Abstract
The objective of the present investigation was to develop and evaluate microemulsion-based gel for the vaginal delivery of clotrimazole (CMZ). The solubility of CMZ in oils and surfactants was evaluated to identify components of the microemulsion. The ternary diagram was plotted to identify the area of microemulsion existence. Various gelling agents were evaluated for their potential to gel the CMZ microemulsion without affecting its structure. The bioadhesive potential and antifungal activity of the CMZ microemulsion-based gel (CMZ-MBG) was determined in comparison to the marketed clotrimazole gel (Candid-V® gel) by in vitro methods. The chemical stability of CMZ in CMZ-MBG was determined as per the International Conference on Harmonization guidelines. The CMZ microemulsion exhibited globule size of 48.4 nm and polydispersity index of 0.75. Carbopol® ETD 2020 could successfully gel the CMZ microemulsion without disturbing the structure. The CMZ-MBG showed significantly higher (P < 0.05) in vitro bioadhesion and antifungal activity as compared to that of Candid-V® gel. The stability studies indicated that CMZ undergoes acidic pH-catalyzed degradation at all the storage conditions at the end of 3 months.
Key words: clotrimazole, microemulsion, microemulsion-based vaginal gel, stability studies, vaginal delivery
INTRODUCTION
Vulvovaginal candidiasis is one of the most common gynecological disorders. Approximately 75% of women experience vulvovaginal candidiasis during their life and about 40% to 50% of them suffer from multiple episodes (1,2). For the treatment of vulvovaginal candidiasis, local treatment with antifungal agents is a first resort. The local (vaginal) delivery not only gives site-specific treatment but also avoids toxic side effects of antifungal agents that are encountered on oral administration. The most commonly prescribed treatment for vaginal candidiasis has been the topical application of clotrimazole (CMZ), an imidazole antifungal agent. CMZ is known to be very effective locally and presents no major side effects (3). Clotrimazole is available in several conventional dosage forms such as creams, gels, pessaries, and ovules for vaginal application. However, these conventional dosage forms do not offer prolonged duration of action which compromises the efficacy of the CMZ (4). Furthermore, poor water solubility of CMZ (0.49 μg/ml) (5) also presents a hindrance for the local availability of CMZ and limits the effective antifungal therapy. In order to overcome these disadvantages, several delivery strategies such as liposomes (4), microspheres (6), sustained-release bioadhesive tablets (7), and polycarbophil gels (8) have been proposed for the vaginal delivery of CMZ. However, potential of microemulsions has not been explored for the delivery of CMZ.
Microemulsions have demonstrated a great potential for improving the systemic and local bioavailability of an array of hydrophobic therapeutic agents (9–11). In view of this, the solubilization of CMZ in microemulsions would improve its vaginal availability. However, as discussed earlier, it is also essential to have a dosage form which adheres to the vaginal mucosa and increases the residence time of CMZ in vagina. This functionality can be imparted by gelling of the CMZ microemulsion using bioadhesive agent. The success of mucoadhesive microemulsions has already been described in the literature for the nasal delivery (12). Thus in the present investigation, formulation of CMZ microemulsion-based gel (CMZ-MBG) was attempted for vaginal delivery. The developed CMZ-MBG was evaluated for in vitro release, in vitro bioadhesive time and in vitro antifungal activity. Furthermore, the chemical stability of CMZ in the formulated gel was evaluated as per the International Conference on Harmonization (ICH) guidelines.
MATERIALS AND METHODS
Materials
Clotrimazole was kindly gifted by Cipla Pharmaceuticals Ltd., Mumbai, India. Cremophore- EL (polyoxyl 35 castor oil), Solutol HS 15 (macrogol 15 hydroxystearate; BASF India Ltd., Mumbai, India), Carbopol ETD-2020 (Noveon India Ltd., Mumbai, India), Gattefosse excipients such as Capryol 90 (propylene glycol monocaprylate), Plurol Oleique (polyglyceryl oleate), Lauroglycol 90 (propylene glycol monolaurate), Labracfac CC (caprylic–capric acid triglycerides), Labrafil 1944 CS (oleoyl macrogolglycerides), Methocel K4M (hydroproyl methylcellulose; Colorcon Asia Ltd., Mumbai, India), Manugel DMB (sodium alginate; Anshul Agencies Ltd., Mumbai, India), and Captex 8000 (glyceryl tricaprylate, Indchem International, Mumbai, India) were received as gift samples. Methanol (high-performance liquid chromatography (HPLC) grade), Tween 80, citric acid anhydrous, disodium hydrogen phosphate, chlorocresol, dimethyl sulfoxide (DMSO), and benzyl alcohol (all AR grade) were purchased from s.d. Fine Chemical Ltd., Mumbai, India. Sabaraud dextrose agar was purchased from HiMedia Ltd., Mumbai India.
Candid-V® gel (clotrimazole 2% w/w, Glenmark Pharmaceuticals Ltd., Mumbai, India), a marketed vaginal gel of clotrimazole was purchased from local market. Double-distilled water was used whenever required.
Solubility Studies
The solubility of CMZ in various oils and surfactants was determined by using shake-flask method (n = 3). Briefly, an excess amount of CMZ was added to each vial containing 5 ml of the selected vehicle, i.e., either oil or surfactant. After sealing, the mixture was vortexed using a cyclomixer for 10 min in order to facilitate proper mixing of CMZ with the vehicles. Mixtures were shaken for 72 h in an isothermal shaker (Remi, Mumbai, India) maintained at 37 ± 1°C. Mixtures were centrifuged at 5,000 rpm for 15 min, followed by filtration through membrane filter (0.45 μm, 13 mm, Pall Life sciences, Mumbai, India). The concentration of CMZ in the supernatant was determined by HPLC method.
HPLC Analysis of CMZ
The solubility of CMZ in various excipients was determined by a stability-indicating validated reverse-phase HPLC method developed in-house. The HPLC apparatus consisted of Jasco PU-2080 Plus Intelligent HPLC pump (Jasco, Japan) equipped with a Jasco UV-2075 Intelligent UV/VIS detector (Jasco, Japan), a Rheodyne 7725 injector (Rheodyne, USA), a Jasco Borwin Chromatography Software (version 1.50) integrator software, and a Hi-Q-Sil RP-18 (4.6 × 250 mm and 10-μm particle size) column. The mobile phase consisted of a mixture of methanol: dipotassium hydrogen phosphate (0.025 M) buffer (75:25 v/v) at a flow rate of 1.5 ml/min that led to retention time of 12.5 min when detection was carried out at 254 nm. The assay was linear (r2 = 0.999) in the concentration range 10–250 μg/ml with the lowest detection limit of 1.33 μg/ml of CMZ. The method was validated with respect to accuracy and interday and intraday precision as per ICH guidelines and the relative standard deviation was less than 2% in both the cases.
Phase Diagram
An oil titration method was employed in the present investigation to construct phase diagrams (13). Briefly, mixtures of the double-distilled water with Cremophore EL were prepared at ratios (% w/w) of 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, and 1:9 into different vials. A small amount of Capryol 90 in 0.5% (w/w) increments was added into the vials. Following each addition, the mixtures in vials were vortexed for 2 to 3 min and were allowed to equilibrate at 25°C for 30 min. After equilibration, the mixtures were examined visually for phase separation, transparency, and flow properties. In addition, the mixtures were observed through crossed polarizers (fabricated in-house by using polarizing lenses, Nikkon, Japan) for determining the optical isotropy of the systems. The point at which the mixture became turbid or showed signs of phase separation was considered as the end point of the titration. The area of microemulsion existence was determined and denoted as ME.
Formulation of Microemulsion
From the phase diagrams, suitable composition was chosen for further studies. The composition is shown in Table I. Briefly, CMZ (200 mg) was dissolved in Capryol 90 (14.0 g) by using overhead stirrer at 1,000 rpm. To this solution, Cremophore EL (43.5 g), benzyl alcohol (2.0 g), and chlorocresol (0.1 g) were added and the mixture was stirred further to yield a homogenous solution. To this solution, water (38.4 g) was added and stirred further to yield microemulsion.
Table I.
Composition of CMZ Microemulsion
| Ingredient | Content (% w/w) |
|---|---|
| Clotrimazole | 2 |
| Capryol 90 | 14 |
| Cremophor EL | 43.5 |
| Benzyl alcohol | 2 |
| Chlorocresol | 0.1 |
| Water to make (g) | 100 |
Globule Size Analysis of the Microemulsion
The average globule size and polydispersity index of the CMZ microemulsion were determined in duplicate by the photon correlation spectroscopy (Beckman Coulter N4 plus, Wipro, India). Measurements were carried at an angle of 90° at 25°C. Microemulsion was diluted with double-distilled water to ensure that the light-scattering intensity was within the instrument’s sensitivity range. Double-distilled water was filtered through 0.45-μm membrane filters (Pall Life Sciences, Mumbai, India) prior to globule size determination.
Formulation of Microemulsion-Based Gel of CMZ
Various gelling agents, namely, sodium alginate (Manugel DMB), hydroxypropyl methylcellulose (Methocel K4 M), and Carbopol® ETD 2020, were evaluated for their ability to gel CMZ microemulsion. Briefly, the nonaqueous part of the microemulsion was prepared as described above (“Formulation of Microemulsion”); gelling agent was dispersed/dissolved in water by using overhead stirrer at 1,000 rpm. Aqueous part was mixed with nonaqueous part by stirring. The suitable gelling agent was selected on the basis of compatibility with microemulsion structure, feel, and ease of spreadability. The optimized composition of CMZ-MBG is shown in Table II.
Table II.
Composition of CMZ-MBG
| Ingredient | Content (% w/w) |
|---|---|
| Clotrimazole | 2 |
| Capryol 90 | 14 |
| Cremophor EL | 43.5 |
| Benzyl alcohol | 2 |
| Chlorocresol | 2.0 |
| Carbopol ETD-2020 | 0.8 |
| Water to make (g) | 100 |
Characterization of the CMZ-MBG
Determination of CMZ Content, pH, and Spreadability
For determination of drug content, about 1 g of the gel was weighed in a 100-ml volumetric flask and dissolved in methanol; it was diluted appropriately and analyzed by the HPLC method described earlier. The spreadability of the gel was determined using the following technique: 0.5 g gel was placed within a circle of 1-cm diameter premarked on a glass plate over which a second glass plate was placed. A weight of 500 g was allowed to rest on the upper glass plate for 5 min. The increase in the diameter due to spreading of the gels was noted. The pH of the 10% (w/w) gel was determined using Equip-tronic Digital pH meter Model EQ 610, standardized using pH 4.0 and 7.0 standard buffers before use.
Rheological Studies on the MBG
Brookfield Synchro-Lectric Viscometer (Model RVT) with helipath stand was used for rheological studies. The sample (30 g) was placed in a beaker and was allowed to equilibrate for 5 min before measuring the dial reading using a T–C spindle at 0.5, 1, 2.5, and 5 rpm. At each speed, the corresponding dial reading on the viscometer was noted. The spindle speed was successively lowered and the corresponding dial reading was noted. The measurements were carried in duplicate at ambient temperature. Direct multiplication of the dial readings with factors given in the Brookfield viscometer catalog gave the viscosity in centipoises.
In Vitro Bioadhesion Study
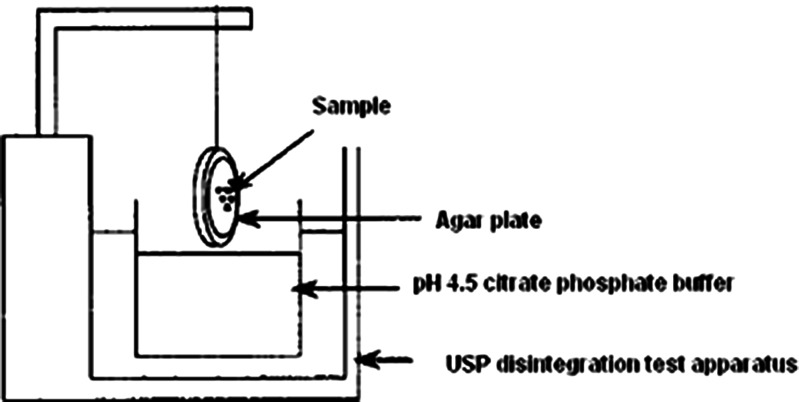
The bioadhesive potential of the CMZ-MBG was evaluated in comparison with the marketed clotrimazole gel (Candid-V® gel) by an in vitro method reported by Nakamura et al. (14). Briefly, an agar plate (1% w/w) was prepared in pH 4.5 citrate phosphate buffer. Test sample of 50 mg was placed at the center of plate. After 5 min, the agar plate was attached to a US Pharmacopeia disintegration test apparatus (Fig. 1) and moved up and down in pH 4.5 citrate phosphate buffer at 37 ± 1°C. The sample on the plate was immersed into the solution at the lowest point and was out of the solution at the highest point. The residence time of the test samples on the plate was noted visually. The experiments were performed in triplicate.
Fig. 1.
Apparatus used for in vitro bioadhesion study
In Vitro Dissolution
In vitro release profiles of CMZ-MBG and Candid-V® were studied using modified USP XXIII apparatus I at 37 ± 0.5° C with a rotating speed of 25 rpm in buffer pH 4.5 citrate phosphate buffer as a dissolution medium. A watch dish containing 1.0 g of the developed formulation was tightly secured with a stainless steel wire screen (350 µ mesh size sinker). The dish was then dipped in 500 ml pH 4.5 citrate phosphate buffer, contained in a vessel of USP dissolution test apparatus. During the study, 2 ml of aliquots were removed at predetermined time intervals (0.5, 1, 2, 3, 4, 6, 8, 10, and 12 h) from the dissolution medium and replaced with fresh media. The amount of CMZ released in the dissolution medium was determined by a HPLC method described earlier. Samples were filtered through 0.45 µ nylon membrane filter.
In Vitro Antifungal Activity
Antifungal activity of CMZ-MBG, Candid-V® gel, and CMZ standard (CMZ dissolved in DMSO) was evaluated against Candida albicans ATCC 10231 by using a cup plate method. Briefly, the concentration of C. albicans ATCC 10231 in inocula was equivalent to 5 × 1015 CFU/ml. CMZ-MBG, Candid-V® gel (500 mg each), and 1 ml of CMZ dissolved in DMSO (10 mg/ml) was added to agar plate and plates were kept in the dark conditions at room temperature for 48 h. After incubation, the mean zone of inhibition was recorded for all the test samples (n = 3).
Stability Studies
Chemical stability of CMZ in CMZ-MBG was assessed at various storage conditions viz. 25°C/60% relative humidity (RH), 30°C/65% RH, and 40°C/75% RH for a period of 3 months. CMZ-MBG was packed in 10-g aluminum ointment tubes. Samples (n = 3) were removed at 0, 30, 60, and 90 days and were assessed for CMZ content by a stability-indicating HPLC method described earlier. The statistical significance of differences in the data was analyzed utilizing analysis of variance followed by Bonferroni’s test (GraphPad InStat Demo Version). Differences were considered statistically significant at P < 0.05.
RESULTS AND DISCUSSION
Solubility Studies
The results of the solubility studies are shown in Table III. It is evident from Table III that the Capryol 90 exhibited the highest solubilizing potential for the CMZ as compared to the other oils. Among the various surfactants used in the study, Labrasol exhibited the highest solubilizing potential for CMZ followed by Solutol HS 15, Cremophore EL, and Tween 80 (Table III). However, Labrasol has poor emulsification ability for Capryol 90 as compared to the other surfactants (15). Among the surfactants employed in the study, Cremophor EL exhibited excellent emulsification ability for Capryol 90 (15) and fairly good solubilizing potential for the CMZ. Hence, Cremophore EL was selected for the further studies.
Table III.
Solubility of CMZ in Various Oils and Surfactants (n = 3)
| Excipient | Solubility (mg/ml) |
|---|---|
| Capryol 90 | 103.1 ± 6 |
| Lauroglycol 90 | 97.4 ± 5 |
| Captex 8000 | 10.6 ± 2 |
| Plurol Oleique | 3.0 ± 0.5 |
| Labrafil 1944 CS | 57.0 ± 1.5 |
| Cremophor EL | 45.0 ± 2.0 |
| Solutol HS-15 | 48.7 ± 3.2 |
| Tween 80 | 36.4 ± 3.5 |
| Labrasol | 58.0 ± 4.0 |
Phase Diagram and Globule Size Analysis of the Microemulsion
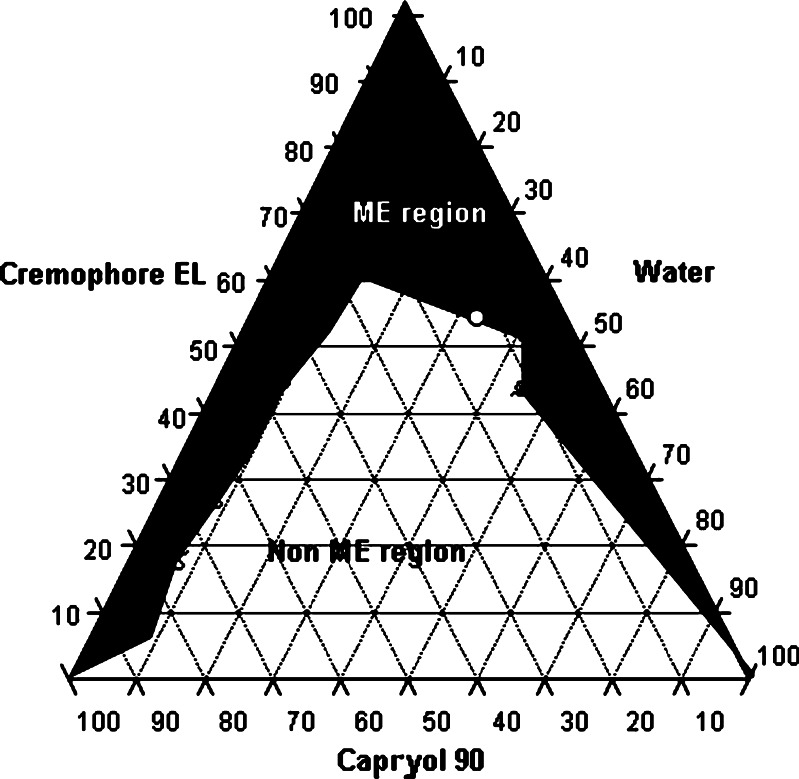
It is reported that nonionic surfactants alone can yield microemulsion without help of cosurfactant (9–11). The phase diagram of Cremophore EL-Capryol 90-water system is shown in Fig. 2. Black region in the figure indicates microemulsion region (ME region) and white region is non-ME region. It is evident from the figure that Cremophore alone could give considerable microemulsification region (>30%). White circle in the black region of Fig. 2 indicates ME composition selected for formulation. Selection of this ME composition was based on solubility of CMZ in Capryol 90 and amount of Cremophor EL required for emulsification to produce a stable microemulsion. The selected microemulsion system was composed of maximum amount of water possible. The microemulsion for further studies was selected from the phase diagram which had globule size of 48.4 nm and polydispersity index of 0.75. The incorporation of CMZ did not have considerable influence on the globule size of the microemulsion.
Fig. 2.
Ternary-phase diagram of Cremophore EL-Capryol 90-Water system
Formulation and Characterization of the MBG
Various gelling agents such as sodium alginate, hydroxypropyl methylcellulose, and Carbopol® ETD 2020 were evaluated for the gelling of CMZ microemulsion. It was observed that sodium alginate affected the structure of the microemulsion and resulted in separation of oily phase. This observation could be attributed to that fact that salts like sodium alginate can affect the structure of the microemulsion (9–11). Hydroxypropyl methylcellulose was unable to yield viscosity desirable for the gel formulations. Only Carbopol® ETD 2020 could yield clear gel without disturbing the microstructure of the CMZ microemulsion. Furthermore, Carbopols are known to have mucoadhesive properties and have been used in the formulation vaginal delivery systems (7,8,16). Hence, Carbopol® ETD 2020 was selected for the formulation of MBG.
The CMZ content of the MBG and Candid-V® gel was found to be 98.4 ± 3.2% and 99.6 ± 0.8%, respectively. The pH value of CMZ-MBG was 4.52 which is equivalent to the vaginal pH; for candid-V®, pH value was 6.8. Spreadability is an important property of topical formulation from patient compliance point of view. The diameter for CMZ-MBG and Candid-V® was found to be 7.2 and 6.20 cm, respectively. High spreadability value of CMZ-MBG compared to Candid-V indicates better spreading ability at the site of application. Both CMZ-MBG and Candid-V® showed pseudoplastic behavior and the viscosity values of CMZ-MBG and Candid-V® at 5 rpm were 9.0 × 106 and 7.8 × 106 mPa s, respectively.
In Vitro Bioadhesion Study
The bioadhesive potential of CMZ-MBG and commercial formulation (Candid-V® gel) was evaluated by in vitro method. The results of the study are shown in Table IV. The CMZ-MBG showed significantly higher retention time as compared to Candid-V® gel (P < 0.05). This clearly indicates that the CMZ-MBG may have higher residence time in vagina as compared to Candid-V® gel. The increased bioadhesivity of CMZ-MBG can be attributed to the presence of Carbopol.
Table IV.
Results of In vitro Bioadhesion Studies (n = 3)
| Formulation | In vitro bioadhesion time (min) |
|---|---|
| FLZ-MBG | 48 ± 3.5* |
| Candid-V® gel | 24 ± 1.5 |
*P < 0.05 as compared to Candid-V® gel
In Vitro Dissolution
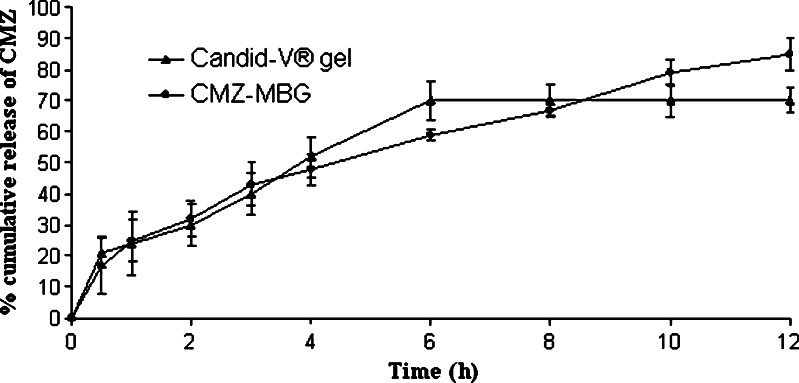
In vitro release profile of CMZ-MBG and Candid-V® gel is shown in Fig. 3. More than 85% of CMZ was released over the period of 12 h from CMZ-MBG and it followed first-order release kinetics. Candid-V gel® showed maximum 70% release of CMZ up to 12 h. The in vitro release pattern of CMZ-MBG and Candid-V® gel was equivalent up to 3 h. After this initial phase, Candid-V® gel produced higher release of CMZ up to 6 h and it was constant at the end of 12 h. CMZ-MBG provided controlled release of CMZ up to 12 h. The difference between the release pattern of MBG and Candid-V® gel was nonsignificant as determined by F2 test (F2 = 61.99 with standard error of 7.049).
Fig. 3.
In vitro dissolution profile of CMZ formulations (n = 3)
In Vitro Antifungal Activity
The results of antifungal studies are shown in Table V. It is evident that CMZ-MBG showed higher antifungal activity as compared to the marketed Candid-V® gel and CMZ standard (P < 0.01). The enhanced in vitro antifungal activity of CMZ-MBG may be attributed to enhanced penetration of oil globules containing CMZ through fungal cell walls to inhibit ergosterol synthesis.
Table V.
Antifungal Activity of Various Samples Against C. albicans ATCC 10231 (n = 3)
| Formulation | Zone of inhibition (mm) |
|---|---|
| CMZ standard | 4.2 ± 0.1* |
| Candid-V® gel | 3.0 ± 0.1* |
| CMZ-MBG | 5.4 ± 0.2 |
CMZ-MBG showed significantly higher in vitro antifungal activity
*P < 0.05 as compared to CMZ-MBG
Stability Studies
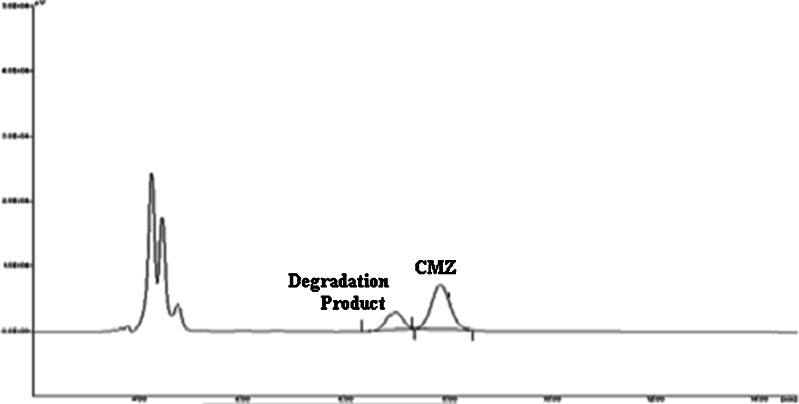
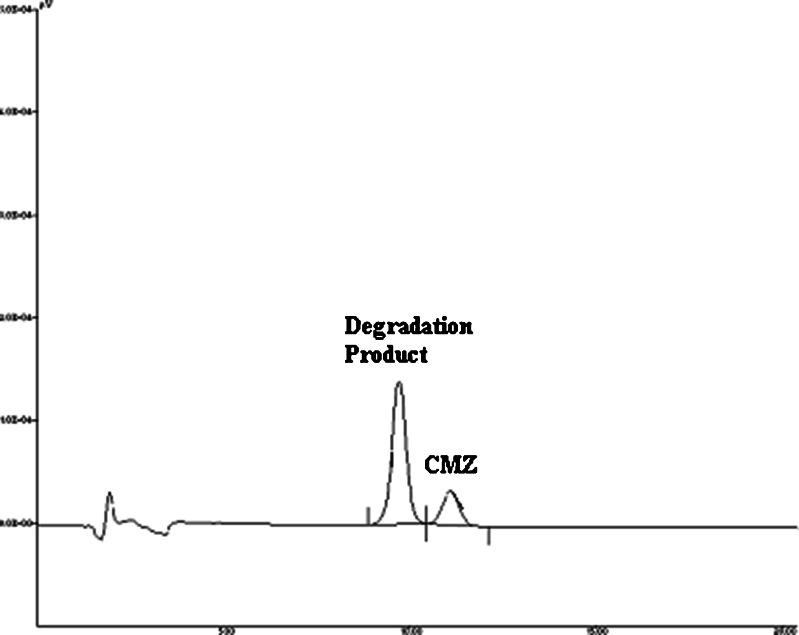
The results of stability studies are shown in Table VI. It is evident from the table that CMZ showed a significant degradation (P < 0.05) at all the storage conditions at the end of 3 months. This observation was totally unexpected. The rate of degradation increased with the increase in the temperature. Hence, at 40°C/75% RH, around 38% of CMZ was degraded at the end of the 3 months. The chromatogram of the sample (stored at 40°C/75% RH) at the end of the third month is shown in Fig. 4 and it clearly shows the well-resolved degradation product of the CMZ. Interestingly, this chromatogram is similar to the chromatogram obtained after forced degradation of CMZ in 1 N HCl (Fig. 5). This clearly indicates that degradation of CMZ in MBG is due to the acidic pH of the formulation. The acidic pH of the MBG could be attributed to the presence of the free fatty acids (such as caprylic acid) in the Capryol 90.
Table VI.
Chemical Stability of CMZ in CMZ-MBG During Stability Testing (n = 3)
| Month | % CMZ content in gel (±SD) | ||
|---|---|---|---|
| 25°C/60% RH | 30°C/60% RH | 40°C/75% RH | |
| 0.5 | 101.2 (±4.2) | 98.7 (±0.5) | 94.7 (±0.9) |
| 1 | 98.1 (±2.5) | 95.1 (±4.1) | 84.8 (±1.7) |
| 2 | 92.1 (±4.2) | 93.7 (±0.7) | 71.1 (±1.1) |
| 3 | 84.6 (±2.9) | 87.2 (±1.36) | 62.4 (±2.6) |
Fig. 4.
Chromatogram of the CMZ-MBG subjected to 40°C/75% RH at the end of 3 months
Fig. 5.
Chromatogram of forced degradation of CMZ in acidic conditions (1 N HCl)
It was observed that commercial formulation of CMZ (Candid-V® gel) has pH value of 6.81 and it claims stability of up to 1.5 years. This corroborates the inferences drawn about the acid-catalyzed degradation of CMZ.
Our preliminary investigations on the stabilization of the CMZ in CMZ-MBG indicated that the increase in the pH of the gel results in the loss of transparency and also disturbs the structure of the microemulsion (data not shown). Hence, it may be necessary to reformulate the developed MBG. The future efforts would be directed towards identifying an oily phase that preserves the integrity of CMZ on long-term storage and has good solubilizing potential for CMZ. Nonetheless, this investigation clearly indicates that MBG could be a viable alternative to the conventional vaginal formulations.
CONCLUSION
The microemulsion-based gel could be successfully developed for the vaginal delivery of clotrimazole. The developed gel showed promising in vitro performance with respect to bioadhesivity and antifungal activity. However, CMZ showed a considerable degradation on long-term storage in the developed microemulsion-based gel which was attributed to the pH of the formulation.
Acknowledgements
YGB thanks the University Grants Commission, New Delhi India for the financial support. The authors are thankful to Cipla Pharmaceuticals, BASF, Indchem International, Noveon India, Anshul Agencies and Colorcon Asia Pvt. Ltd. for the gift samples of the drug and excipients. Authors would like to acknowledge Mr. Abhijit Date for his help in the preparation of the manuscript and also for the technical discussion.
References
- 1.Lanchares JL, Hernandez ML. Recurrent vaginal candidiasis changes in etiopathogenical patterns. Int J Gynecol Obstet. 2000;71:S29–S35. doi: 10.1016/S0020-7292(00)00352-0. [DOI] [PubMed] [Google Scholar]
- 2.Ferrer J. Vaginal candidosis: epidemiological and etiological factors. Int J Gynecol Obstet. 2000;71:S21–S27. doi: 10.1016/S0020-7292(00)00350-7. [DOI] [PubMed] [Google Scholar]
- 3.Ritter W, Patzschke K, Krause U, Stettendorf S. Pharmacokinetic fundamentals of vaginal treatment with clotrimazole. Chemotherapy. 1982;28:37–42. doi: 10.1159/000238150. [DOI] [PubMed] [Google Scholar]
- 4.Pavelic Z, Skalko-Basnet N, Jalsenjak I. Characterisation and in vitro evaluation of bioadhesive liposome gels for local therapy of vaginitis. Int J Pharm. 2005;301:140–148. doi: 10.1016/j.ijpharm.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen M, Bjerregaard S, Jacobsen J, Sørensen AM. A genuine clotrimazole γ-cyclodextrin inclusion complex -isolation, antimycotic activity, toxicity and an unusual dissolution rate. Int J Pharm. 1998;176:121–131. doi: 10.1016/S0378-5173(98)00310-X. [DOI] [Google Scholar]
- 6.Richardson JL, Whetstone J, Fisher AN, Watts P, Farraj NF, Hinchcliffe M, et al. Gamma scintigraphy as a novel method to study distribution and retention of a bioadhesive vaginal delivery in sheep. J Control Rel. 1996;42:133–142. doi: 10.1016/0168-3659(96)01451-4. [DOI] [Google Scholar]
- 7.Sharma G, Jain S, Tiwary K, Kaur G. Once daily bioadhesive vaginal clotrimazole tablets: design and evaluation. Acta Pharm. 2006;56:337–345. [PubMed] [Google Scholar]
- 8.Knuth K, Amiji M, Robinson J. Hydrogel delivery systems for vaginal and oral applications. Adv Drug Deliv Rev. 1993;11:137–167. doi: 10.1016/0169-409X(93)90030-8. [DOI] [Google Scholar]
- 9.Tenjarla S. Microemulsions: an overview and pharmaceutical applications. Crit Rev Ther Drug Carrier Syst. 1999;16:461–521. [PubMed] [Google Scholar]
- 10.Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Del Rev. 2000;45:89–121. doi: 10.1016/S0169-409X(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 11.Date AA, Patravale VB. Microemulsions: applications in transdermal and dermal delivery. Crit Rev Ther Drug Carrier Sys. 2007;24:547–596. doi: 10.1615/critrevtherdrugcarriersyst.v24.i6.20. [DOI] [PubMed] [Google Scholar]
- 12.Vyas TK, Babbar AK, Sharma RK, Misra A. Intranasal mucoadhesive microemulsions of zolmitriptan: preliminary studies on brain-targeting. J Drug Target. 2005;13:317–324. doi: 10.1080/10611860500246217. [DOI] [PubMed] [Google Scholar]
- 13.Corswant CV, Engstrom S, Söderman O. Microemulsions based on soybean phosphatidylcholine and triglycerides. Phase behavior and microstructure. Langmuir. 1997;13:5061–5070. doi: 10.1021/la9702897. [DOI] [Google Scholar]
- 14.Nakamura F, Ohta R, Machida Y, Nagai T. In vitro and in vivo nasal mucoadhesion of some water-soluble polymers. Int J Pharm. 1996;134:173–181. doi: 10.1016/0378-5173(95)04416-7. [DOI] [Google Scholar]
- 15.Date AA, Nagarsenker MS. Design and evaluation of self-nanoemulsifying drug delivery systems (SNEDDS) for cefpodoxime proxetil. Int J Pharm. 2007;329:166–172. doi: 10.1016/j.ijpharm.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 16.Baloglu E, Özyazıcı M, Hızarcıoglu SY, Karavana HA. An in vitro investigation for vaginal bioadhesive formulations: bioadhesive properties and swelling states of polymer mixtures. Il Farmaco. 2003;58:391–396. doi: 10.1016/S0014-827X(03)00044-2. [DOI] [PubMed] [Google Scholar]