Abstract
The aim of this study was to formulate and characterize a microparticulate system of progestin-only contraceptive. Another objective was to evaluate the effect of gamma radio-sterilization on in vitro and in vivo drug release characteristics. Levonorgestrel (LNG) microspheres were fabricated using poly(lactide-co-glycolide) (PLGA) by a novel solvent evaporation technique. The formulation was optimized for drug/polymer ratio, emulsifier concentration, and process variables like speed of agitation and evaporation method. The drug to polymer ratio of 1:5 gave the optimum encapsulation efficiency. Speed of agitation influenced the spherical shape of the microparticles, lower speeds yielding less spherical particles. The speed did not have a significant influence on the drug payloads. A combination of stabilizers viz. methyl cellulose and poly vinyl alcohol with in-water solvent evaporation technique yielded microparticles without any free drug crystals on the surface. This aspect significantly eliminated the in vitro dissolution “burst effect”. The residual solvent content was well within the regulatory limits. The microparticles passed the test for sterility and absence of pyrogens. In vitro dissolution conducted on the product before and after gamma radiation sterilization at 2.5 Mrad indicated no significant difference in the drug release patterns. The drug release followed zero-order kinetics in both static and agitation conditions of dissolution testing. The in vivo studies conducted in rabbits exhibited LNG release up to 1 month duration with drug levels maintained within the effective therapeutic window.
Key words: contraceptive, gamma radiation, in vivo, levonorgestrel, poly(lactide-co-glycolide)
INTRODUCTION
Poly(lactide-co-glycolide) (PLGA) is the polymer of choice for parenteral systems (1–3). Depending upon the type and ratio of lactide and glycolide, the biodegradation behavior can be altered. Injectable contraceptives offer several advantages. This administration is highly effective with reliable reversibility. The miniaturized systems that employ biodegradable polymers have attracted the attention of many researchers since these systems bypass the surgical complications associated with the implantable devices (4–8). In comparison to the oral formulation-like tablet, the parenteral system would require lesser amounts of the drug. Since the injectable system would require a lower dose, this would in turn lead to fewer unwanted side effects associated with the molecule. Further, a system that would release the active agent for prolonged periods would be more patient-adherent (9). One can avoid the problems associated with missing a dose during the ongoing therapy.
In this study, we employed levonorgestrel (LNG) as the model progestin drug. LNG is 13-ethyl-17-ethynyl-17-hydroxy-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydrocyclopenta[a] phenanthren-3-one, a levorotatory enantiomer derived from 19-nortestosterone. It is the drug of choice by most medical practitioners for contraception. Microparticles of LNG using poly(lactide-co-glycolide) polymer have been reported (10). LNG microspheres that released the drug continuously for more than 8 months in female baboons have been reported. However, no further progress has been reported on this system (11). However, these studies do not account the sterilization step and the consequence of post-sterilization effects on the performance of the product.
The primary objective of our study was to develop a progestin-only contraceptive preparation for monthly release of LNG. The microparticles were prepared using a solvent evaporation method. Sterility is an important parameter for parenteral systems. It is imperative to examine the effects of radiation sterilization on the stability of the system from its developmental stages. The effect of gamma radiation terminal sterilization on the in vitro and in vivo release of the steroid was studied. This significant study was undertaken to critically investigate the suitability of the radiation technique for sterilization of this developed system. The formulation was optimized for different formulation factors and process variables. The product was characterized for in vitro drug release kinetics. The proof-of-concept of the developed parenteral system was successfully demonstrated by in vivo studies in rabbits.
MATERIALS AND METHODS
Materials
LNG was a gift sample from Wyeth Laboratories (Mumbai, India). Methyl cellulose (Methocel®-400, MC) was obtained from Colorcon Asia Pvt. Ltd. (Mumbai, India). Polyvinyl alcohol (PVA, partially hydrolyzed 88%), sodium azide, thiomerosal, potassium dihydrogen orthophosphate, sodium dihydrogen phosphate (anhydrous), disodium hydrogen phosphate (anhydrous), sodium dodecyl sulfate, and sodium chloride were purchased from S.D. Fine Chemicals (Mumbai, India). Poly(lactide-co-glycolide) 53/47 (PLGA; Mw 14,000 Da; inherent viscosity 0.2 dL/g) was a gifted from Purac Biochem (Holland). A regular hypodermic disposable syringe was purchased from Becton Dickinson and Company, USA. The fluid thyoglycollate medium was purchased from Hi Media Pvt. Ltd. (Mumbai, India). Sodium carboxymethylcellulose (USP grade; medium viscosity polymer, 2% solution at 25°C has a viscosity of 400–800 cps) was purchased from Sigma Chemicals (USA). Bacillus subtilis ATCC No. 6633 (NCIM 2063) and Candida albicans ATCC No. 10231 (NCIM 3471) were obtained form National Chemical Laboratory (Pune, India). Bacteroides vulgatus ATCC No. 8482 (MTCC 1350) was procured from Institute of Microbial Technology (India). High performance thin layer chromatography (HPTLC) precoated silica gel 60 F254 plates were purchased from Merck (Germany). Potassium bromide (KBr) was purchased from Ranbaxy Chemicals (Mumbai, India). Karl Fisher (KF) reagent was purchased from Merck (Germany). Female albino Belgium rabbits were obtained from the Institute for Research in Reproduction, Mumbai, India. Radioimmunoassay (RIA) kit was a gift from the World Health Organization, London. All other reagents used were of analytical grade and were purchased from Ranbaxy Chemicals (Mumbai, India).
Methods
Fabrication and Optimization of LNG–Poly(Lactide-co-Glycolide) Microparticles (LNG–PLGA)
Microparticles containing LNG were formed by the modified o/w emulsion in-water interrupted solvent evaporation technique. The procedure involved placing 200 mL purified water containing polymeric emulsifier/s in a beaker. The stabilizers namely MC and PVA were employed in varying concentrations. The drug and PLGA (mg 40:200, 80:200, and 160:200) dissolved in 4 mL of dichloromethane was gradually added into the aqueous continuum. The resulting emulsion was stirred at a constant rate at a temperature of 23–24°C. The semisolid droplets (embryonic microparticles) were then separated from the system followed by resuspension in 30 mL of stabilizer-free water. The product was harvested after the methylene chloride evaporation proceeded to completion (about 10 h). The drug-loaded microspheres were further lyophilized at −0.004 mbar pressure, −40°C temperature (Labconco Corporation, England, UK). The two-step solvent evaporation process eliminated the formation of free drug crystals in the aqueous phase or on the microparticle surface. The formulation was optimized by a series of microsphere trials as described in subsequent sections. All the formulation variables and process parameters have been listed in tabular form in Table I. All experiments were conducted in triplicate runs.
Table I.
The Influence of Formulation and Process Parameters on the Quality of LNG–PLGA Microparticles
| Batch code | D/P ratio wt/wt | Emulsifier concentration (% wt/wt) | Speed of agitation | Mean particle size (μm) | Free drug crystals | EE (%) mean ± SD | Sphericity (%) | Moisture content (% wt/wt) |
|---|---|---|---|---|---|---|---|---|
| LP 1 | 1:5 | PVA (0.27) | C | 45.30 | − | 82.45 ± 1.25 | 93.63 | 0.1402 |
| LP 2 | 1:5 | PVA (0.27) | B | 49.25 | − | 79.82 ± 1.09 | 84.96 | 0.1621 |
| LP 3 | 1:5 | PVA (0.27) | A | − | − | − | Aggregation | 0.1713 |
| LP 4 | 1:5 | PVA (1.0) | C | 51.82 | − | 78.59 ± 1.33 | 90.61 | 0.1321 |
| LP 5 | 1:5 | PVA (2.0) | C | 55.89 | + | 80.95 ± 1.40 | 100 | 0.1384 |
| LP 6 | 1:5 | PVA (3.0) | C | − | + | − | Precipitation | 0.1223 |
| LP 7 | 1:5 | MC (0.1) | C | 48.56 | + | 79.55 ± 1.23 | 93.66 | 0.1408 |
| LP 8 | 1:5 | MC (0.3) | C | 50.55 | + | 82.01 ± 1.09 | 92.97 | 0.1404 |
| LP 9 | 1:5 | MC (0.5) | C | 49.99 | + | 81.46 ± 1.19 | 91.89 | 0.1335 |
| LP 10 | 1:5 | PVA (0.27) and MC (0.05) | C | 46.26 | − | 80.99 ± 1.38 | 100 | 0.1218 |
| LP 11 | 2:5 | PVA (0.27) and MC (0.05) | C | 59.23 | + | 81.33 ± 1.27 | 88.80 | 0.1409 |
| LP 12 | 4:5 | PVA (0.27) and MC (0.05) | C | 54.25 | + | 82.99 ± 1.42 | 85.92 | 0.1556 |
Speed of agitation: A = 270 rpm, B = 550 rpm, and C = 910 rpm. Values given are for triplicate samples
LNG–PLGA levonorgestrel–poly(lactide-co-glycolide), D/P drug/polymer, EE encapsulation efficiency, PVA polyvinyl alcohol, MC methyl cellulose, + presence of drug crystals, − absence of drug crystals
Drug to Polymer Ratio
The drug to polymer ratio combination trials were taken in order to get optimum drug encapsulation efficiency. Various combinations of drug and polymer were chosen in the preparation of microparticles by the solvent evaporation technique. Drug to polymer ratios tried were 1:5, 2:5, and 4:5 wt/wt. The ratio that would give optimum encapsulation efficiency with microparticles having the desired characteristics without crystallization of the free drug was investigated.
Emulsifier Concentration
Various emulsifiers were tested to obtain a product without any free drug crystals on the microsphere surface. The influence of the emulsifier concentration in the aqueous phase on maintaining the sphericity of the microparticles and on the drug crystallization behavior was studied. The following formulations were prepared using different concentrations and mixtures of the emulsifiers:
PVA: 0.27, 1.0, 2.0 and 3.0% wt/wt
MC: 0.1, 0.3 and 0.5% wt/wt
PVA (0.27% wt/wt) + MC (0.05% wt/wt)
Speed of Agitation
Stirring speed trials were undertaken with an objective of getting perfectly spherical microsphere. The speed of agitation was altered and its influence on the sphericity of the product was studied. Three speeds tried were 270 rpm (speed A), 550 rpm (speed B), and 910 rpm (speed C). The speed was monitored using a digital photo tachometer DT-2234A (Lutron, Taiwan).
Mode of Solvent Evaporation
Two processes were followed for the evaporation of the organic solvent. In one method, the solvent was evaporated to completion as the microparticles were still in the emulsifier containing aqueous phase. This method gave rise to crystallization of the drug. Hence, this method was not followed in subsequent optimization trials. The second method involved decanting the nascent microparticles before total solvent evaporation and resuspending them in plain water (without the emulsifier). The remainder of the residual solvent was then evaporated to completion in this medium. This technique was employed in further optimization of the formulation.
Gamma Radiation Sterilization
The LNG–PLGA system (batch code LP 10) was subjected to cobalt-60 radiation at 2.5 Mrad. The samples to be irradiated were filled into 5-mL glass vials, stoppered with rubber closure, and sealed with aluminum overseal closure. Vials were packed in dry ice into polyurethane container to assure a low temperature during the irradiation process. Although gamma radiation causes a minimal temperature rise, keeping the temperature low avoids the possibility of hydrolytic degradation of the PLGA polymer. Placebo microspheres, plain LNG, physical mixture of polymer and LNG, and drug-loaded microparticles were subjected to gamma radiation sterilization. The gamma radiation sterilization process was carried out in the presence of air and at ambient temperature. The essential parameter in gamma radiation sterilization is the measurement of radiation dose. During the gamma radiation, the dosimetric control was performed using Fricke dosimeters (ferrous sulfate dosimeter, Bhabha Atomic Research Centre, India) which were placed inside the gamma chamber. Non-irradiated samples served as control. The study utilized a gamma chamber GC-900 (Bhabha Atomic Research Centre, Bombay, India). A dose rate of 0.13 Mrad/h was employed. The drug content after radiation was determined and compared with the non-radiated system. The cumulative effect of all the parameters discussed above on the final quality of the microspheres viz. the sphericity, absence of free drug crystals, process yields, particle size as well as the drug encapsulation efficiency was critically investigated.
Characterization of the LNG–PLGA System
Particle Size Analysis
The particle size was determined by measuring the diameter of individual particle using an optical microscope (Nikon YS100, Nikon Instruments Inc., USA). The diameter of 100 particles was measured and the mean particle size determined.
Drug Encapsulation Efficiency
Microspheres (10 mg) were accurately weighed and dissolved in 10 mL of dichloromethane. The polymer was precipitated using ethanol (volume made up to 25 mL). After centrifugation, the clear supernatant was subjected to HPTLC analysis. The analysis was conducted in triplicates. The encapsulation efficiency was calculated by the actual and theoretical drug-loading values.
Analytical Method (HPTLC Method)
The drug loading in microspheres was estimated by the HPTLC method. Briefly, the stationary phase used was HPTLC precoated silica gel 60 F254 plates (Merck, Germany; size 10 cm × 10 cm). An autosampler (Camag Linomat IV, Switzerland) was used. The mobile phase consisted of benzene/methanol (9:1 vol/vol). Plate development was linear ascending in Camag twin trough chamber (Switzerland). Spectrodensitometric analysis (Camag TLC scanner II, Switzerland) was done with a scanning speed of 1 mm/s at a wavelength of 240 nm. Integration was done in Perkin Elmer integrator system LCI-100 (Switzerland). Drug-loaded microspheres (100 mg) were dissolved in 10 mL of methylene chloride. The solution was further diluted suitably and spotted on HPTLC plates. The blank consisted of microparticulate placebo system treated in a similar manner. Representative standard curve of LNG was constructed by plotting the peak area as well as peak height versus drug concentration.
Sphericity
The sphericity of the microspheres was computed by the Lovgren and Lundberg technique (12). Briefly, individual microparticle was viewed on a projection microscope (model MP3Nr 3725, Poland). The longest diameter (L) and that perpendicular to it at its mid-point (b) were measured. Fifty readings were taken. The ratio of L/b was put into class intervals and the percent sphericity calculated using the formula, 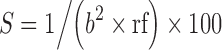 , where b is the lower class interval limit, and rf is the relative frequency.
, where b is the lower class interval limit, and rf is the relative frequency.
Moisture Content
The residual water content in the microparticles was found by the KF technique using a Karl Fischer/Autotitrator (model 831 KF Coulometer, Metrohm, UK). Dehydrated methanol (Merck, 20 mL) was titrated to the electrometric end point with the KF reagent (Merck). The microsphere sample was then carefully transferred to the titration vessel and after stirring for 1 min titrated again using the KF reagent till the characteristic end-point.
In Vitro Drug Release Kinetics
In the in vitro release studies, 25 mg of microspheres (batch LP 10) was placed into a 100-mL stoppered conical flask containing 20 mL dissolution medium that consisted of 0.9% wt/vol sodium chloride solution in distilled water. To maintain the sink conditions, 0.5% wt/vol sodium dodecyl sulfate was added, and to prevent microbial growth, sodium azide (0.02% wt/vol) was employed. Dissolution testing was done on the sample subjected to gamma radiation as well as on the non-radiated system. Two methods used in the study included the static and the shaking method. In the shaking technique, samples were agitated (80 strokes/min) using a constant temperature shaker water bath. The temperature was maintained at 37 ± 0.5°C. The test involved withdrawing 2 mL of microsphere-free samples at predetermined time intervals of 4, 8, 12, 16, 20, 24, 28, and 32 days (replacing with fresh medium after every sampling) and measuring the drug release by a sensitive high performance liquid chromatography (HPLC) method.
The drug release data were fitted to various kinetic models viz. zero-order, first-order, and Higuchi kinetics (13). Higuchi equation describes the release of a drug from an insoluble matrix as the square root of a time-dependent process. This is essentially based on Fickian diffusion as given below:
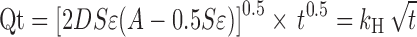 |
Where Qt is the amount of drug released in time t, D is the diffusion coefficient, S is the solubility of the drug in the dissolution medium, ɛ is the porosity, A is the drug content per cubic centimeter of matrix tablet, and kH is the release rate constant for the Higuchi model. The release data were also fitted to Baker–Lonsdale kinetic model (14). This model is for diffusion-controlled release and more specific from spherical matrix systems and is given below.
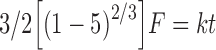 |
Where F is the fraction of drug released, t is the time, and k is the rate constant. It has been reported that drug release from controlled drug delivery systems using typically hydrophilic matrices shows a time-dependent profile. Further, the drug release decreases with time due to increased diffusion path length due to a gel layer formed on the surface that retards further the ingress of fluid and subsequent drug release (15). This leads to first-order release kinetics depicted in following equation:
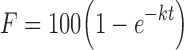 |
Where F is the percentage of drug released at time t. Zero-order kinetics is obtained when the drug release from a system is independent of the concentration of the drug (16). When the percent drug release was plotted as a function of time, a linear relationship was obtained with the r value closet to unity.
Analytical Method (HPLC)
A validated stability indicating HPLC method was used. Analysis was conducted on Jasco intelligent unit (Japan) using Lichrospher RP-18 column (5 μm, length 125 mm, Merck) column and mobile phase consisting of acetonitrile/water/acetic acid (40:5:5 vol/vol/vol). The flow rate was kept at 1 mL/min. Detection wavelength was 360 nm using Jasco UV-975 UV/VIS detector coupled with Borwin V 1.21 chromatography software. The analysis was conducted in triplicates. Calibration curves were obtained from the plots of peak area versus drug concentration for the concentration range of 10 to 80 μg/mL. The limit of detection for the drug was estimated to be 100 ng, the level at which the drug could be detected without any noise. The limit of quantitation was found to be 350 ng.
Surface Morphology
Scanning electron microscopy was performed to examine the surface morphology of the microparticles (model S-570, Hitachi, Japan). The microparticles were placed on a metal stud coated with adhesive label. The sample was sputter-coated with conductive gold palladium (Edwards Sputter Coater).
Infra-red Spectroscopy
IR spectra were taken for LNG, gamma-sterilized LNG at 2.5 Mrad dose, LNG–PLGA microspheres, physical mixture of LNG + PLGA (1:5 wt/wt ratio), PLGA polymer, and blank placebo microspheres prepared by the same manufacturing process. The possibility of excipient–drug interaction was investigated by infra-red absorption spectrophotometry. A FT-IR spectrophotometer (Jasco FT/IR 5300 instrument, Japan) was employed in this study (KBr disc method was used).
Differential Scanning Calorimetry
Differential scanning calorimetric thermograms were obtained for LNG, polymer PLGA, physical mixture of drug and polymer (1/5 wt/wt ratio), and the LNG–PLGA microparticulate system. Microparticulate samples were separately sealed in aluminum cells and set in a Shimadzu thermal analyzer DT-40 apparatus (Japan). Thermal analysis was performed at a heating rate maintained at 10°C/min in nitrogen atmosphere. Alumina was employed as the reference substance.
X-ray Diffraction Studies
X-ray diffractograms were obtained for LNG, PLGA, and LNG–PLGA microsphere system at a scanning rate of 2°/min. The polymorphic state of the drug in the microparticulate system was examined by X-ray diffraction studies. An automatic X-ray diffractometer (Siemens 5000, Germany) equipped with an X-ray generator was used. Nickel-filtered Cu Kα1 radiation having a wavelength of 1.5106 Å, operating at 35 kW and 20 mA in the range (2θ) of 5° to 70° was employed.
Residual Solvent Content
The residual methylene chloride content in the LNG–PLGA microspheres was determined by head space gas chromatography using CHEMITO 8610 HT gas chromatograph using BPX5 capillary column equipped with the head space system. The oven, injection port, and detector temperatures were kept at 40°C, 80°C, and 240°C, respectively. Nitrogen (flow rate of 5 mL/min) was used as carrier gas and a flame ionization detector was employed in the study. The microparticles (200 mg) were dissolved into 5 mL of distilled water and sonicated for 5 min. The sample was then placed in the head space tube in the heating block and heated at 80°C for 10 min, and the head space was subsequently injected. The standard employed in the analysis consisted of 0.1 mL (132.5 mg) of methylene chloride (99.5%) suitably diluted in distilled water to obtain a final concentration of 53 and 8.6 ppm. The above two standard solutions (4 mL) were taken separately in the head space tube in the heating block and heated to 80°C for 10 min. The head space was injected and gas chromatograms of the samples and standards were recorded. The residual solvent content was determined from the peak areas.
Sterility Testing
The test for sterility was done on the optimized batch according to the United States Pharmacopoeia method (17). Briefly, the sample was added to the fluid thyoglycollate medium and incubated at 32.5 ± 2.5°C for 14 days. The contents were observed for any microorganism growth. The formulation subjected to gamma radiation was subjected to the sterility testing.
Test for Pyrogens
A test was carried out to confirm the absence of pyrogens (18). Three healthy, adult rabbits of either sex weighing not less than 1.5 kg were selected for the study. Basal body temperature was documented. Sterilized microspheres were diluted with pyrogen-free saline solution and injected slowly into the marginal ear vein of each rabbit. Rectal temperature of each rabbit was monitored every 30 min for 3 h.
Pharmacokinetic Study
The study protocol was approved by the Institutional Animal Ethics Committee (IAEC, India) prior to the study. The guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA, India) were followed during the study. To evaluate the efficacy of the formulations in biological milieu, in vivo studies were conducted on non-rodent species. Healthy female albino Belgium rabbits (Institute for Research in Reproduction, Mumbai, India) selected from the same colony with body weight of 1.5–2.0 kg were used in the study. The performance of the product was assessed by monitoring blood levels of the drug released from the system. The animals were quarantined in a well-ventilated room with defined room temperature, humidity, and lighting conditions. Each group contained six animals. The randomization technique was used in grouping the animals. For identification purpose, the animals were tattooed using a suitable color coding scheme. Each animal received one formulation. For the purpose of comparison, a control group comprising of equal number of animals as that of the test group was taken. The animals did not receive any formulation. They were subjected to mating with male rabbits. This served as the positive control. All the animals received standard balanced diet and free access to water throughout the study.
Based on the in vitro release profiles, the LNG–PLGA microsphere (batch LP 10) was selected to evaluate the therapeutic performance of the designed dosage form. The LNG-loaded microparticles (previously sterilized by gamma radiation at 2.5 Mrad) were suspended in sterile saline (1 mL volume). The product after reconstitution was injected intramuscularly into proven fertile female rabbits. The injection was done using sterile disposable hypodermic syringe equipped with a 22-gauge needle. Before injecting the product, blood samples were taken and this served as the initial zero day reading. The serum separated served as “serum blanks” during the RIA study. Blood samples were withdrawn at predetermined time intervals of 1, 3, 4, 8, 12, 16, 20, 24, and 28 days from the marginal ear vein of the rabbit. The serum separated was collected and stored in stoppered vials at −20°C until further analysis. The samples were analyzed by a sensitive RIA technique. In addition to the measurement of the drug in the body, the performance of the formulation as an effective contraceptive was assessed by examination of conception in rabbits. The animals were kept with male rabbits and tested periodically for indication of conception/pregnancy.
Drug Analysis (Radioimmunoassay Technique)
A sensitive radioimmunoassay method was employed in estimating the drug in the serum samples. For the standard curve, ethanolic solution of LNG was suitably diluted with anhydrous ethanol and buffer to obtain concentration levels of 1,500, 750, 375, 188, 94, 47, and 23 fmol/tube. The solvent was evaporated and 200 μL of blank serum was added to each of the tubes. The extraction was done with 2 mL of ether and vortex mixing for 2 min. The tubes were then transferred to a mixture of solid carbon dioxide and acetone. The ether layer was carefully decanted into assay tubes after the aqueous layer was frozen. The tubes were left standing inside the fume cup-board till the solvent evaporated. A buffer (200 μL) was added to each of the assay tube and vortex mixed, allowing the buffer to roll around the tubes. The tubes were put in a water bath at 40°C and allowed to stand for 5 min. The contents were then removed and vortexed. To each of the tube, 50 μL of antiserum and 50 μL of the working tracer were added and mixed.
The serum samples collected from the rabbits (200 μL each) were subjected to extraction procedure as described for the standard curve. To each extraction tube, 50 μL of antiserum and 50 μL of the working tracer were added and vortexed. The vials were then transferred into a beta counter (Wallac 1409 liquid scintillation counter). An equilibration time of 24 h was allowed before the counting. Background counts of the vials were taken prior to use. The counting time was 180 cpm (beta spectrum). For the calibration curve, a dose–response curve was plotted. The method consisted of plotting the logits on the y-axis and log-dose on the x-axis. The concentration of the drug in the serum samples was read from the calibration plot. Pharmacokinetic parameters viz. Cmax, AUC0–t, AUCinf, and Kel were calculated by the WINNONLIN® program version 5.2 (Pharsight). Mean residence time (MRT) was computed by the statistical moment theory method.
Statistical Analysis
All the results are expressed as mean values ± standard deviation (SD). For curve fitting to different kinetic models in dissolution studies, the polynomial regression method was employed. To check whether there was any significant difference in the mean of the treated groups, comparison of the mean values of various groups was performed by one-way analysis of variance. Statistical significance was defined at P > 0.05.
RESULTS AND DISCUSSION
Optimization of LNG–Poly(Lactide-co-Glycolide) Microparticles (LNG–PLGA)
Two methods of solvent evaporation, i.e., continuous method and interrupted evaporation process, were studied. It was observed that crystal formation occurred when solvent evaporation was performed according to the continuous evaporation technique. In the interrupted solvent evaporation (when the microspheres were removed from the aqueous continuum and further evaporation was carried out in stabilizer-free water), this crystallization phenomenon was eliminated. However, in the latter case, if microparticles were removed at an early stage, they tend to agglomerate due to excess of methylene chloride. On the other hand, if the emulsifier was removed too late, then this led to the formation of microcrystals. Hence, it was necessary to define the precise time at which the transfer should be executed. The typical average time window for microsphere transfer was 45 ± 10 min. An optimized ratio of polymer and drug (ratio of 5:1, batch LP 10) gave improved encapsulation efficiency of 80.99% without free drug crystallization. An impeller agitation speed of 910 rpm gave microparticles with sphericity of 100%. The effect of formulation and process parameters on the morphology is shown in Table I.
Among all the ratios of the dispersed phase to the aqueous phase, a ratio of 1:5 vol/vol was found to give spherical particles, and hence, this ratio was selected for further optimization of the system. In all the batches D50% was about 51.82 μm and D90% was about 40.82 μm. The drug to polymer ratio affected sphericity and formation of free drug crystals. Higher drug to polymer ratios viz. drug to polymer ratios of 2:5 wt/wt and 4:5 wt/wt yielded microparticles with less sphericity (85–88%) along with free drug crystal formation. At low speeds of agitation, the sphericity of the microparticles was disturbed and also the process yield was less due to agglomeration tendencies. Higher stirring speeds produced microspheres in good yields (about 80%). The speed did not have a significant influence on the drug payloads. For a constant rate of agitation, the mean particle size increased as the PVA concentration increased. This was due to the increase in viscosity of the aqueous phase. The type and concentration of the emulsifier played a deciding role in maintaining the sphericity of the microparticles. The microspheres exhibited perfect sphericity when PVA was used in 2% wt/wt concentration. However, there was crystallization of the drug. Lower concentrations of PVA gave ovoid-shaped microparticles. Methocel in concentrations of 0.1% to 0.5% wt/wt gave a product with sphericity ranging from 91% to 93%. Also, in all the cases, there was drug precipitation. The emulsifying agents employed in the solvent evaporation process played a key role in successful formation of spherical microparticles. Methyl cellulose and partially hydrolyzed polyvinyl alcohol used in the study gave suitable microspheres having the desired qualities. A combination of PVA (0.27% wt/wt) and MC (0.05% wt/wt) yielded perfectly spherical microparticles (100% sphericity) without free drug crystallization. Hence, this formulation was selected for further studies. Thus, a novel interrupted o/w emulsification-solvent evaporation technology was successfully designed for the production of PLGA microspheres of LNG. The moisture content determined in all the formulations is given in Table I.
In Vitro Release Studies
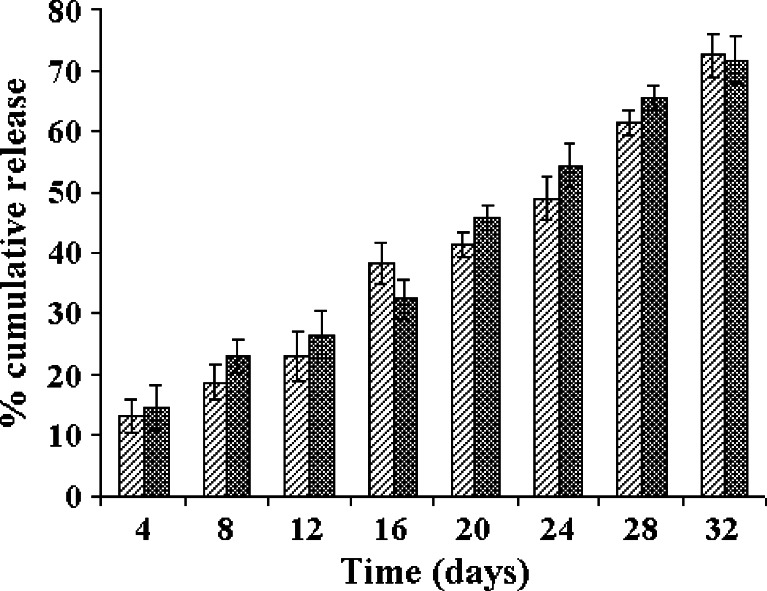
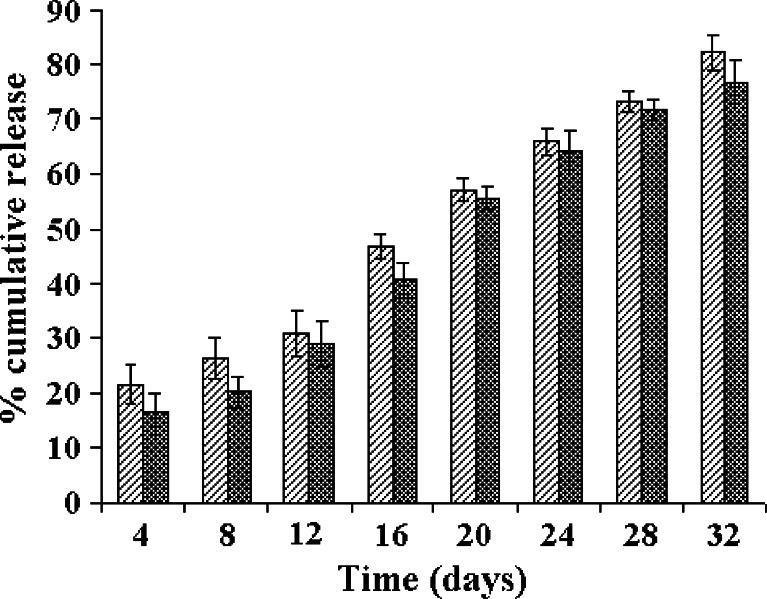
The in vitro drug release profiles of the LNG–PLGA microparticles are shown in Fig. 1 (by the static method) and Fig. 2 (using the agitation method). There was no major difference seen in the dissolution profiles with both the methods used for dissolution. Thus, agitation of the dissolution medium did not affect the dissolution rate and extent. The release data were fitted into different kinetic models viz. zero-order, first-order, Higuchi kinetics and Baker–Lonsdale equation. The corresponding coefficients of determination (r) and slopes were also computed. The fit for various kinetic models and comparison of calculated coefficient of determination indicated that the drug release kinetics followed predominantly a zero-order release profile with r2 value of 0.9826 for the gamma-radiated sample and 0.9854 for the non-irradiated LNG–PLGA microspheres. Thus, gamma radiation sterilization did not affect the in vitro drug release kinetics. Many research groups have studied the effect of gamma radiation on the product characteristics. Some experiments have noted significant changes in drug release rate (either an increase or decrease in the drug profile was observed after subjecting the microparticles to gamma radiation) (19–23). On the other hand, there are reports where the in vitro release rates from drug delivery devices remained unaffected by gamma radiation (24–28). In our studies, the drug release data from gamma-irradiated LNG–PLGA microparticles indicate that the cobalt-60 radiation did not influence the dissolution profile characteristics. Thus, it can be reasonably concluded that the radiation gamma dose of 2.5 Mrad can be safely used to terminally sterilize the product.
Fig. 1.
In vitro dissolution profiles of LNG–PLGA microspheres before and after gamma sterilization (n = 6) in static dissolution conditions. Values indicate mean ± SD (for triplicate samples)
Fig. 2.
In vitro dissolution profiles of LNG–PLGA microspheres before and after gamma sterilization (n = 6) in agitation dissolution conditions. Values indicate mean ± SD (for triplicate samples)
Gamma Radiation Studies
The product retained its physical characteristics after exposure to gamma radiation of 2.5 Mrad. The color of the product did not change after exposure to cobalt-60 irradiation. The microsphere system was free-flowing and no clumping and/or aggregation behavior was observed. The drug content was found to be 99.78 ± 1.22% which indicated that the microparticulate system was stable to cobalt-60 radiation. The mean particle size of the product was also not affected by gamma radiation (48.20 ± 2.53 μm).
Surface Morphology
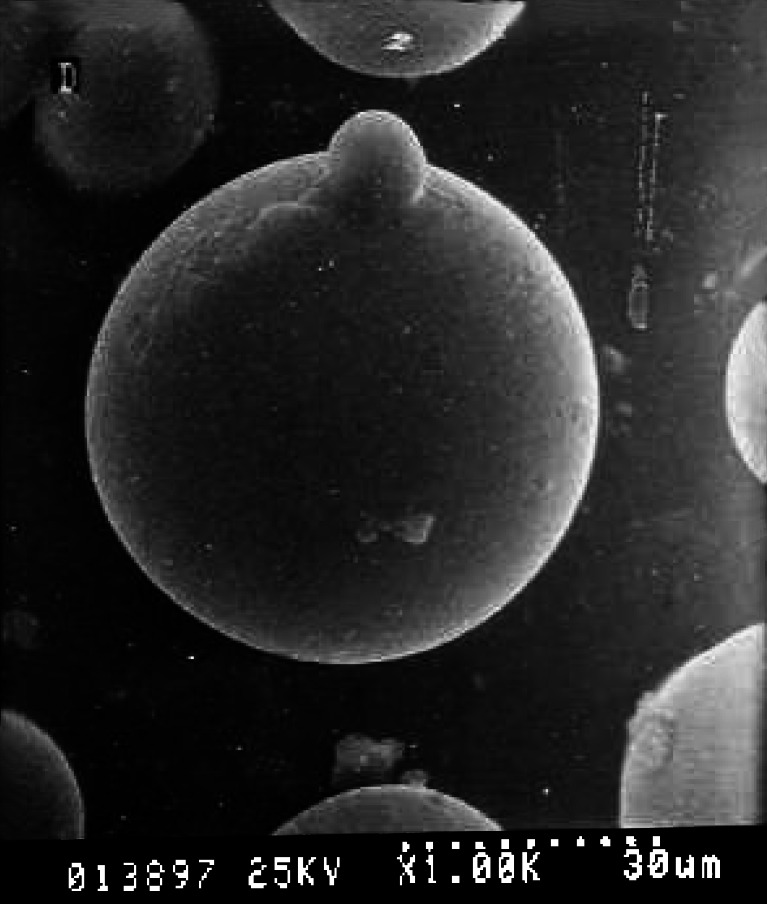
The scanning electron photomicrographs of microspheres of batch LP 10 is illustrated in Fig. 3. The miniaturized particles had a smooth surface texture. The shape of the microspheres remained unaltered after gamma radiation at 2.5 Mrad. There was no evidence of microscopic pores, and free drug crystals were absent. The microparticulate system was free from any structural defects.
Fig. 3.
Scanning electron photomicrograph of LNG–PLGA microspheres. There are no free drug crystals on the microsphere surface
Infra-red Spectroscopy
In case of LNG, gamma-sterilized LNG, and LNG–PLGA microspheres, there was no shift or changes observed in the wave numbers (conjugated C=O stretching at 1,655 cm−1, C=C stretching at 1,618 cm−1, methyl C–H asymmetric bending at 1,444 cm−1, alcoholic C–O stretching at 1,066 cm−1, and acetylene C–H bending at 692 cm−1). The IR spectra of PLGA polymer and blank microspheres also did not show any shift or additional peaks (1,763 cm−1 for C=O stretch, 1,387 cm−1 for C–H stretch, 1,088 and 1,196 cm−1 for C–O stretch). The presence of characteristic peaks of LNG in the physical mixture and drug-loaded microspheres indicates the chemical stability of LNG in the developed system. The IR spectra of the gamma-radiated drug showed the same absorption bands as the non-radiated LNG. Further similar absorption peaks in the PLGA polymer and placebo microspheres indicated that the manufacturing process did not influence the polymer characteristics. Thus, the above data suggest that there is a fairly good compatibility of the drug and polymer in the developed system.
Differential Scanning Calorimetry
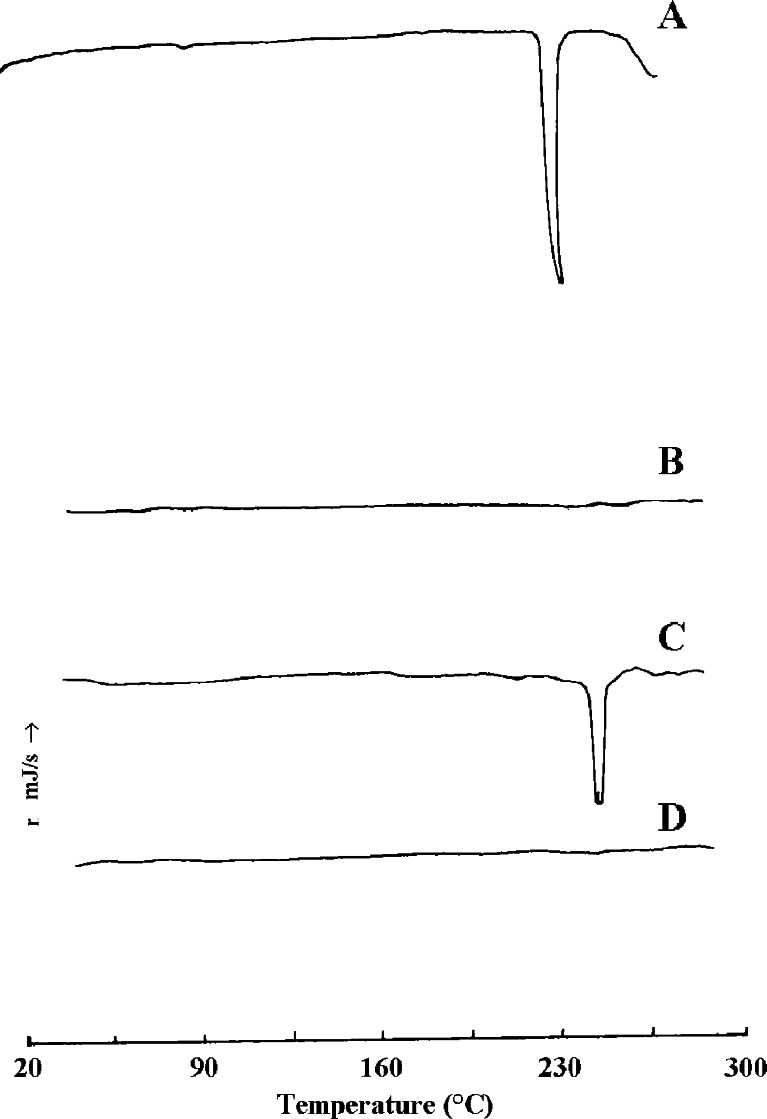
LNG exhibited a characteristic sharp endotherm at 241.9°C (Fig. 4). The bulk PLGA did not show any melting endotherm as seen in plot B. The physical mixture of the drug and polymer gave an endotherm at 240.1°C. However, the drug-loaded microsphere system showed no peaks originating from LNG. The above findings suggest that the drug is uniformly distributed in the polymer matrix.
Fig. 4.
Differential scanning calorimetry. A levonorgestrel, B PLGA, C physical mixture of LNG and PLGA, D LNG–PLGA microparticles
X-ray Diffraction Studies
The X-ray diffractogram of the pure drug shows a characteristic pattern of crystalline nature. The intensity of the diffraction peaks of PLGA was not sharp (data not shown). Taking into consideration the diffraction pattern of the LNG–PLGA system after gamma radiation, X-ray diffraction peaks of the drug were retained. The intensity of the diffraction peaks of LNG in the microsphere system coincided with those of the pure drug. It is reasonable to conclude that the solvent evaporation technique employed in microsphere formation might have resulted in some amounts of the drug being present in crystalline form and partly in the amorphous state. The above results can also be justified by the fact that methylene chloride is a good solvent for LNG and even a small quantity could affect crystallization of the drug in a given microparticle sample. Changes in polymorphic state may also be attributable to the gradual loss of small amounts of residual methylene chloride from microspheres on storage.
Residual Solvent Content
The residual methylene chloride content was found to be 14 ppm. This was well below the reported limit of 500 ppm (29).
Sterility Testing
The product (in all the experimental doses of gamma radiation) complied with the test for sterility.
Test for Pyrogens
The product passed the test since the summed response in the rabbits’ body temperature did not exceed 1.15°C.
Pharmacokinetic Study
In the RIA method, a representative standard curve of LNG was constructed by plotting the log(concentration) versus logit value. The polynomial regression for the calibration plots showed good linear relationship with coefficient of correlation −0.9998, slope −1.0993, and intercept 5.1739 over the concentration range studied.
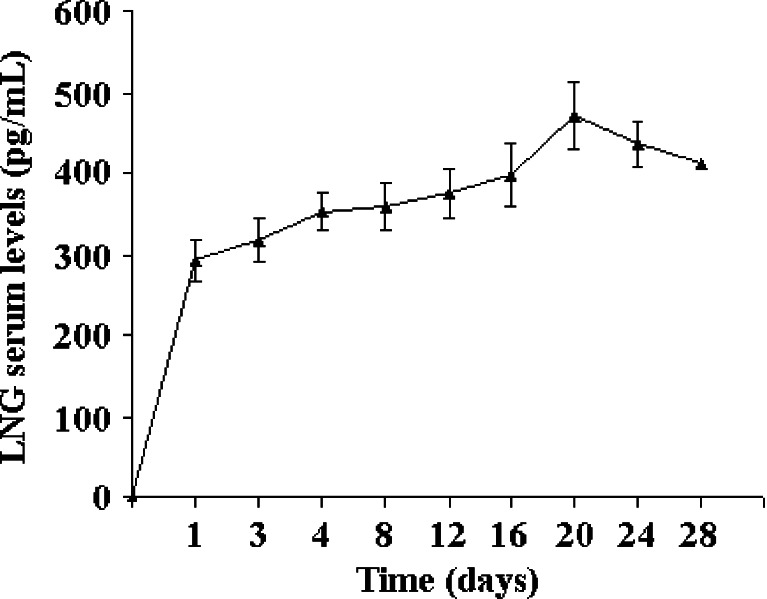
The plasma concentration–time profile is depicted in Fig. 5. Just like in the case of in vitro dissolution profile, there was no dose dumping in the in vivo profile as well. This may be attributed to the modified formulation process of microspheres that gave the product without free drug crystals on the surface. Pharmacokinetic parameters were calculated using a non-compartment model by processing with WinNonlin statistical software program version 5.2 (Pharsight). The terminal elimination rate constant (Kel) was calculated by the linear least square regression analysis of the log-linear phase of the concentration–time curve with the last three experimental points. The AUC0–t was calculated by the linear trapezoidal rule from 0 to the last measured plasma concentration. AUC0–inf was calculated by adding Ct/Kel to AUC0–t. Individual pharmacokinetic parameters were calculated for each of the plasma concentration–time profile for each animal. The mean and SD were then computed and the mean plot of the plasma concentration–time profile was obtained. The AUC0–t was 10,765.1 ± 2,150.3 pg/mL day−1 (coefficient of variation, CV = 0.19), AUC0–inf was 36,058.76 ± 6,490.4 pg/mL day−1 (CV = 0.17) with Cmax of 469.6 ± 84.42 pg/mL (CV = 0.17), and Kel was 0.02 ± 0.004 day−1 (CV = 0.2). The MRT was calculated to be about 15 days. The control group exhibited signs of conception. No pregnancies occurred in all test animals injected with the microparticles during the 1-month study period. Also, there was reversibility of fertility in the rabbits after about 5–6 months time.
Fig. 5.
Plasma concentration–time profile LNG release from the microparticle system after intramuscular injection in female rabbits (n = 6). Values indicate mean ± SD (value for triplicate samples)
It has been reported in the literature that inhibition of ovulation occurs at LNG concentration of 500–800 pg/mL (30). In a comparative study, the article claims that serum LNG levels during the 12th month of use were 500–800 pg/mL in the polydimethylsiloxane (silastic) rod users, while in the capsule users, they were 150–300 pg/mL (31). However, it should be noted that suppression of ovulation may not be critical for contraceptive efficacy. Progestin-only contraceptive systems have achieved acceptable rates of contraception without inhibition of ovulation but by rendering the endometrium inhospitable to nidation or cervical mucus impermeable to sperm migration (32). A randomized clinical trial provides evidence that the minimum LNG concentration necessary to protect against pregnancy is below 200 pg/mL and possibly below 175 pg/mL. This study also indicates that drug concentration in the upper part of the range 151–200 pg/mL is protective against pregnancy (33). In our studies, there was no tissue inflammation or erythemia observed at the site of injection, which indicates that this dosage form is biocompatible with the formulation excipients. Comparison of drug release observed in the 1-month period in the in vitro dissolution studies and similar plasma profile where the drug levels start to decrease after day 28 indicates good in vitro/in vivo correlation. More significantly, the gamma irradiation effects did not influence the in vivo release kinetics and performance of the formulation. It has been reported in the literature that there is good correlation of the in vivo drug release from microsphere systems in rabbits and humans (34,35).
There are very few commercially available products for contraceptives that utilize long-term controlled release technology. A silicone-based implantable device using non-biodegradable polymer is available for the delivery of LNG by the trade name Norplant® (marketed by Population Council). The formulation needs surgical manipulations for the insertion and retrieval of the product. Since we have employed a biodegradable polymer to develop the formulation, it is not required to be removed after cessation of therapy. Our attempt was successful in formulating a pharmaceutically acceptable system that avoids the above shortcomings with ease of single intramuscular administration of the product. Oral route of administration of contraceptive steroids requires higher quantities of the active moieties. This leads to increase in side effects. The undesired side effects observed after oral administration of LNG in clinical trials range from minor ones like headache/dizziness, nausea, abdominal pain, vomiting, to more significant like high blood pressure (severe headache, flushing, blurred vision), fatigue, menstrual changes, and liver damage. Oral contraceptives have been associated with thrombophlebitis, arterial thromboembolism, pulmonary embolism, myocardial infarction, cerebral hemorrhage, cerebral thrombosis, hypertension, gallbladder disease, hepatic adenomas, carcinomas, or benign liver tumors. The daily dose of oral tablet is 0.1–0.15 mg which is generally given in combination with ethinyl estradiol. The serum concentration of LNG after daily oral administration of the tablet formulation is much higher than that required for contraception. Here, we present an injectable system with lesser dose in the formulation as compared to the oral tablets. Thus, a transformation from oral delivery system to a long-term parenteral product using lower amounts of the drug would lead to reduced unwanted side effects, which is an additional feature of the developed system.
CONCLUSIONS
The present investigation demonstrates that gamma radiation sterilization can be a method of choice for steroid-based microparticulate systems of poly(lactide-co-glycolide). The drug release can be controlled for a 1-moth period with reliable reversibility of fertility. Further, the dose dumping phenomena observed with these systems can be avoided by a change in the preparative technique of the microspheres.
Acknowledgments
The authors would like to thank Purac Biochem for providing poly(lactide-co-glycolide), Wyeth Laboratories for LNG, and World Health Organization (London) for providing radioimmunoassay kit. The authors acknowledge the valuable help of Dr. Dixit in preparation of this manuscript.
References
- 1.Jiang W, Gupta RK, Deshpande MC, Schwendeman SP. Biodegradable poly(lactide-co-glycolide) microparticles for injectable delivery of vaccine antigens. Adv Drug Deliv Rev. 2005;57:391–410. doi: 10.1016/j.addr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissues. Adv Drug Deliv Rev. 2003;55:329–47. doi: 10.1016/S0169-409X(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 3.Mundargi RC, Ramesh Babu V, Rangaswamy V, Patel P, Aminabhavi TM. Nano/micro technologies for delivering macromolecular therapeutics using poly(D,L-lactide-co-glycolide) and its derivatives. J Control Release. 2008;125:193–209. doi: 10.1016/j.jconrel.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Heya T, Okada H, Ogawa Y, Toguchi H. Factors influencing the profiles of TRH release from copoly(d,l-lactic/glycolic acid) microspheres. Int J Pharm. 1991;72:199–205. doi: 10.1016/0378-5173(91)90108-Z. [DOI] [Google Scholar]
- 5.Sah HK, Chien YW. Evaluation of a microreservoir-type biodegradable microcapsule for controlled release of proteins. Drug Dev Ind Pharm. 1993;19:1243–63. doi: 10.3109/03639049309074399. [DOI] [Google Scholar]
- 6.Urata T, Arimori K, Nakano M. Modification of release rates of cyclosporin A from polyl(L-lactic acid) microspheres by fatty acid esters and in-vivo evaluation of the microspheres. J Control Release. 1999;58:133–41. doi: 10.1016/S0168-3659(98)00146-1. [DOI] [PubMed] [Google Scholar]
- 7.Chattaraj SC, Rathinavelu A, Das SK. Biodegradable microparticles of influenza viral vaccine: comparison of the effects of routes of administration on the in vivo immune response in mice. J Control Release. 1999;58:223–32. doi: 10.1016/S0168-3659(98)00161-8. [DOI] [PubMed] [Google Scholar]
- 8.Viswanathan NB, Thomas PA, Pandit JK, Kulkarni MG, Mashelkar RA. Preparation of non-porous microspheres with high entrapment efficiency of proteins by a (water-in-oil)-in-oil emulsion technique. J. Control Release. 1999;58:9–20. doi: 10.1016/S0168-3659(98)00140-0. [DOI] [PubMed] [Google Scholar]
- 9.Okada HY, Heya IT, Uneo H, Ogawa Y, Toguchi H. Pharmacokinetics of once-a-month injectable microspheres of leuprolide acetate. Pharm Res. 1991;8:787–91. doi: 10.1023/A:1015818504906. [DOI] [PubMed] [Google Scholar]
- 10.Wang SH, Zhang LC, Lin F, Sa XY, Zuo JB, Shao QX, Chen GS, Zeng S. Controlled release of levonorgestrel from biodegradable poly(d,l-lactide-co-glycolide) microspheres: in vitro and in vivo studies. Int J Pharm. 2005;301:217–25. doi: 10.1016/j.ijpharm.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 11.Beck LR, Pope VZ, Tice TR, Gilley RM. Long-acting injectable microsphere formulation for the parenteral administration of levonorgestrel. Adv Contracept. 1985;1:119–29. doi: 10.1007/BF01849793. [DOI] [PubMed] [Google Scholar]
- 12.Lovgren K, Lundberg P. Determination of sphericity of pellets prepared by extrusion/spheronization and the impact of some process parameters. Drug Dev Ind Pharm. 1989;15:2375–92. doi: 10.3109/03639048909052536. [DOI] [Google Scholar]
- 13.Higuchi T. Mechanism of sustained action medication: theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52:1145–9. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- 14.Doshi CC, Bhalla HL. In vitro release studies of levonorgestrel loaded biodegradable microspheres. Ind J Pharm Sci. 1999;61:39–43. [Google Scholar]
- 15.Shah MV, De Gennaro MD, Suryakasuma H. An evaluation of albumin microcapsules prepared using a multiple emulsion technique. J Microencapsul. 1987;4:223–38. doi: 10.3109/02652048709021816. [DOI] [PubMed] [Google Scholar]
- 16.Ranga Rao KV, Padmalatha DK, Buri PK. Cellulose matrices for zero-order release of soluble drugs. Drug Dev Ind Pharm. 1988;14:2299–320. doi: 10.3109/03639048809152017. [DOI] [Google Scholar]
- 17.United States Pharmacopoeia, Microbiological tests: Sterility Tests, USP 29-NF 24, The United States Pharmacopoeial Convention, Inc., Rockville, 2006.
- 18.European Pharmacopoeia, Appendix XIVD Test for pyrogens, Ph. Eur. method 2.6.8, Council of Europe, 2005.
- 19.Beck LR, Pope VZ. Controlled-release delivery systems for hormones. A review of their properties and current therapeutic use. Drugs. 1984;27:528–47. doi: 10.2165/00003495-198427060-00002. [DOI] [PubMed] [Google Scholar]
- 20.Sanders LM, Kent JS, McRae GI, Vickery BH, Tice TR, Lewis DH. Controlled release of a luteinizing hormone-releasing hormone analogue from poly(d,l-lactide-co-glycolide) microspheres. J Pharm Sci. 1984;73:1294–7. doi: 10.1002/jps.2600730927. [DOI] [PubMed] [Google Scholar]
- 21.Spenlehauer G, Vert M, Benoit JP, Chabot F, Veillard M. Biodegradable cisplatin microspheres prepared by the solvent evaporation method: morphology and release characteristics. J Control Release. 1988;7:217–29. doi: 10.1016/0168-3659(88)90054-5. [DOI] [Google Scholar]
- 22.Spenlehauer G, Vert M, Benoit JP, Boddaert A. In vitro and in vivo degradation of poly(D,L lactide/glycolide) type microspheres made by solvent evaporation method. Biomaterials. 1989;10:557–63. doi: 10.1016/0142-9612(89)90063-X. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz JM, Busnel JP, Benoit JP. Influence of average molecular weights of poly(DL-lactic acid-co-glycolic acid) copolymers 50/50 on phase separation and in vitro drug release from microspheres. Pharm Res. 1990;7:928–34. doi: 10.1023/A:1015945806917. [DOI] [PubMed] [Google Scholar]
- 24.Wise DL, Trantolo DJ, Marino RT, Kitchell JP. Opportunities and challenges in the design of implantable biodegradable polymeric systems for the delivery of antimicrobial agents and vaccines. Adv Drug Deliv Rev. 1987;1:19–39. doi: 10.1016/0169-409X(87)90067-6. [DOI] [Google Scholar]
- 25.Hartas SR, Collett JH, Booth C. The influence of gamma-irradiation on the release of melatonin from poly(lactide-coglycolide) microspheres. Proc Int Symp Control Release Bioact Mater. 1992;19:321–2. [Google Scholar]
- 26.Crossan IM, Whateley TL. Microspheres of dexamethasone as a biodegradable controlled release drug delivery system. Proc Int Symp Control Release Bioact Mater. 1994;21:184–5. [Google Scholar]
- 27.Fallon PA, Whateley TL. The development and evaluation of poly(lactic-glycolic acid) implants for sustained delivery of dexamethasone to the brain. Proc Int Symp Control Release Bioact Mater. 1994;21:264–5. [Google Scholar]
- 28.Yoshioka S, Aso Y, Otsuka T, Kojima S. The effect of γ-irradiation on drug release from poly(lactide) microspheres. Radiat Phys Chem. 1995;46:281–5. doi: 10.1016/0969-806X(95)00025-S. [DOI] [Google Scholar]
- 29.ICH harmonized tripartite guideline, Q3C(R3) Impurities: guideline for residual solvents (http://www.ich.org/lob/media/media423.pdf).
- 30.Roy S, Stanczyk FZ, Mishell DR, Lumkin M, Gentzschein E. Clinical and endocrinologic study of continuous levonorgestrel administration from subcutaneous solid polydimethylsiloxane rods. Contraception. 1980;21:595–615. doi: 10.1016/0010-7824(80)90033-5. [DOI] [PubMed] [Google Scholar]
- 31.Moore DE, Roy S, Stanczyk FZ, Mishell D. Bleeding and serum d-norgestrel, estradiol and progesterone patterns in women using d-norgestrel subdermal polysiloxane capsules for contraception. Contraception. 1978;17:315–28. doi: 10.1016/0010-7824(78)90078-1. [DOI] [PubMed] [Google Scholar]
- 32.Ory SJ, Hammond CB, Yancy SG, Hendren RW, Pitt CG. The effect of a biodegradable contraceptive capsule (Capronor) containing levonorgestrel on gonadotropin, estrogen, and progesterone levels. Am J Obstet Gynecol. 1983;145:600–5. doi: 10.1016/0002-9378(83)91204-8. [DOI] [PubMed] [Google Scholar]
- 33.Sivin I, Lähteenmäki P, Ranta S, Darney P, Klaisle C, Wan L, Mishell DR, Lacarra M, Viegas OA, Bilhareus P, Koetsawang S, Piya-Anant M, Diaz S, Pavez M, Alvarez F, Brache V, LaGuardia K, Nash H, Stern J. Levonorgestrel concentrations during use of levonorgestrel rod (LNG ROD) implants. Contraception. 1997;55:81–5. doi: 10.1016/S0010-7824(96)00276-4. [DOI] [PubMed] [Google Scholar]
- 34.Lancranjan I, Bruns C, Grass P, Jaquet P, Jervell J, Kendall-Taylor P, Lamberts SW, Marbach P, Orskov H, Pagani G, Sheppard M, Simionescu L. Sandostatin LAR: a promising therapeutic tool in the management of acromegalic patients. Metabolism. 1996;45:67–71. doi: 10.1016/S0026-0495(96)90087-6. [DOI] [PubMed] [Google Scholar]
- 35.Comets E, Mentre F, Kawai R, Nimmerfall F, Marbach P, Vonderscher J. Modeling the kinetics of release of octreotide from long-acting formulations injected intramuscularly in rabbits. J Pharm Sci. 2000;89:1123–33. doi: 10.1002/1520-6017(200009)89:9<1123::AID-JPS4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]