Abstract
The purpose of the work was to investigate correlation between disintegration and dissolution for immediate release tablets containing a high solubility drug and to identify formulations where disintegration test, instead of the dissolution test, may be used as the acceptance criteria based on International Conference on Harmonization Q6A guidelines. A statistical design of experiments was used to study the effect of filler, binder, disintegrating agent, and tablet hardness on the disintegration and dissolution of verapamil hydrochloride tablets. All formulation variables, i.e., filler, binder, and disintegrating agent, were found to influence tablet dissolution and disintegration, with the filler and disintegrating agent exerting the most significant influence. Slower dissolution was observed with increasing disintegration time when either the filler or the disintegrating agent was kept constant. However, no direct corelationship was observed between the disintegration and dissolution across all formulations due to the interactions between different formulation components. Although all tablets containing sodium carboxymethyl cellulose as the disintegrating agent, disintegrated in less than 3 min, half of them failed to meet the US Pharmacopeia 30 dissolution criteria for the verapamil hydrochloride tablets highlighting the dependence of dissolution process on the formulation components other than the disintegrating agent. The results identified only one formulation as suitable for using the disintegration test, instead of the dissolution test, as drug product acceptance criteria and highlight the need for systematic studies before using the disintegration test, instead of the dissolution test as the drug acceptance criteria.
Key words: disintegration test, dissolution test, ICH Q6A, specification
INTRODUCTION
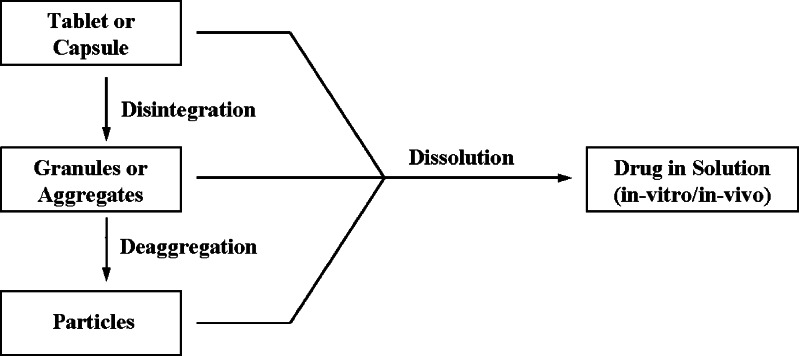
Immediate release oral dosage forms, i.e., tablets and capsules, are most widely used drug delivery systems available. These products are designed to disintegrate in the stomach followed by their dissolution in the fluids of the gastrointestinal tract (Fig. 1). Dissolution of the drug substance, under physiological conditions, is essential for its systemic absorption. For this reason, dissolution testing is typically performed on solid dosage forms to measure the drug release from the drug product as a test for product quality assurance/product performance and to determine the compliance with the dissolution requirements when stated in the individual monograph (1). In limited number of cases, an in vitro–in vivo correlation is established between the drug release and drug product absorption necessary for therapeutic effects. Disintegration test is also a standardized test and is primarily used as a quality assurance tool to confirm complete disintegration of solid oral dosage forms within the prescribed time when placed in a liquid medium under the experimental conditions described in their respective official monographs (1). Disintegration test neither implies nor tests for the complete solution of the drug or the dosage form. The difference between these two tests is that in case of the disintegration test, the test measures the time required for a product to disintegrate and de-aggregate into multiparticulate system in a given medium, while in case of the dissolution test, the test measures the concentration of the drug product in a given medium at a specified time. Thus, disintegration of a solid dosage form may not be a measure of dissolution of the drug substance in the dosage form.
Fig. 1.
Relationship between dissolution and disintegration of an oral solid dosage form
The recent International Conference on Harmonization (ICH) Q6A guideline provides specifications and acceptance criteria which should be established for all new drug substances and new drug products that have not been registered previously in the ICH region (2). The guidance recommends using a single-point measurement test to measure the release of drug substance from immediate-release drug products. This guidance also provides an option where dissolution testing may be replaced by disintegration testing for some immediate release solid oral drug products, containing high solubility drug over a pH range. In such cases, ICH allows disintegration time with an upper time limit to be used as the drug release acceptance criteria if following conditions are satisfied (2):
The drug product is not designed to produce modified release.
The solubility of drug is high enough at 37 ± 0.5°C so that dose/solubility <250 mL through out the physiological pH range (1.2–6.8).
Greater than 80% dissolution is achieved in 15 min at pH 1.2, 4.0, and 6.8.
A relationship has been determined and established between disintegration and dissolution or disintegration is more discriminating than dissolution.
Dissolution does not affect bioavailability.
Changes in formulation or manufacturing variables do not affect dissolution.
There was a concern of using disintegration as a universal surrogate for dissolution for immediate release dosage forms (3). The current study focuses on determining the relationship, if any, between the disintegration time and the dissolution time, and identifying formulations where dissolution testing may be replaced by disintegration testing as the acceptance criteria for the release of the drug product. Verapamil hydrochloride, a high solubility drug with dose/solubility <250 mL throughout the physiological pH range (1.2–6.8), was used (4). Filler, binder, and disintegrating agent were selected as the formulation variables, while manufacturing conditions were varied by compressing the resulting formulations at two compression pressures.
MATERIALS AND METHODS
Chemicals
Verapamil hydrochloride (Fermion Oy, Espoo, Finland) was used as the model drug. Lactose monohydrate (LMH; Foremost Farms USA, Rothschild, WI, USA) or dicalcium phosphate dihydrate (DCP; Di-Cafos, CFB KG, Budenheim, Germany) was used as the filler. Hypromellose (HPMC; Methocel E15 LV, Dow Chemical, Midland, MI, USA) or polyvinyl pyrollidone (PVP; Kollidon 30, BASF Corp, Florham Park, NJ, USA) was used as the binder. Microcrystalline cellulose (MCC; Emcocel 50 M, JRS Pharma, Cedar Rapids, IA, USA) or sodium carboxymethyl cellulose (NaCMC; Ac-di-sol, FMC Biopolymer, Newark, DE, USA) was used as the disintegrating agent. Talc (Spectrum Chemicals Mfg. Corp., Gardena, CA, USA) and magnesium stearate (Riedel-de Haen, Seelze, Germany) were used as glidant and lubricant, respectively. All materials were used “as received” after passing through a standard US standard No. 20 sieve. The concentrations are listed in Table I.
Table I.
Concentration of the Formulation Components Used to Prepare Tablets
| Ingredient | Function | Concentration (%) | Code (V*1*2*3 #)a |
|---|---|---|---|
| Verapamil HCl | Drug | 10.0 | |
| DCP/LMH | Filler | 75.0 | *1 = D/L |
| PVP/HPMC | Binder | 4.0 | *2 = P/H |
| NaCMC/MCC/none | Disintegrating agent | 10.0/10.0/0.0 | *3 = A/M/X |
| Talc | Glidant | 0.5 | |
| Magnesium stearate | Lubricant | 0.5 |
HCl hydrochloride, DCP/D dicalcium phosphate dihydrate, LMH/L lactose monohydrate, PVP/P polyvinyl pyrollidone, HPMC/H hypromellose, NaCMC/A sodium carboxymethyl cellulose, MCC/M microcrystalline cellulose, X none
a# = 1 or 2; used to distinguish tablets from each formulation with low or high hardness, respectively
Preparation of Formulations Using Design of Experiments
A 2 × 2 × 3 × 2 full factorial design was used to evaluate the influence of filler, binder, disintegrating agent, and hardness on the dissolution and disintegration time of tablets. Appropriate quantities of drug and filler were weighed and blended together in a low shear bench-top mixer (Model N50, Hobart Canada, North York, Ontario, Canada) for 5 min and transferred to a Strea-1 fluid-bed granulator (Niro Inc., Columbia, MD, USA). The batch size was 1,500 g. Granulation was performed by spraying a 6.0% w/v aqueous binder solution on to the fluidized powder blend at spray rate of 7 ± 1 g/min. The inlet air temperature and air flow were kept constant at 55 ± 1°C and 140 ± 10 m3/h, respectively. Subsequent to the binder addition, granules were dried to moisture content of less than 2.0% w/w in the fluid bed. The dried granules were screened though a US standard No. 20 sieve and blended with the disintegrating agent (if present), lubricant, and glidant in a 2.0-L V-blender (GlobePharma, New Brunswick, NJ, USA) at 30 rpm for 5 min.
The 12 formulations, obtained by varying the filler, binder, and disintegrating agent, were compressed into tablets on a 10-station instrumented tablet press (MiniPress-I, GlobePharma, New Brunswick, NJ, USA) fitted with a single set of 12-mm flat-faced, beveled-edged, round punches and cylindrical die. The press speed was kept constant at 20 rpm. Approximately 60 tablets were compressed from each formulation at low (6 KgF) and high (10 KgF) hardness settings, resulting in 24 set of tablets. The die and punch were cleaned using methanol after compressing each formulation. The 24 set of tablets were assigned a five-digit alpha-numeric code “V*1*2*3#”, with “*1”, “*2”, “*3”, and “#” representing filler, binder, disintegrating agent, and tablet hardness, respectively. The codes used for each digit are listed in Table I. Thus, VDPA1 represent tablets with 10 KgF hardness and containing DCP, PVP, and NaCMC as filler, binder, and disintegrating agent, respectively.
Dissolution Test
A VanKel VK 7000 dissolution system (Varian, Cary, NC, USA) automated with a VanKel peristaltic pump that flows sample from the dissolution vessels to an 18 cell Cary UV–visible spectrophotometer (Varian, Cary, NC, USA) was used. Testing was performed according to the US Pharmacopeia (USP)30 procedure for the verapamil hydrochloride tablets by monitoring the absorbance at 278 nm (5). Dissolution testing of selected tablets was also performed in pH 1.2, 4.0, and 6.8 buffer media.
Disintegration Test
An Electrolab ED-2 L Disintegration Tester (GlobePharma, New Brunswick, NJ, USA) was used to measure the disintegration time of the tablets, in accordance with USP30 <701> Disintegration procedures for the uncoated tablets using 900 mL of distilled water at 37°C. Six tablets were dropped into individual tubes of the basket-rack assembly. Disks were not mounted on the tubes and the time at which all six tablets had disintegrated was recorded.
Data Analysis
Data analysis was performed using JMP software (ver 7.0.1, SAS Institute Inc, Cary, NC, USA). A p value of <0.05 was considered as statistically significant.
RESULTS AND DISCUSSION
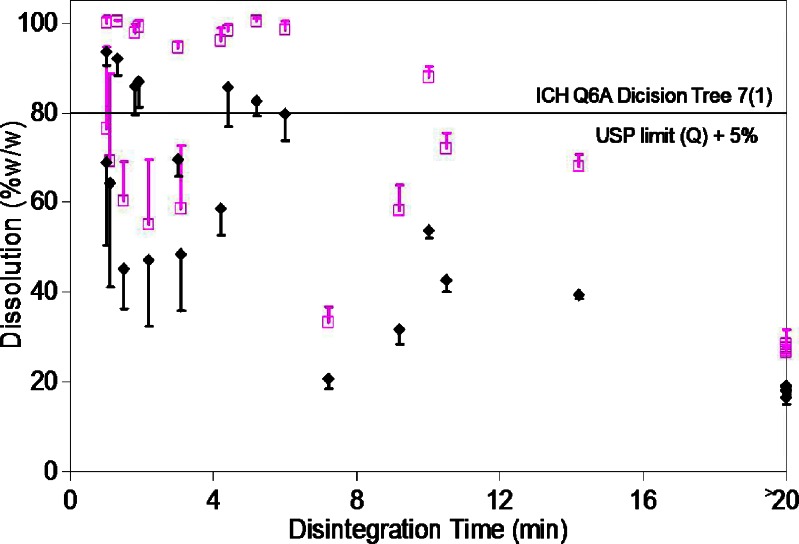
The 24 set of tablets prepared using different filler, binder, and disintegrating agent and compressed into tablets to different hardness showed large variation in the dissolution and disintegration time. The percentage dissolution ranged from 16.4% to 93.5 % at 15 min and from 26.7% to 100.5% at 30 min, while the disintegration time ranged from under 1 to over 20 min. However, no relationship between tablet dissolution and tablet disintegration time was observed across the 24 set of tablets (Fig. 2).
Fig. 2.
Variation in the tablet dissolution in 15 min (closed diamond) and at 30 min (open square) as function of the tablet disintegration time. Results are expressed as mean ± standard deviation for n = 6
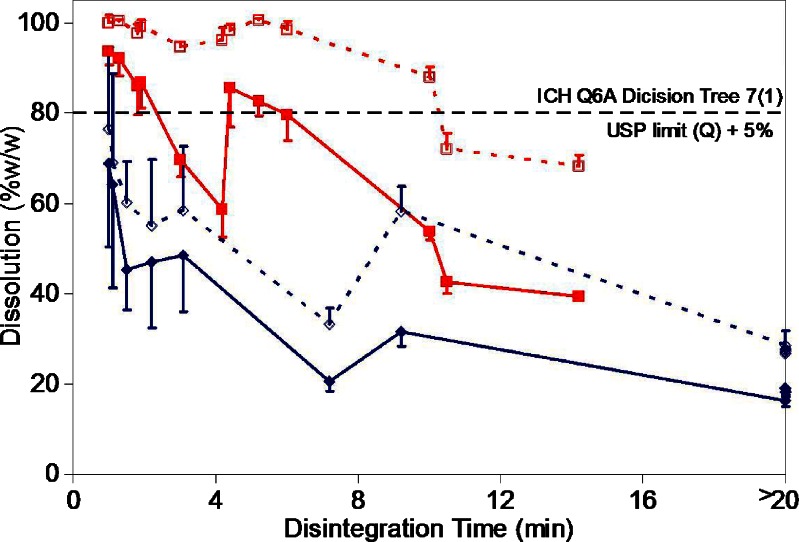
Fourteen out of 24 set of tablets failed to meet the USP dissolution criterion of >80% (Q + 5%) dissolution in 30 min for the verapamil tablets (4). These included all 12 set of tablets prepared using DCP as the filler. Only two set of tablets containing LMH as the filler failed to meet the USP dissolution requirement. These tablets were formulated using HPMC as the binder and were without any disintegrating agent and compressed at the low and the high hardness settings (VLHX1 and VLHX2). The remaining ten set of tablets containing LMH as the filler showed >85% dissolution at 30 min. These results show a strong dependence of dissolution on the type of filler used to prepare the tablets. Statistical analysis of the dissolution results confirmed a significant faster dissolution (p < 0.0001) at 30 min in presence of LMH as filler. A statistically significant faster disintegration (p < 0.0002) was also observed for tablets prepared using LMH as the filler as compared to those prepared using DCP as the filler (Fig. 3).
Fig. 3.
Relationship between disintegration time and the tablet dissolution in 15 min (closed symbols) and at 30 min (open symbols) as function of filler used in the formulation (red squares—LMH; blue diamonds—DCP). Results are expressed as mean ± standard deviation for n = 6
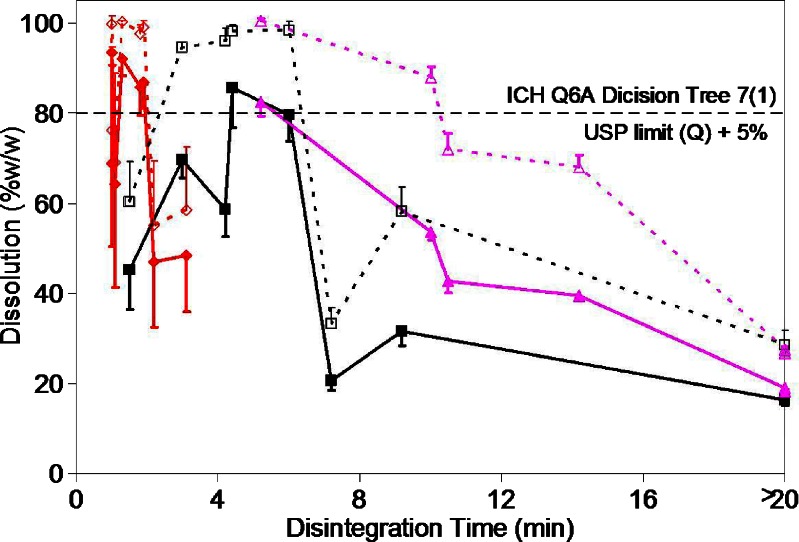
A significant influence of disintegrating agent was also observed on the tablet dissolution (p < 0.002) and the disintegration time (p < 0.0001; Fig. 4). Although all tablets formulated using NaCMC disintegrated in less than 3 min, those containing DCP as the filler failed to meet the USP dissolution criterion of >80% (Q + 5%) dissolution in 30 min. On the other hand, tablets formulated without any disintegrating agent required at least 5 min for complete disintegration, yet met the USP dissolution criterion when formulated using LMH as the filler and PVP as the binder. Disintegrating agent accelerates tablet disintegration into smaller fragments increasing the surface area exposing to the medium for dissolution of the drug to occur. The results highlight the importance and influence of other formulation components, e.g., filler, binder, etc., on the dissolution process and cautions against relying solely on the disintegrating agent to accelerate tablet dissolution
Fig. 4.
Relationship between disintegration time and the tablet dissolution in 15 min (closed symbols) and at 30 min (open symbols) as function of disintegrating agent used in the formulation (red diamond—NaCMC; black squares—MCC; pink triangles—none). Results are expressed as mean ± standard deviation for n = 6
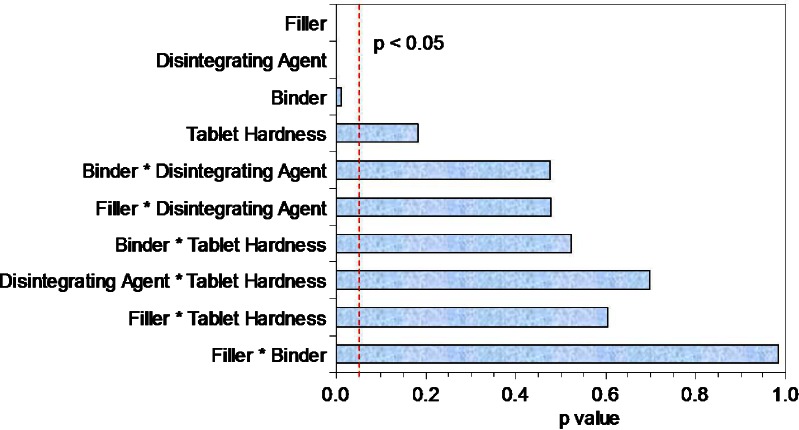
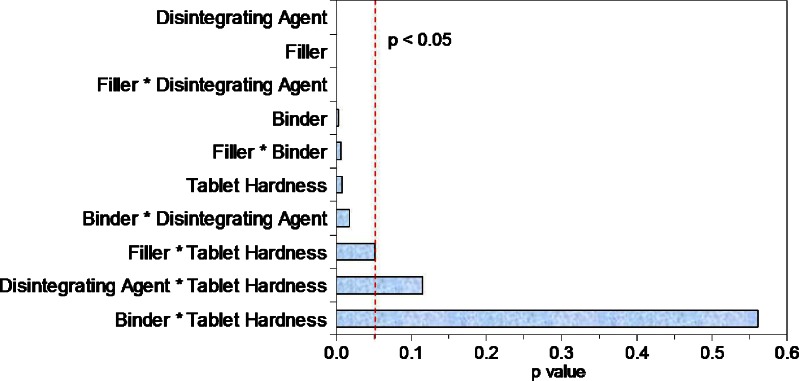
Tablets formulated using PVP showed faster dissolution (p < 0.015) and faster disintegration (p < 0.004) as compared with those formulated using HPMC as the binder. Although no influence of hardness was observed on the dissolution, tablets compressed to lower hardness disintegrated faster as compared to those compressed to higher hardness values (p < 0.02). Significant influences of filler, disintegrating agent, and binder were also observed on the tablet dissolution at 15 min (p < 0.0001, p < 0.002, and p < 0.012, respectively) as shown in Pareto plot in Fig. 5 confirming the strong correlation of the tablet dissolution with these three variables. Significant two-way interaction effects between filler-disintegrating agent, filler-binder, and binder-disintegrating agent (p < 0.0005, p < 0.01, p < 0.03, respectively) were observed on the tablet disintegration time (Fig. 6). The presence of these two-way interactions resulted in slower disintegration and hence slower dissolution of the VLPA1 and VLPA2 tablets as compared to the VLHA1 and VLHA2 tablets, although the effect analysis of individual variable showed faster dissolution and faster disintegration for tablets formulated using PVP as the binder. These results highlight the importance of understanding the effects of interaction occurring among different formulation components in addition to identifying their individual effects and are in line with the Food and Drug Administration’s quality-by-design initiative (6).
Fig. 5.
Pareto plot for dissolution at 15 min. A p value of <0.05 was considered statistically significant
Fig. 6.
Pareto plot for disintegration time. A p value of <0.05 was considered statistically significant
Out of the 12 formulations, tablets prepared from two formulations at both hardness settings (VLHA1, VLHA2, VLPA1, and VLPA2) had disintegration time of less than 2 min and average dissolution of >80% within 15 min when tested under the USP30 conditions. However, one tablet each of VLPA1 and VLPA2 had dissolution <80% at 15 min. One of the ICH Q6A Decision Trees #7(1) criterion for using disintegration test for drug release is >80% dissolution in 15 min across the physiological pH range (pH 1.2–6.8). Dissolution testing of these four set of tablets in pH 1.2, 4.0, and 6.8 buffer solutions also gave similar results. Dissolution of all VLHA1 and VLHA2 tablets exceeded 80%, while at least one tablet of VLPA1 and VLPA2 had dissolution <80% at 15 min under each of the three pH conditions making only the VLHA1 and VLHA2 tablets suitable for replacing, dissolution testing by disintegration testing.
CONCLUSIONS
A significant influence of different formulation components was observed on the tablet dissolution and disintegration with the filler and disintegrating agent exerting the most significant influence. At constant filler or disintegrating agent, an increase in disintegration time led to slower tablet dissolution. However, no relationship was observed between the disintegration and dissolution across all formulations due to the interactions between different formulation components. Tablets, prepared at both hardness settings, of only one out of the 12 formulations met the ICH Q6A criteria of >80% dissolution across the physiological pH range between 1.2 and 6.8. These tablets were formulated using LMH as filler, HPMC as binder, NaCMC as disintegrating agent, and may be suitable for using the disintegration test instead of the dissolution test as the drug product acceptance criteria. Thus, systematic studies are needed to determine the relationship between the disintegration and dissolution before using the disintegration test, instead of the dissolution test as the drug acceptance criteria.
Abbreviations
- DCP
Dicalcium phosphate dihydrate
- HPMC
Hypromellose
- ICH
International Conference on Harmonization
- LMH
Lactose monohydrate
- MCC
Microcrystalline cellulose
- NaCMC
Sodium carboxymethyl cellulose
- PVP
Polyvinyl pyrollidone
- USP
United States Pharmacopeia
- VLHX1
Tablets containing LMH, HPMC, without disintegrating agent, and hardness of 6 Kgf
- VLHX2
Tablets containing LMH, HPMC, without disintegrating agent, and hardness of 10 Kgf
- VLPA1
Tablets containing LMH, PVP, NaCMC, and hardness of 6 Kgf
- VLPA2
Tablets containing LMH, PVP, NaCMC, and hardness of 10 Kgf
- VLHA1
Tablets containing LMH, HPMC, NaCMC, and hardness of 6 Kgf
- VLHA2
Tablets containing LMH, HPMC, NaCMC, and hardness of 10 Kgf
Footnotes
The opinions expressed in this work are only of authors and do not necessarily reflect the policy and statements of the FDA.
References
- 1.United States Pharmacopeia 30/National Formulary 25. 2008. The U.S. Pharmacopeial Convention, Inc. Rockville, MD. (www.USP.org).
- 2.ICH Q6A Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances. Available at www.ich.org. Accessed January 24, 2008. [PubMed]
- 3.Sayeed V. Disintegration test as a surrogate for dissolution: some practical considerations. In: AAPS Workshop on the Role of Dissolution in QbD and Drug Product Life Cycle. Arlington, VA: AAPS Workshop, 2008.
- 4.Vogelpoel H, Welink J, Amidon GL, Junginger HE, Midha KK, Moller H, et al. Biowaiver monographs for immediate release solid oral dosage forms based on Biopharmaceutics Classification System (BCS) literature data: verapamil hydrochloride, propranolol hydrochloride, and atenolol. J Pharm Sci. 2004;93:1945–1956. doi: 10.1002/jps.20131. [DOI] [PubMed] [Google Scholar]
- 5.Verapamil hydrochloride tablets monograph. United States Pharmacopeia 30/National Formulary 25. 2008. The U.S. Pharmacopeial Convention, Inc. Rockville, MD. (www.USP.org).
- 6.Chen C. A FDA perspective on Quality by Design. Pharmtech, Dec 5, 2007. Available at http://pharmtech.findpharma.com/pharmtech/Article/A-FDA-Perspective-on-Quality-by-Design/ArticleStandard/Article/detail/469915. Accessed May 15, 2008.