of the microencapsulation process on the stability of Hd, long-term studies were performed on all microsystems produced. The formulations were analyzed for hesperidin content over 12 months, including four time points (0, 3, 6, and 12 months) by HPLC method. No decrease of Hd content as well as no drug degradation product were recorded.
CONCLUSION
CAP may be used as polymeric carrier with adequate coating properties able to protect Hd in the gastric medium. The spray-drying technique and process conditions selected were effective in microencapsulating and stabilizing the labile flavonoid giving satisfactory encapsulation efficiency, product yield, microparticles morphology, and drug stability over 12 months. The presence of the enhancers such as CMC, SDBS, and Tween 85 in the formulation seems to be essential to obtain complete drug release in the simulated intestinal fluid. This approach might be suitable for delivering flavonoids such as Hd to the intestine, avoiding gastric degradation of the molecule and improving its bioavailability from solid oral dosage forms.
References
- 1.Barthe GA, Jourdan PS, McIntosh CA, Mansell RL. Radioimmunoassay for the quantitative determination of hesperedin and analysis of its distribution in Citrus sinensis. Phytochemistry. 1988;27:249–254. doi: 10.1016/0031-9422(88)80625-3. [DOI] [Google Scholar]
- 2.Garg A, Garg S, Zaneveld LJD, Singla AK. Review article: chemistry and pharmacology of the citrus bioflavonoid hesperedin. Phytother Res. 2001;15:655–669. doi: 10.1002/ptr.1074. [DOI] [PubMed] [Google Scholar]
- 3.Bok SH, Lee SH, Park YB, Bae KH, Son KH, Jeong TS, et al. Plasma and hepatic cholesterol and hepatic activities of 3-hydroxy-3-methyl-glutaryl-CoA reductase and acyl CoA: cholesterol transferases are lower in rats fed citrus peel extract or a mixture of citrus bioflavonoids. J Nutr. 1999;129:1182–1185. doi: 10.1093/jn/129.6.1182. [DOI] [PubMed] [Google Scholar]
- 4.Hirata A, Murakami Y, Shoji M, Kadoma Y, Fujisawa S. Kinetics of radical-scavenging activity of hesperetin and hesperidin and their inhibitory activity on COX-2 expression. Anticancer Res. 2005;25:3367–3374. [PubMed] [Google Scholar]
- 5.Horcajada MN, Coxam V. Hesperidin, a citrus flavanone, improves bone acquisition and prevents skeletal impairment in rats in nutritional aspects of osteoporosis. 2. New York: Elsevier; 2004. pp. 103–120. [Google Scholar]
- 6.Yang M, Tanaka T, Hirose Y, Deguchi T, Mori H, Kawada Y. Chemopreventive effects of diosmin and hesperidin on N-butyl-N-(4-hydroxybutyl)nitrosamine induced urinary bladder carcinogenesis in male ICR mice. Int J Can. 1997;73:719–724. doi: 10.1002/(SICI)1097-0215(19971127)73:5<719::AID-IJC18>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 7.Franke AA, Cooney RV, Custer LJ, Mordan LJ, Tanaka Y. Inhibition of neoplastic transformation and bioavailability of dietary flavonoid agents. Adv Exp Med Biol. 1998;439:237–228. doi: 10.1007/978-1-4615-5335-9_17. [DOI] [PubMed] [Google Scholar]
- 8.Choi JS, Park KY, Moon SH, Rhee SH, Young HS. Antimutagenic effects of plant flavonoids in the Salmonella assay system. Arch Pharm Res. 1994;17:71–75. doi: 10.1007/BF02974226. [DOI] [PubMed] [Google Scholar]
- 9.Calomme M, Pieters L, Vlietinck A, Berghe DV. Inhibition of bacterial mutagenesis by Citrus flavonoids. Planta Med. 1996;62:222–226. doi: 10.1055/s-2006-957864. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka T, Makita H, Kawabata K, Mori H, Kakumoto M. Modulation of N-methyl-N-amylnitrosamine induced tumorigenesis by dietary feeding of diosmin and hesperidin, alone and in combination. Carcinogenesis. 1997;18:957–965. doi: 10.1093/carcin/18.5.957. [DOI] [PubMed] [Google Scholar]
- 11.Beninati S. Transglutaminase activity and protein polyamine binding capacity in animal and plants cells. In: Pandalai G, editor. Recent developments in phytochemistry. vol 1. Kerala: Research Signpost; 1997. pp. 243–253. [Google Scholar]
- 12.Lentini A, Forni C, Provengano B, Beninati S. Enhancement of transglutaminase activity and polyamine depletion in B16-F10 melanoma cells by flavonoids naringenin and hesperitin correlate to reduction of the in vivo metastatic potential. Amino Acids. 2007;32:95–100. doi: 10.1007/s00726-006-0304-3. [DOI] [PubMed] [Google Scholar]
- 13.Thacher SM, Rice RH. Keratinocyte-specific transglutaminase of cultured human epidermal cells: relation to cross-linked envelope formation and terminal differentiation. Cell. 1985;40:685–695. doi: 10.1016/0092-8674(85)90217-X. [DOI] [PubMed] [Google Scholar]
- 14.Benedetti L, Grignani F, Scicchitano BM, Jetten AM, Diverio D, Lococo F, et al. Retinoid-induced differentiation of acute promyelocytic leukemia involves PML-RAalpha-mediated increase of type II transglutaminase. Blood. 1996;87:1939–1950. [PubMed] [Google Scholar]
- 15.Arani I, Adler-Storthz K, Trying SK, Brysk H, Brysk MM. Differentiation markers in oral carcinoma cell lines and tumors. Anticancer Res. 1997;17:4607–4610. [PubMed] [Google Scholar]
- 16.Kanaze FI, Kokkolou E, Niopas I, Georgarakis M, Stergiou A, Bikiaris D. Thermal analysis study of flavonoid solid dispersion having enhanced solubility. J Therm Anal Calorim. 2006;83:283–290. doi: 10.1007/s10973-005-6989-9. [DOI] [Google Scholar]
- 17.Lauro MR, Maggi L, Conte U, De Simone F, Aquino RP. Quercetin gastro-resistant microparticles obtained by spray-drying technique. J Drug Del Sci Tech. 2005;15:363–369. [Google Scholar]
- 18.Lauro MR, De Simone F, Sansone F, Iannelli P, Aquino RP. Preparation and release chacteristics of naringin and naringenin gastro-resistant microparticles by spray-drying. J Drug Del Sci Tech. 2007;17:119–124. [Google Scholar]
- 19.Palmieri GF, Bonacucina G, Di Martino P, Martelli S. Gastro-resistant microspheres containing ketoprofene. Journal of Microencapsulation. 2002;19:111–119. doi: 10.1080/02652040110065477. [DOI] [PubMed] [Google Scholar]
- 20.Giunchedi P, Conte U. Spray drying as preparation method of microparticulate drug delivery systems: an overview. STP Pharm Sci. 1990;5:276–290. [Google Scholar]
- 21.Gaylord N, Schor LM. Controlled release solid drug dosage forms based on mixture of water soluble non-ionic cellulose ethers and anionic surfactants. US Patent 1989;4849229.
- 22.Tommasini S, Calabrò ML, Raneri D, Ficarra P, Ficarra R. Combined effect of pH and polysorbates with cyclodextrins on solubilization of naringenin. J Pharm Biomed Anal. 2004;36:327–333. doi: 10.1016/j.jpba.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Sangalli ME, Giunchedi P, Colombo P, Gazzaniga A, La Manna A. Cross-linked sodium carboxymethylcellulose as a carrier for dissolution rate improvement of drugs. Boll Chim Farm. 1989;128:242–247. [PubMed] [Google Scholar]