Abstract
The objective of this study was to synthesize anhydride prodrugs for carboxylic-acid-bearing agents such as non-steroidal anti-inflammatory drugs, shield the carboxylic acid group from irritative effects, and obtain sustained release patterns. Ibuprofen was used as a representative drug for anhydride derivatization. Conjugates of ibuprofen with carboxylic acid moieties of different acrylic polymers were prepared by dehydration reaction using acetic anhydride. Products were characterized by infrared spectroscopy, nuclear magnetic resonance, and scanning electron microscopy followed by preparation of microspheres with different sizes from the conjugate Eudragit® L-100-ibuprofen. The drug release was monitored by high-performance liquid chromatography. Ibuprofen was bound to the polymers via an anhydride bond in high reaction yields (75–95%) with drug loading of up to 30% (w/w). These anhydride derivatives hydrolyzed and release the drug at different periods ranging from 1 to 5 days, depending on the hydrophobicity and the cross-linking of the conjugates. The release of drug from the microspheres was correlated to their size and ranged from 2 to almost 8 days. This study demonstrates the promise of anhydride prodrug for extending drug action while shielding the carboxylic acid group.
Key words: anhydride, controlled release, ibuprofen, microspheres, prodrugs
INTRODUCTION
A wide variety of compounds having carboxylic acid groups are biologically active, for example, the non-steroidal anti-inflammatory drugs, such as ibuprofen, naproxen, indomethacin, and diclofenac. The presence of free acid groups in these compounds produces local irritation on interaction with mucosal tissues, and at the same time, they ionize at physiological pH, which makes the drug poorly absorbed through biological membranes (1).
The general approach to overcome these limitations is esterification of the carboxylic acid groups to produce non-irritating prodrug forms, provided that the parent bioactive agent can be released from the prodrug at its sites of activity (2). However, several aliphatic and aromatic esters of carboxylic acid drugs are not sufficiently labile in vivo to ensure a sufficiently high rate and extent of prodrug conversion (3,4). As a result, plasma enzymes may not hydrolyze there esters fast enough to obtain the necessary conversion of the ester to the free acid (5). In another consideration, ester derivative prodrugs have been found cleavable enzymatically to release their bioactive forms and, thus, are dependent on the enzymatic activity, which may vary among individuals or even in the same individuals at various times during the day or in various sites where the drug is administered. This fact may result in a large variation of drug bioavailability (6,7). To overcome the aforementioned limitations, anhydride derivatives of carboxylic-acid-bearing drugs are suggested.
Unlike the ester bond used in prodrugs, the anhydride bond is more susceptible to hydrolysis to its carboxylic acid counterparts in a predictable rate and pattern and is less sensitive to enzymolysis than the esters or amides (8). Studies on polyanhydrides as drug carriers show that they degrade in a controlled manner and are biocompatible with the human body tissues (9). Interestingly, not much attention has been given to the formation of prodrugs based on the hydrolytically degradable anhydride bonds, although biodegradable polymers based on these bonds have been developed and used as erodible carriers for drugs both in animals and in humans (10).
Our previous study (11) focused on the synthesis of mixed anhydrides of ibuprofen with various fatty acids for improved bioavailability. It was found that anhydride conjugated drugs having longer alkyl chain provide higher absorption through lipid–water membrane. In addition, this anhydride prodrug approach can be applied for manipulating the release rate of a free drug by using different types of fatty acids.
Polyacrylic acid (PAA)-based polymers are largely used in various technological applications due to their viscoelastic properties, bioadhesiveness, safety, and easy administration. They can be found in drug delivery systems (12), mucoadhesive forms (13), synthesis of nanoparticles by the sol–gel method (14), and in ophthalmic and vaginal preparations (15), to name a few of their diverse applications. Eudragit® L 100 and Eudragit® S 100 are anionic copolymers based on methacrylic acid and methyl methacrylate. The ratio of the free carboxyl groups to the ester groups is approximately 1:1 in Eudragit® L 100 and approximately 1:2 in Eudragit® S 100. Their average molecular weight is approximately 135,000. Eudragit acrylic resins are widely used in enteric coated and controlled release formulations due to their pH-dependent solubility (16,17).
The objective of this work was to design a prodrug that relies on anhydrides of ibuprofen with carboxylic-acid-bearing polymers. These prodrugs are predicted to be less irritating to stomach or intestinal mucosal membranes and can be used for various topical dosage forms.
MATERIALS AND METHODS
Materials
Ibuprofen was a gift from Ethyl Corp. (NJ, USA). Acetic anhydride was purchased from Aldrich (Milwaukee, WI, USA). PAA with Mw of 2,000 and 5,000 Da and phenolphthalein solution and polyvinyl alcohol (PVA, 30–70 kDa) were purchased from Sigma-Aldrich (Rehovot, Israel). Eudragit® S-100 and L-100 were purchased from Rohm Pharma (GmbH, Germany). Cross-linked polyacrylic acid (Carbopol 934) was obtained from Goodrich Co., Ltd. (Cleveland, OH, USA). All solvents were analytical grade from Biolab (Jerusalem, Israel) and were used as received.
Synthesis of Mixed Anhydrides
Linear Polymers
Polymer powder (1 g) and ibuprofen (0.25 g) were refluxed in acetic anhydride (10 mL) under dried nitrogen atmosphere for 3 h followed by evaporation of the acetic anhydride using an oil pump (0.3 mmHg at 70°C). The products were dissolved in dichloromethane and precipitated from dry diethyl ether. The final product was received after drying in a desiccator for 6 h.
Cross-linked Polymer
Cross-linked PAA (1 g) and ibuprofen (0.25 g) were refluxed in acetic anhydride (10 mL) and dichloromethane (10 mL) for 6 h followed by evaporation of the acetic anhydride using an oil pump (0.3 mmHg at 70°C). The dry residue was washed several times with dry diethyl ether to remove access of ibuprofen symmetric anhydride. Then, the powder was left to dry in a desiccator for 3 h. Cross-linked PAA-based anhydride was received as off-white powder.
Preparation of Ibuprofen Anhydride
Ibuprofen (10 g) was refluxed in acetic anhydride (100 mL) for 3 h followed by the evaporation of acetic anhydride to dryness, and the residue was distilled under vacuum (0.3 mmHg at 70°C) to yield (70%) a viscous liquid of symmetric ibuprofen anhydride.
Characterization
Quantitative analysis of carboxylic acid groups was determined by dissolving 0.25 g of each of the polymers in sufficient amount of water (Eudragit® S-100 and L-100 were dissolved in 3:1 DDW/ethanol solution) followed by titration with 0.1 volumetric solution of sodium hydroxide using phenolphthalein solution as indicator. After a blank titration was performed to each of the solvents, the amount of free carboxylic acids was calculated.
Infrared (IR) spectroscopy (Perkin-Elmer System 2000 FT-IR) was performed on polymers and conjugates. Conjugates were measured on NaCl plates using dichloromethane as solvent. Each sample was recorded between 4000 and 500 cm−1 by 16 scans. 1H-NMR spectra were recorded on a Varian 300-MHz instrument using DMSO-D6 or CDCl3 as solvents. Values were recorded as parts per million relative to internal standard (TMS). Drug loading was detected after hydrolyses in 1 N NaOH for 24 h. The free drug content was detected by high-performance liquid chromatography (HPLC) at 270 nm.
Ibuprofen Analysis
Quantitative analysis of ibuprofen was performed by HPLC using a C18 Lichospher 100 column (Merck, Dorstadt, Germany, 250 × 4 mm, 5 μm) using a 70:30 (v/v) mixture of acetonitrile/water and 0.2% phosphoric acid as mobile phase at a flow rate of 1 mL/min. Ibuprofen was detected by UV at 270 nm, and its typical retention time was 4.9 min.
In Vitro Release of Ibuprofen
Thirty milligrams of each conjugate was suspended in a vial containing 30 mL of 0.1 M, pH 7.4, phosphate buffer. The vials were incubated at 37°C with constant shaking at 75 rpm. At time intervals, each vial was centrifuged at 3,000 rpm for 5 min and samples (100 μL) from the releasing medium were withdrawn for HPLC analysis. The withdrawn volume was replaced with fresh buffer followed by resuspending before continuing incubation. The results are a mean of three experiments.
Preparation of Microspheres
Ibuprofen-loaded microspheres were produced from Eudragit L-100 anhydride conjugate loaded with 4.2% (w/w) ibuprofen. One hundred milligrams of the conjugate was dissolved in 1 mL dichloromethane. The solution was dispersed in 300 mL of 1% (w/v) aqueous solution of PVA (pH 2). The resulting emulsion was stirred for 3 h at 750 rpm by a mechanical stirrer. Microspheres were then collected by centrifugation and dried at room temperature under vacuum until required for use.
In a second assay, conjugate of the same batch was dissolved in dichloromethane (100 mg in 1 mL). This was then emulsified in an aqueous phase (300 mL, pH 2) of a 1% (w/v) solution of PVA using a homogenizer Silverson L4R (Silverson Machines, Ltd., Bucks, UK) at 8,000 rpm for 30 min. Emulsion was left to mix by a magnetic stirrer in a hood for additional 3 h before centrifugation, collecting the microspheres and drying at room temperature under vacuum. Ibuprofen content was over 90%.
Characterization
The morphology of microspheres was analyzed by scanning electron microscopy (SEM, Quanta 2000). Before the examination, the surfaces were coated with a thin layer of gold so as to improve the conductivity and prevent charging.
Microsphere diameter and distribution size were calculated using Image Analyzer software (Digimizer -MedCalc Software, Mariakerke, Belgium).
The release pattern of each of the microspheres was analyzed as described in “In Vitro Release of Ibuprofen.”
Statistical Analysis
Statistical comparisons of the findings were made by one-way analysis of variance. Comparison of means was performed by the least significant difference test. Data analysis was performed using a statistical software package (Instat; GraphPad Software, San Diego, CA, USA). The significance level was set at p < 0.05.
RESULTS AND DISCUSSION
Synthesis of Mixed Anhydrides
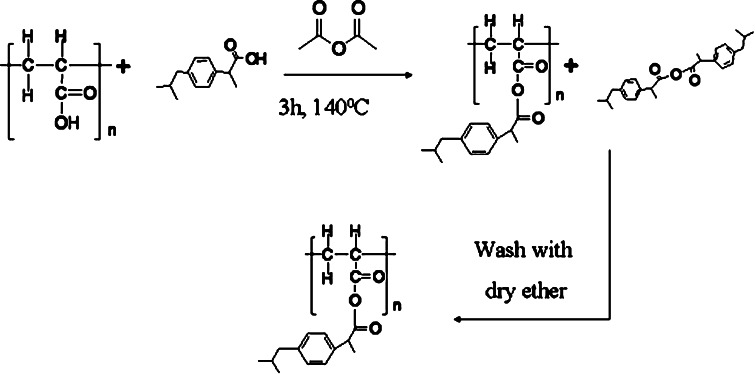
The common way of producing anhydride bonds is by activating the carboxylic acid with acetic anhydride [18]. Mixed anhydrides of ibuprofen and carboxylic acid moieties of acrylic polymers were synthesized from the corresponding polymers in acetic anhydride for 3 h (Fig. 1). All mixed anhydrides were solid at room temperature. The anhydride parameters are summarized in Table I.
Fig. 1.
a Schematic illustration of the methods for the synthesis of anhydrides conjugate
Table I.
Anhydride’s Parameters
| Polymer in conjugate | Polymer weight (g) | Ibuprofen (g) | Yield (%) | Drug loadinga (% w/w) | Ibuprofen to carboxylic acidb (%) |
|---|---|---|---|---|---|
| PAA-2000 | 1 | 0.05 | 82 | 1.1 | 0.5 |
| PAA-2000 | 1 | 0.25 | 73 | 7.8 | 4.3 |
| PAA-2000 | 1 | 1 | 34 | 14.1 | 8.3 |
| PAA-2000 | 1 | 4 | 11 | 30.8 | 22.7 |
| PAA-5000 | 1 | 0.25 | 77 | 6.2 | 5 |
| Eudragit L-100 | 1 | 0.25 | 95 | 4.2 | 4.1 |
| Eudragit S-100 | 1 | 0.25 | 94 | 3.6 | 5.8 |
| Cross-linked PAA | 1 | 0.25 | 88 | 7.3 | 4.2 |
aCalculated after hydrolysis in 1 N NaOH followed by drug detection by HPLC
bCalculated after quantitative analysis of carboxylic acid groups of each of the polymers followed by titration with 0.1 volumetric solution of sodium hydroxide using phenolphthalein solution as indicator
The production yields of the anhydride conjugate were between 73% and 95% for the 1:0.25 polymers to drug feed ratio. As can be seen in Table I, increasing the ratio of drug to polymer considerably decreases the yield and increases the drug loading and the drug to carboxylic acid ratio. The rank order of drug loading was PAA > cross-linked PAA > Eudragit® L-100 > Eudragit® S-100, corresponding to the amount of carboxylic acids in the polymer, its solubility, and the Mw of the polymers.
The percentage of drug loading and the ibuprofen to carboxylic acid ratio increases as drug concentration increases in the reaction. For the PAA with Mw of 2,000, ibuprofen loading score increased rapidly at concentrations below 0.25 to 1 g polymer and less at higher concentrations.
Characterization
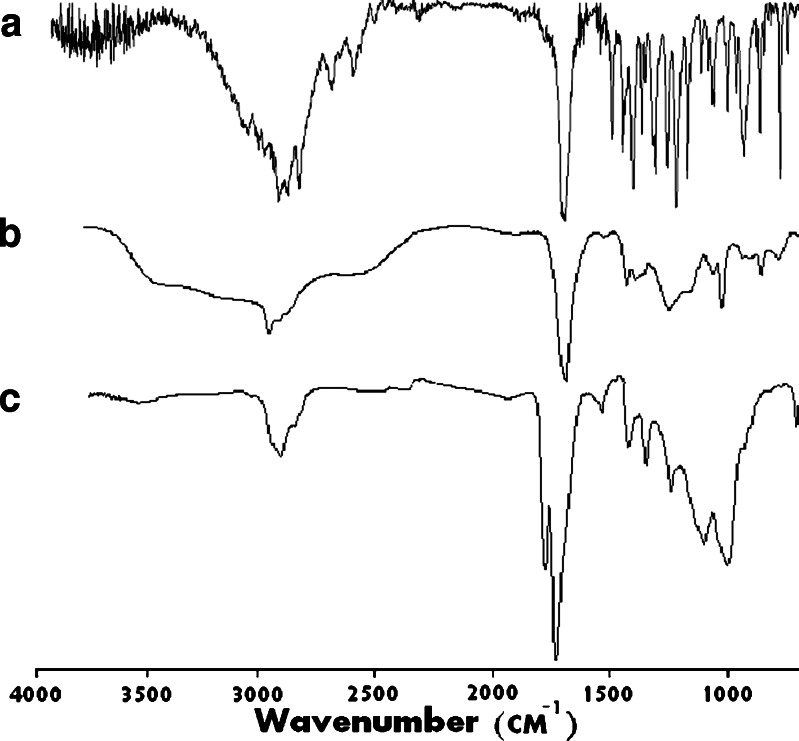
Figure 2 shows the IR spectra of ibuprofen, PAA-2000, and their mixed anhydride containing 7.8% ibuprofen. It can be seen that while the IR spectra of the drug and the polymer show carbonyl absorbance at 1,710 cm−1, the mixed anhydride demonstrate anhydride absorbance at 1,800 and 1,780 cm−1. Additionally, the stronger carboxylic acid absorbance of the parent polymer at 3,500–2,500 cm−1 comparing to the weak peak of the conjugate confirms anhydride bond formation. Similar results were also reported by Jiang et al. (19).
Fig. 2.
Infrared spectrum (NaCl method) of a ibuprofen; b polyacrylic acid (M w 2,000 Da); and c anhydride-based conjugate from ibuprofen and polyacrylic acid (M w 2,000 Da)
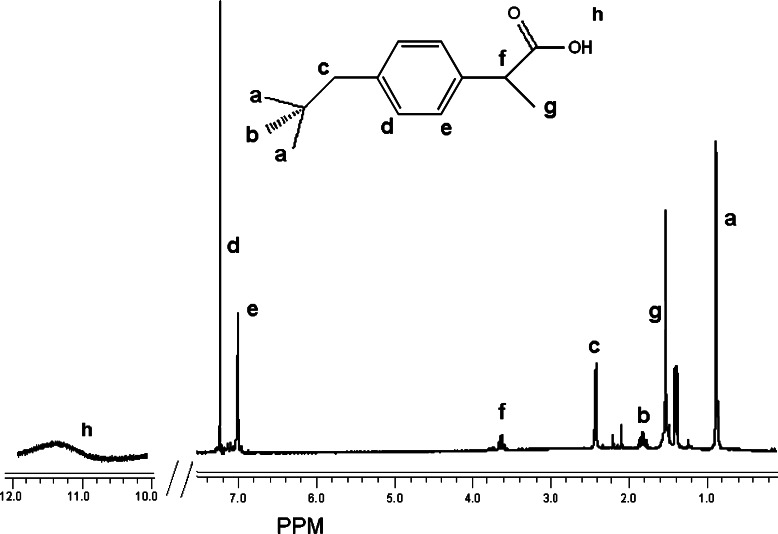
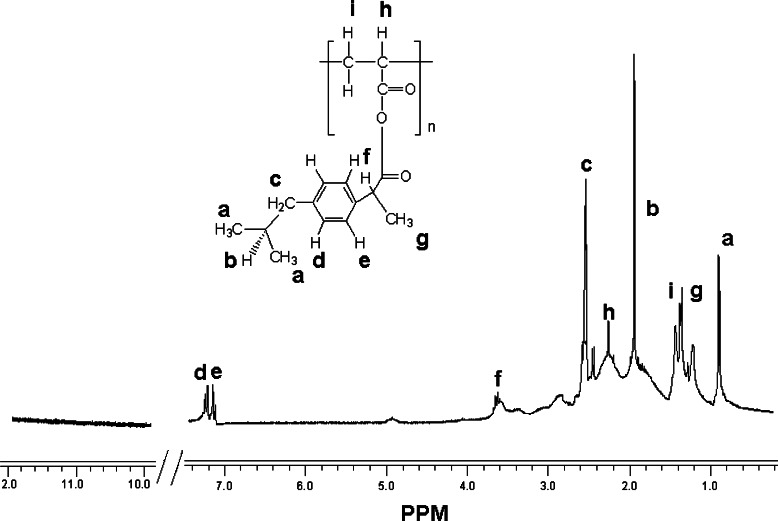
The 1H-NMR spectra of ibuprofen and conjugate from ibuprofen and PAA-2000 are shown in Figs. 3 and 4, respectively. While the spectrum of the conjugate correlates with the NMR data of the free ibuprofen, its carboxylic acid’s hydrogen peak at 11.6 ppm was not observed, suggesting the formation of anhydride.
Fig. 3.
1H NMR spectra (in CDCl3) of ibuprofen
Fig. 4.
1H NMR spectra (in DMSO-D6) of anhydride-based conjugate from ibuprofen and polyacrylic acid 2,000 Da
When ibuprofen was exclusively refluxed in acetic anhydride (10 mL) followed by the evaporation of the acetic anhydride, a yield of 75% was obtained. This symmetric ibuprofen anhydride product was received as a viscous liquid soluble in ether. Similar to the spectra of the conjugate, its NMR spectra (not shown) revealed the disappearance of the carboxylic acid’s hydrogen at 11.6 ppm. This symmetric ibuprofen anhydride had the following parameters: IR, (cm−1) 1,812, 1,748; 1H NMR (300 MHz, CDCl3), 7.14–7.01 (m, 8H), 3.71–3.62 (m, 2H), 2.45 (d, J = 7.2 Hz, 4H), 1.92–1.79 (m, 2H), 1.43 (dd, J = 4.2, 7.2 Hz, 4H), and 0.91 (d, 6.6 Hz, 12H).
Release of Ibuprofen
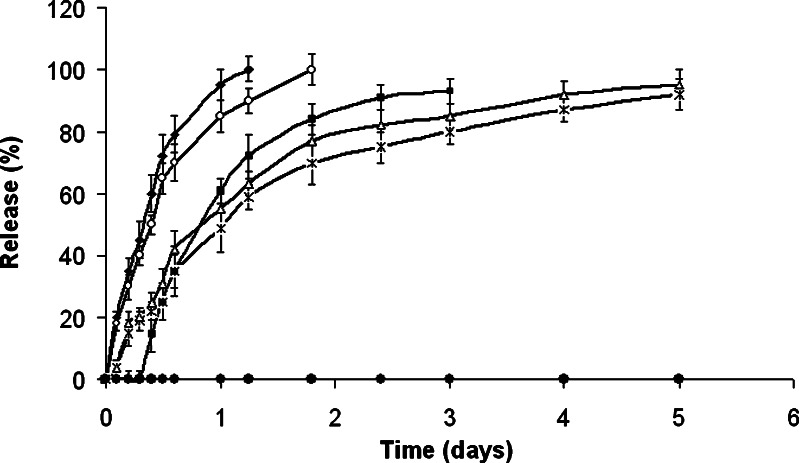
The release properties of ibuprofen from these mixed anhydrides were examined in vitro in a 0.1 M, pH 7.4, phosphate buffer at 37°C. As can be seen in Fig. 5, the release rate of ibuprofen was a function of the polymer chain length, hydrophilicity, and cross-linking. Typically, 80% of the drug was constantly released for 14 h from the ibuprofen–PAA 2000 derivative, whereas only 35% from the Eudragits L-100 and the cross-linked PAA during the same time period. PAA 2000 and 5000 showed similar pattern of release with a total release of the ibuprofen within 30 and 38 h, respectively. In the same manner, the Eudragit copolymers showed comparable patterns with release of almost 90% of the drug content within 5 days.
Fig. 5.
In vitro release of ibuprofen from anhydride conjugates of ibuprofen and polymers as measured by high-performance liquid chromatography. The polymers were: polyacrylic acid 2,000 Da (diamond), polyacrylic acid 5,000 Da (open circle), cross-linked polyacrylic acid (square), Eudragit® L-100 (triangle), Eudragit® S-100 (ex), polyacrylic acid 2,000 Da at pH 2 (filled circle). Experiments were performed in phosphate buffer (pH 7.4) at 37°C
A comparison study of these anhydrides in buffer pH 2 was also performed; however, no ibuprofen could be monitored (representative PAA-2000 mix anhydride in pH 2 is shown in Fig. 5). This can be explained by the slower anhydride hydrolysis (20,21) and the poor solubility of ibuprofen in acidic solutions (11).
Preparation of Microspheres, Characterization, and Release
Ibuprofen-loaded microspheres were produced using conjugate of Eudragit L-100 loaded with 4.2% (w/w) ibuprofen. The technique involved oil-in-water emulsion followed by the solvent evaporation method (22). Particle size decreased from 90.1 ± 1 to 5 ± 0.3 μm with the increase of stirring speed from 750 to 8,000 rpm, respectively.
SEM photomicrographs of the conjugate and its microspheres are depicted in Fig. 6a–c. Figure 6a represents the conjugate before the formation of microspheres. It can be seen that the particles present nodular cluster morphology with varying density networks. On the other hand, microspheres made from the same conjugate are spherical and smooth, as seen in Fig. 6b, c.
Fig. 6.
Scanning electron microscopy conjugate anhydride. a Eudragit L-100 loaded with 4.2% (w/w) of ibuprofen. Microspheres prepared from the same conjugate as in a with a diameter of b 5 and c 100 μm
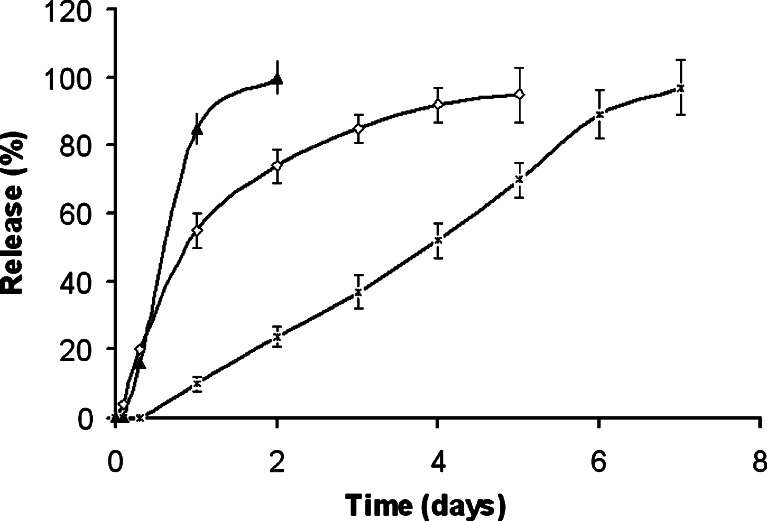
Figure 7 shows the drug release profiles from the conjugate and the microspheres. The drug in 5-μm microspheres was rapidly released within 2 days. This was likely due to the increased surface-to-volume ratio of the smaller particles. Non-microspheric conjugates moderately sustained the drug release to 5 days, whereas 100-μm particles could prolong to almost 8 days.
Fig. 7.
In vitro release from anhydride conjugate from Eudragit L-100 loaded with 4.2% (w/w) ibuprofen as measured by high-performance liquid chromatography. Microspheres of 5 μm in diameter (triangle), conjugate as received (diamond), microspheres of 100 μm in diameter (ex). Experiments were performed in phosphate buffer (pH 7.4) at 37°C
The results obtained suggest that anhydride-based prodrugs may overcome some of the problems of carboxylic-acid-bearing drugs mentioned in “INTRODUCTION”. First, protecting the carboxylic function can be temporarily masked by anhydride bond to overcome stomach and mucosal irritation. Secondly, this approach can be applied for extended action/release of acidic drugs after administration to the eye, skin, vagina, or oral intake where these polymers are already widely used. Thus, using these polymers is ideal due to their contribution to various systemic and topical delivery systems.
CONCLUSIONS
This work provides a simple synthetic method for producing anhydride-based conjugates of carboxylic-acid-bearing drugs and carboxylic-acid-bearing polymers. In this approach, the release rate of a free drug can be manipulated using different types of polymer and/or by controlling the particle size. For systemic or localized delivery via oral administration, a sustained release of drug can be obtained with the only degradation by-product being commonly used biocompatible biopolymer. Nevertheless, chemical and physical stability in biological relevant media as well as clinical studies are required in order to assess the healing potential of these conjugates. Further in vitro and in vivo evaluations are required to investigate the therapeutic potential of the above-described anhydride-based drug conjugates.
Acknowledgments
We thank Dr. Alfonso Bentolila and Dr. Christopher Levins for the help in NMR data analysis.
References
- 1.Bruesewitz C, Funke A, Kuhland U, Wagner T, Lipp R. Comparison of permeation enhancing strategies for an oral factor Xa inhibitor using the Caco-2 cell monolayer model. Eur J Pharm Biopharm. 2006;64(2):229–237. doi: 10.1016/j.ejpb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Bundgaard H. Design of prodrugs. Amsterdam: Elsevier; 1985. [Google Scholar]
- 3.Harrison IT, Lewis B, Nelson P, Rooks W, Roszkows A, Tomoloni A, et al. Nonsteroidal antiinflammatory agents. 1. 6-Substituted 2-naphthylacetic acids. J Med Chem. 1970;13(2):203–205. doi: 10.1021/jm00296a008. [DOI] [PubMed] [Google Scholar]
- 4.Child RG, Osterberg AC, Sloboda AE, Tomcufcik AS. Fenbufen, a new anti-inflammatory analgesic—synthesis and structure–activity-relationships of analogs. J Pharm Sci. 1977;66(4):466–476. doi: 10.1002/jps.2600660403. [DOI] [PubMed] [Google Scholar]
- 5.Hansen LB, Christrup LL, Bundgaard H. Ketobemidone prodrugs for buccal delivery—prediction of the extent of saliva-catalyzed hydrolysis of various ester prodrugs under simulated in vivo conditions. Int J Pharm. 1992;88(1–3):229–235. doi: 10.1016/0378-5173(92)90320-2. [DOI] [Google Scholar]
- 6.Goetz RM, Holtz J. Enhanced angiotensin-converting enzyme activity and impaired endothelium-dependent vasodilation in aortae from hypertensive rats: evidence for a causal link. Clin Sci. 1999;97(2):165–174. doi: 10.1042/CS19990003. [DOI] [PubMed] [Google Scholar]
- 7.Higashikawa F, Murakami T, Kaneda T, Kato A, Takano M. Dose-dependent intestinal and hepatic first-pass metabolism of midazolam, a cytochrome P450 3A substrate with differently modulated enzyme activity in rats. J Pharm Pharmacol. 1999;51(1):67–72. doi: 10.1211/0022357991771971. [DOI] [PubMed] [Google Scholar]
- 8.Kumar N, Edlund U, Teomim D, Rasiel A, Domb AJ. Biopolymers: polyesters (Part-B) Germany: Wiley-VCH; 2002. [Google Scholar]
- 9.Domb AJ, Israel ZH, Elmalak O, Teomim D, Bentolila A. Preparation and characterization of carmustine loaded polyanhydride wafers for treating brain tumors. Pharm Res. 1999;16(5):762–765. doi: 10.1023/A:1011995728760. [DOI] [PubMed] [Google Scholar]
- 10.Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Prog Polym Sci. 2007;32(8–9):762–798. doi: 10.1016/j.progpolymsci.2007.05.017. [DOI] [Google Scholar]
- 11.Shaaya O, Magora A, Sheskin T, Kumar N, Domb AJ. Anhydride prodrugs for nonsteroidal anti-inflammatory drugs. Pharm Res. 2003;20(2):205–211. doi: 10.1023/A:1022214919481. [DOI] [PubMed] [Google Scholar]
- 12.Mizrahi B, Domb AJ. Mucoadhesive tablet releasing iodine for treating oral infections. J Pharm Sci. 2007;96(11):3144–3150. doi: 10.1002/jps.20876. [DOI] [PubMed] [Google Scholar]
- 13.Mizrahi B, Golenser J, Wolnerman JS, Domb AJ. Adhesive tablet effective for treating canker sores in humans. J Pharm Sci. 2004;93(12):2927–2935. doi: 10.1002/jps.20193. [DOI] [PubMed] [Google Scholar]
- 14.Michalski S. Cleaning, retouching and coatings. Preprints to the contributions to the Brussels congress. London; 1990.
- 15.Weyenberg W, Bozdag S, Foreman P, Remon JP, Ludwig A. Characterization and in vivo evaluation of ocular minitablets prepared with different bioadhesive Carbopol–starch components. Eur J Pharm Biopharm. 2006;62(2):202–209. doi: 10.1016/j.ejpb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Bonacucina G, Palmieri GF. Acrylic polymers as thickening agents for tetraglycol cosolvent. J Pharm Sci. 2006;95(4):726–736. doi: 10.1002/jps.20567. [DOI] [PubMed] [Google Scholar]
- 17.Wade A, Weller P. Handbook of pharmaceutical excipients. London: The Pharmaceutical Press; 1994. [Google Scholar]
- 18.Fessenden RJ, Fessenden JS. Organic chemistry. 6. Pacific Grove, CA: Brooks/Cole; 1998. [Google Scholar]
- 19.Jiang HL, Zhu KJ. Synthesis, characterization and in vitro degradation of a new family of alternate poly(ester-anhydrides) based on aliphatic and aromatic diacids. Biomaterials. 2001;22(3):211–218. doi: 10.1016/S0142-9612(00)00176-9. [DOI] [PubMed] [Google Scholar]
- 20.Ege SN. Organic chemistry—structure and reactivity. Lexington, MA: Heath & Co.; 1994. [Google Scholar]
- 21.Liu WP, Lee HK. Degradation study of unsaturated anhydrides by reversed-phase high-performance liquid chromatography. J Chromatogr A. 1998;805(1–2):109–118. doi: 10.1016/S0021-9673(97)01269-7. [DOI] [Google Scholar]
- 22.Andersson N, Kronberg B, Corkery R, Alberius P. Combined emulsion and solvent evaporation (ESE) synthesis route to well-ordered mesoporous materials. Langmuir. 2007;23(3):1459–64. doi: 10.1021/la0622267. [DOI] [PubMed] [Google Scholar]