Abstract
The objective of the present investigation was to develop and evaluate self-microemulsifying drug delivery system (SMEDDS) for improving the delivery of a BCS class II antidiabetic agent, glyburide (GLY). The solubility of GLY in oils, cosurfactants, and surfactants was evaluated to identify the components of the microemulsion. The ternary diagram was plotted to identify the area of microemulsion existence. The in vitro dissolution profile of GLY SMEDDS was evaluated in comparison to the marketed GLY tablet and pure drug in pH 1.2 and pH 7.4 buffers. The chemical stability of GLY in SMEDDS was determined as per the International Conference on Harmonisation guidelines. The area of microemulsion existence increased with the increase in the cosurfactant (Transcutol P) concentration. The GLY microemulsion exhibited globule size of 133.5 nm and polydispersity index of 0.94. The stability studies indicated that GLY undergoes significant degradation in the developed SMEDDS. This observation was totally unexpected and has been noticed for the first time. Further investigations indicated that the rate of GLY degradation was highest in Transcutol P.
Key words: degradation, glyburide, SMEDDS, stability studies, Transcutol P
INTRODUCTION
Glyburide (GLY) or glibenclamide is a second-generation sulfonylurea used in the treatment of noninsulin-dependent diabetes. It is one of the most prescribed long-acting antihyperglycemic agents (1). GLY is classified as BCS class II drug, which means it has high permeability and poor water solubility (2). The poor water solubility of GLY is responsible for its poor dissolution rate, which ultimately leads to variable absorption of GLY. Furthermore, there are reports which have documented that GLY shows large variations in interindividual bioavailability and bioequivalence of the marketed products (3,4). Thus, it can be concluded that the bioavailability and in vivo performance of GLY is dependent on its dissolution rate. In view of this, researchers have attempted various techniques to improve the dissolution rate and, ultimately, the in vivo performance of GLY. To date, the potential of various drug delivery approaches, such as amorphization (5), solid dispersion (6–8), cyclodextrin complexation (9,10), and permeation enhancers (10), has been established in improving in vitro and/or in vivo performance of GLY. However, to date, the potential of microemulsions has not been established in the delivery of GLY.
The potential of microemulsions and/or self-microemulsifying drug delivery systems (SMEDDS) or anhydrous form of microemulsion in the improvement of the bioavailability and therapeutic performance of the hydrophobic agents has been very well-established (11–14). In view of this, the present investigation was aimed at developing SMEDDS for the delivery of GLY. SMEDDS of GLY with globule size <150 nm were successfully developed. The results of stability studies of GLY SMEDDS are also presented in this investigation.
MATERIALS AND METHODS
Materials
Glyburide was kindly provided as a gift sample by Cipla Pharmaceuticals (Mumbai, India). Capryol 90, Lauroglycol 90, Transcutol P, Plurol Oleique CC 497, Labrafil M 1944 CS, Labrasol (Colorcon Asia, Mumbai, India), Capmul MCM (Indchem International, Mumbai, India), hydroxypropyl cellulose (Signet Chemicals, Mumbai, India), and Solutol HS 15 (BASF India, Mumbai, India) were received as gift samples. Tween-80, Tween-20, and PEG-400 (all AR grade) were purchased from Merck (Mumbai, India). Hydrochloric acid, potassium dihydrogen phosphate (all AR grade), and acetonitrile (high-performance liquid chromatography [HPLC] grade) were purchased from s.d. Fine Chemicals (Mumbai, India).
Solubility Studies
The solubility of GLY in various oils, cosurfactants, and surfactants was determined by using the shake flask method. Briefly, an excess amount of GLY was added to each vial containing 5 mL of the selected vehicle, i.e., oil, cosurfactant, and surfactant. After sealing, the mixture was vortexed using a cyclomixer for 10 min in order to facilitate proper mixing of GLY with the vehicles. Mixtures were shaken for 72 h in an isothermal shaker (Remi, Mumbai, India), maintained at 37 ± 1°C, and afterwards, mixtures were centrifuged at 5,000 rpm for 15 min, followed by filtration through membrane filter (0.45 μm, 13 mm; Pall Life Sciences, Mumbai, India). The concentration of GLY in the supernatant was determined by an in-house HPLC method.
HPLC Analysis of GLY
The solubility of GLY in various excipients was determined by a stability-indicating and validated in-house HPLC method. The HPLC apparatus consisted of a Jasco PU-2080 Plus Intelligent HPLC pump (Jasco, Japan) equipped with a Jasco UV-2075 Intelligent UV/VIS detector (Jasco, Japan), a Rheodyne 7725 injector (Rheodyne, USA), a Jasco Borwin Chromatography Software (version 1.50) integrator software, and a Hi-Q-Sil RP-18 (4.6 mm × 250 mm and 10 μ particle size) column. The mobile phase consisted of a mixture of acetonitrile/monobasic ammonium phosphate (0.05 M) buffer (55:45 v/v) at a flow rate of 1.5 mL/min that led to a retention time of 7.1 min when detection was carried out at 225 nm. The assay was linear (r2 = 0.9991) in the concentration range 1–5 μg/mL with the lowest detection limit of 30 ng/mL of GLY. The method was validated with respect to accuracy and interday and intraday precision as per the International Conference on Harmonisation (ICH) guidelines, and the relative standard deviation was <2% in both the cases.
The developed method could successfully resolve the acid, base, and oxidative degradation products of GLY (data not shown). The same method was used for determining chemical stability of GLY in SMEDDS. For determining the solubility of GLY and for determining the GLY content in SMEDDS, the various samples were suitably diluted by the mobile phase and injected in the HPLC system. There was no need for any extraction from the sample as all the components were soluble in the mobile phase.
Phase Diagrams
A water titration method was employed in present investigation to construct phase diagrams (15). Briefly, mixtures of Capryol 90 and Tween-20+Transcutol P were prepared at ratios (in percent w/w) of 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, and 1:9 into different vials. The phase diagrams were plotted at two different Km values (ratio of surfactant to cosurfactant), viz., 0.67 and 0.55. A small amount of double-distilled water in 0.5% (w/w) increment was added into the vials. Following each addition, the mixtures in the vials were vortexed for 2–3 min and were allowed to equilibrate at 25°C for 30 min. After equilibration, the mixtures were examined visually for phase separation, transparency, and flow properties. In addition, the mixtures were observed through crossed polarizers (fabricated in-house by using polarizing lenses; Nikkon, Japan) for determining the optical isotropy of the systems. The point at which the mixture became turbid or showed signs of phase separation was considered as the endpoint of the titration. The area of microemulsion existence was determined and denoted as ME. The phase diagrams were constructed at two different ratios of Tween-20/Transcutol P, namely, 0.55 and 0.67.
Formulation of SMEDDS
The optimized SMEDDS formulation consisted of glyburide/Capryol 90 (oily phase)/Transcutol P (cosurfactant)/Tween-20 (surfactant)/hydroxypropyl cellulose (viscosity builder) (0.75:15.1:53:30.3:0.75) in percent w/w. Briefly, the calculated amounts of GLY that was dissolved in Capryol 90 and Transcutol containing hydroxypropyl cellulose and Tween-20 were added to this solution under vortexing. The homogenous SMEDDS was used for further studies.
Globule Size Analysis
SMEDDS (0.5 g) was diluted with 50 mL double-distilled water. The average globule size and polydispersity index of the GLY microemulsion were determined in duplicate by the photon correlation spectroscopy (Beckman Coulter N4 plus, Wipro, India). Measurements were carried out in triplicate at an angle of 90° at 25°C. If required, microemulsion was diluted with double-distilled water to ensure that the light-scattering intensity was within the instrument’s sensitivity range. Double-distilled water was filtered through 0.45 μm membrane filters (Pall Life Sciences, Mumbai) prior to globule size determination.
In Vitro Dissolution Profile
SMEDDS of GLY (equivalent to 5 mg of GLY) was filled in size “0” hard gelatin capsules. In vitro release profiles of SMEDDS of GLY, pure GLY powder, and marketed GLY formulation (Euglucon Tablets, Nichols-Piramal India, Mumbai, India) were studied using United States Pharmacopeia (USP) XXIII apparatus I at 37 ± 0.5°C with a rotating speed of 100 rpm in buffer pH 1.2 and pH 7.4 as the dissolution media. During the study, 2 mL of aliquots were removed at predetermined time intervals (5, 10, 20, and 30 min) from the dissolution medium and replaced with fresh media. The amount of GLY released in the dissolution medium was determined by an in-house HPLC method described earlier.
Stability Studies
Chemical stability of GLY in SMEDDS was assessed under various storage conditions, viz., 25°C/60% RH, 30°C/65% RH, and 40°C/75% RH as per ICH guidelines. SMEDDS equivalent to 5 mg of GLY was filled in size “0” hard gelatin capsules. Ten such capsules were packed in Alu–Alu strip packs and stored at various aforementioned storage conditions up to 1 month. Samples were removed at 0, 15, and 30 days of interval and the GLY content in the samples was analyzed by an in-house HPLC method described earlier.
Stability of GLY in Individual SMEDDS Components
In order to understand the unusual and unexpected degradation of the GLY, it was decided to monitor the stability of GLY in the components of SMEDDS. Briefly, GLY (5.0 mg) was dissolved in various components of SMEDDS (1.0 mL), viz., Transcutol P, Tween-20, and Capryol 90, whereas in case of hydroxypropyl cellulose, GLY powder was thoroughly mixed with equal amount of hydroxypropyl cellulose to physical mixture. All these samples and a pure GLY powder were stored at 60°C/ambient RH for 15 days. The GLY content of the samples was analyzed by an in-house HPLC at the end of 15 days.
RESULTS AND DISCUSSION
Solubility Studies
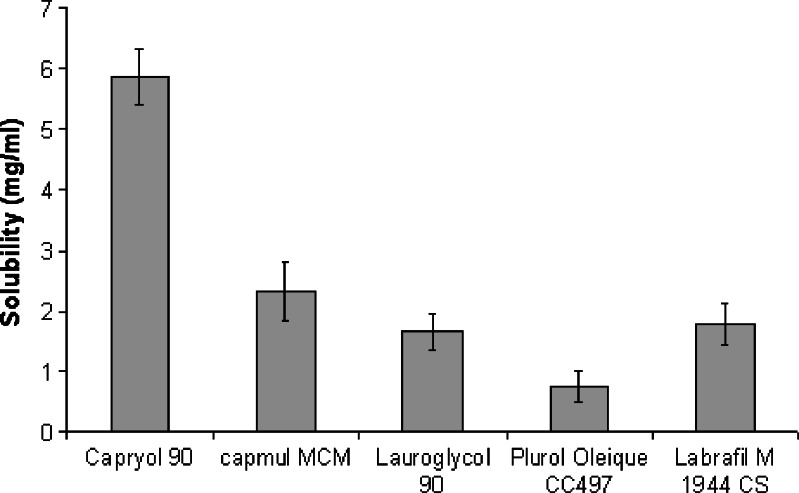
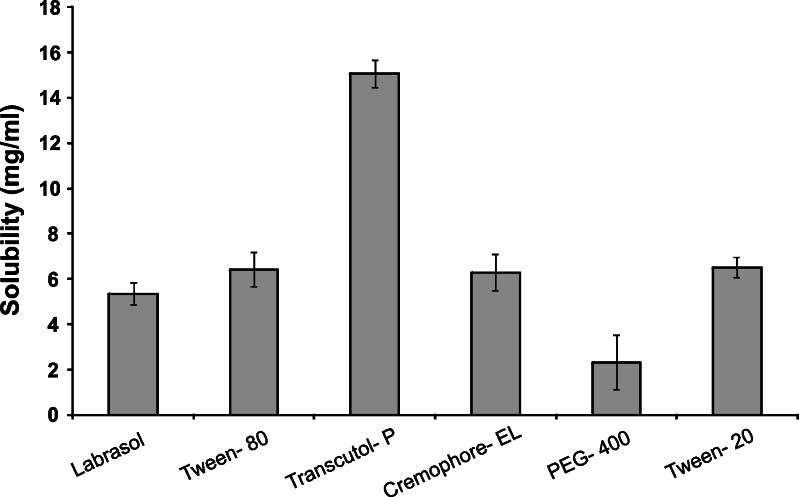
The results of solubility studies of the GLY in various oils, cosurfactants, and surfactants are shown in Figs. 1 and 2. It is evident that GLY has considerably low solubility in oils, surfactants, and cosurfactants despite a high log P value of 4.2. Such a behavior has already been seen with drugs like amphotericin B and carbamazepine. Hence, GLY can be considered as a drug which is poorly soluble in both lipid and water. However, due to the very small dose of GLY, it would still be possible to formulate a SMEDDS formulation that can be filled in a unit dosage form. Among the various oils screened, Capryol 90 showed the highest solubilizing potential for GLY (5.85 ± 0.45 mg/mL) compared to Capmul MCM (2.3 ± 0.5 mg/mL), Labrafil M 1944 CS (1.7 ± 0.3 mg/mL), Lauroglycol 90 (1.6 ± 0.3 mg/mL), and Plurol Oleique CC 497 (0.7 ± 0.2 mg/mL). All the surfactants tested (Cremophor EL, Tween-20, and Tween-80) showed similar solubilization for GLY (6.3–6.5 mg/mL). In all the solubilizers studied, Transcutol P showed the highest solubilizing potential for GLY (15 ± 1.2 mg/mL) compared to Solutol HS 15 (6.5 ± 0.8 mg/mL), Labrasol (5.3 ± 0.6 mg/mL), and PEG-400 (2.3 ± 0.6 mg/mL). Capryol 90, Tween-20, and Transcutol P were selected for further studies.
Fig. 1.
Solubility of GLY (in milligrams per milliliter) in various oils (n = 3; data are expressed as mean±SD)
Fig. 2.
Solubility of GLY (in milligrams per milliliter) in various surfactants and cosurfactants (n = 3; data are expressed as mean±SD)
Phase Diagrams and Globule Size Analysis
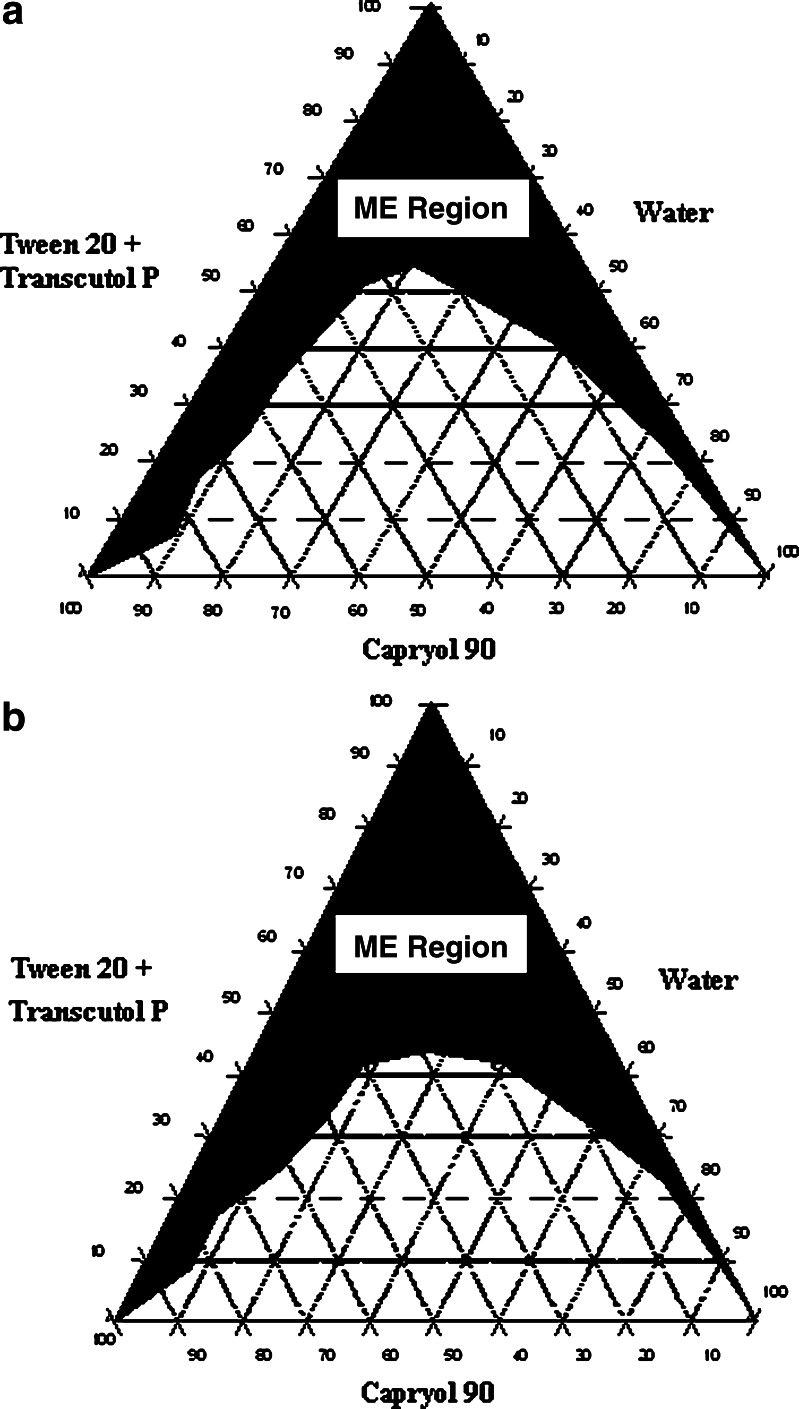
Pseudoternary phase diagrams were constructed to investigate the effect of surfactant to cosurfactant ratio (Km) on the area of microemulsion existence (Figs. 3a, b). It is a well-known fact that the Km value has considerable effect on the area of microemulsion existence. In the present study, it was observed that the area of microemulsion existence was higher at Km = 0.55 compared to that of Km = 0.67. Hence, for the formulation of SMEDDS, a lower Km value (0.55) was employed. The optimized microemulsion had a globule size of 133.5 nm with the polydispersity index of 0.94.
Fig. 3.
Ternary phase diagram of Tween-20+Transcutol P–Capryol 90–water system at a K m = 0.67 and b K m = 0.55
Formulation of SMEDDS
Various SMEDDS compositions were screened (data not shown) and optimal composition which could solubilize the therapeutic dose of GLY was selected.
Hydroxypropyl cellulose was added to increase the viscosity of SMEDDS so as to enable unit dosage form of GLY SMEDDS. There are reports in the literature where cellulosics have been used for this purpose (16,17).
In Vitro Dissolution Study
The results of in vitro dissolution profiles of various GLY formulations are shown in Table I. It is evident from the table that GLY SMEDDS showed a dramatic improvement in the in vitro dissolution profile compared to the marketed GLY tablet and pure GLY in both the dissolution media. GLY SMEDDS showed more than 90% GLY released in 5 min in both the dissolution media, indicating that the release was independent of the pH of the dissolution medium. However, there was decrease in cumulative percent GLY release in pH 1.2 buffer with the increase in time. The reason for this is the extreme insolubility of the GLY in pH 1.2 buffer. Because of the same reason, pure GLY and marketed GLY tablet showed very poor release in pH 1.2 buffer compared to SMEDDS. The pH 1.2 buffer cannot provide sink condition for GLY. The USP recommends pH 7.4 buffer as a dissolution medium for GLY. But since any oral dosage form would be first exposed to acidic conditions, therefore, the in vitro release of GLY was evaluated in pH 1.2 buffer. It is also noteworthy that the in vitro dissolution of GLY formulations in pH 1.2 buffer has been evaluated for the first time as there are no reports in the literature where pH 1.2 buffer is used to evaluate enhanced dissolution rate of GLY. It is also an important observation that decreased dissolution of GLY over the period of time was not observed in the pH 7.4 buffer. The marketed formulation and pure GLY showed very less release (<10%) in both media compared to the GLY SMEDDS.
Table I.
In Vitro Dissolution Profiles of Various GLY Formulations
| Formulation | Cumulative percent GLY released with time | |||||||
|---|---|---|---|---|---|---|---|---|
| pH 1.2 buffer | pH 7.4 buffer | |||||||
| 5 min | 10 min | 20 min | 30 min | 5 min | 10 min | 20 min | 30 min | |
| Pure GLY powder | 0.8 ± 0.2 | 1.0 ± 0.3 | 1.1 ± 0.3 | 1.3 ± 0.1 | 1.15 ± 0.75 | 2.1 ± 1.5 | 5.1 ± 1.5 | 7.3 ± 3.0 |
| Marketed GLY tablets | 1.9 ± 1.5 | 2.2 ± 0.7 | 2.4 ± 0.9 | 3.0 ± 1.3 | 6.12 ± 0.57 | 5.9 ± 0.8 | 7.4 ± 1.3 | 8.5 ± 0.9 |
| GLY SMEDDS | 91.2 ± 7.6 | 100.5 ± 4.6 | 90.1 ± 6.3 | 86.4 ± 5.2 | 95. 0 ± 2.8 | 95.5 ± 3.0 | 96.8 ± 1.3 | 97.3 ± 1.8 |
Stability Studies
The results of stability studies of GLY SMEDDS are shown in Table II. Surprisingly, the content of GLY in SMEDDS started to decline significantly 15 days after the storage at all storage conditions. The degradation was higher at the 40°C/75% RH. On the 30th day, GLY in SMEDDS showed around 50% degradation at 40°C/75% RH. This was a totally unexpected result. There are no reports on the stability of the GLY in the oily phases, surfactants, or the solubilizers like Transcutol P. Furthermore, the GLY is not reported to be a molecule which can easily undergo degradation.
Table II.
Chemical Stability of GLY in SMEDDS During Stability Testing (n = 3)
| Day | Percent GLY content in SMEDDS (±SD) | ||
|---|---|---|---|
| 25°C/60% RH | 30°C/65% RH | 40°C/75% RH | |
| 0 | 98.1 ± 1.1 | 98.1 ± 1.1 | 98.1 ± 1.1 |
| 15 | 90.6 ± 1.7* | 84.7 ± 2.5* | 61.6 ± 0.7* |
| 30 | 81.3 ± 1.9* | 75.3 ± 4.3* | 50.2 ± 2.5* |
*P < 0.05, significantly different compared to 0 day when evaluated by analysis of variance
Lipid excipients, surfactants, and solubilizers are usually thought to be inert. The stability aspects of the active pharmaceutical ingredient (API) in SMEDDS formulations have not been reported in most of the investigations. Paradoxically, all these excipients are routinely used for the formulation of SMEDDS. This observation clearly indicates the need to evaluate the stability of the API in the SMEDDS or SMEDDS components before proceeding to formulation development. In order to understand the degradation behavior of GLY in SMEDDS, it was decided to monitor its stability in the individual SMEDDS components.
Stability of GLY in Components of SMEDDS
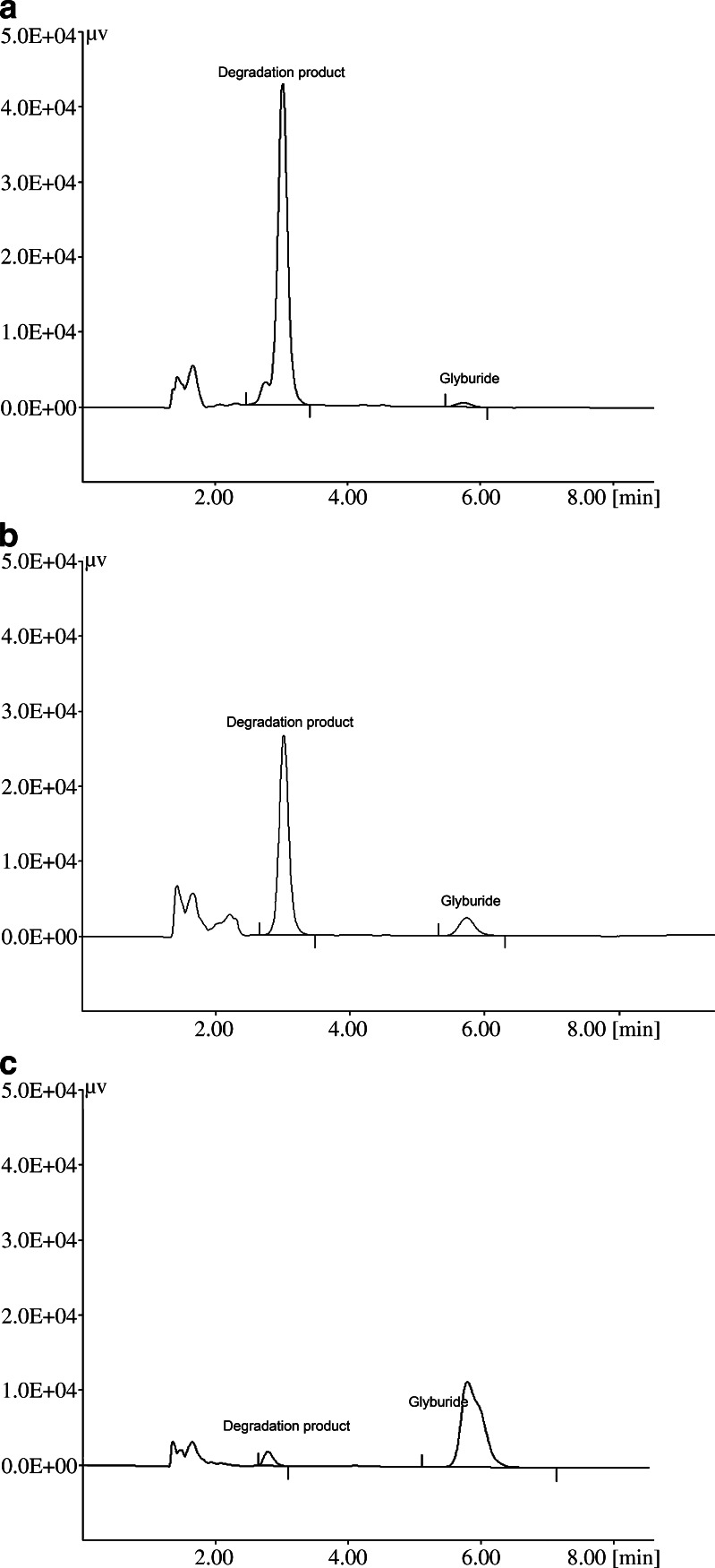
The results of the stability of GLY in the individual SMEDDS components are shown in Table III. It is evident from the table that GLY showed a significant degradation in all the components except hydroxypropyl cellulose at the end of the 15 days. The chromatograms of the samples stored at 60°C/ambient RH for 15 days are shown in Fig 4a–c. It is evident that the rate of degradation of GLY was very fast in Transcutol P compared to the other excipients (Table III). Chemically, Transcutol P is diethylene glycol monoethyl ether, and so far, there are no reports on the degradation of APIs in Transcutol P. At the same time, it should be noted that GLY was not compatible with other components, as degradation of GLY was observed in contact with Capryol 90 and Tween-20. It can be seen from the chromatograms that the degradation product was the same in all the excipients, although the extent of degradation was variable. In the future, studies would be undertaken to understand the mechanism of the degradation of GLY by conducting liquid chromatography–mass spectrometry analysis.
Table III.
Percent GLY Content in Individual SMEDDS Excipients after 15 Days at 60°C of Storage (n = 3)
| Sample | Percent GLY content |
|---|---|
| Pure GLY powder | 99.5 ± 1.5 |
| GLY+hydroxypropyl cellulose | 98.7 ± 2.5 |
| GLY dissolved in Capryol 90 | 68.2 ± 3.5* |
| GLY dissolved in Transcutol P | 2.0 ± 0.5* |
| GLY dissolved in Tween-20 | 44.1 ± 1.3* |
*P < 0.05, significantly different compared to pure GLY when evaluated by analysis of variance
Fig. 4.
Chromatogram of the GLY dissolved in various SMEDDS excipients at the end of 15 days when stored at 60°C: a Transcutol P, b Tween-20, c Capryol 90
CONCLUSION
The SMEEDS of GLY could be successfully developed, and the developed SMEDDS showed dramatic improvement in the dissolution rate of GLY compared to the marketed formulation and pure GLY powder. However, stability studies indicated that GLY showed significant degradation in the developed SMEDDS. This clearly indicates the need to investigate the stability of the API in the SMEDDS components selected for formulation development, an aspect which has not been focused so far in the literature.
Acknowledgement
YGB thanks the University Grants Commission, New Delhi, India for the financial support. The authors would like to thank Cipla Pharmaceuticals, Colorcon Asia Pvt. Ltd., Indchem International, BASF India, and Signet Chemicals for the gift samples of the drug and the excipients. The authors would also like to acknowledge Mr. Abhijit Date for his help in the preparation of the manuscript and also for the technical discussion.
References
- 1.Groop L, Wahlin-Boll E, Totterman KJ, Melander A, Tolppanen EM, Fyhrqvist F. Pharmacokinetics and metabolic effects of glibenclamide and glipizide in type 2 diabetes. Eur J Clin Pharmacol. 1985;28:697–704. doi: 10.1007/BF00607919. [DOI] [PubMed] [Google Scholar]
- 2.Wei H, Lobenberg RL. Biorelevant dissolution media as a predictive tool for glyburide a class II drug. Eur J Pharm Sci. 2006;19:45–62. doi: 10.1016/j.ejps.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Chalk JB, Patterson M, Smith MT, Eadie MJ. Correlations between in vitro dissolution, in vivo bioavailability and hypoglycaemic effect of oral glibenclamide. Eur J Clin Pharmacol. 1986;31:177–182. doi: 10.1007/BF00606655. [DOI] [PubMed] [Google Scholar]
- 4.Blume H, Ali SL, Siewert M. Pharmaceutical quality of glibenclamide products: a multinational postmarket comparative study. Drug Dev Ind Pharm. 1993;19:2713–2741. doi: 10.3109/03639049309050174. [DOI] [Google Scholar]
- 5.Cordes D, Mueller BW. Deactivation of amorphous glibenclamide during dissolution. Eur J Pharm Sci. 1996;4:S187. doi: 10.1016/S0928-0987(97)86577-3. [DOI] [Google Scholar]
- 6.Varma MM, Jayaswal SB, Singh J. In vitro and in vivo evaluation of fast release solid dispersions of glibenclamide. Indian Drugs. 1992;29:608–611. [Google Scholar]
- 7.Betageri GV, Makaria KR. Enhancement of dissolution of glyburide by solid dispersion and lyophilization techniques. Int J Pharm. 1995;126:155–160. doi: 10.1016/0378-5173(95)04114-1. [DOI] [Google Scholar]
- 8.Tashtoush BM, Al-Qashi ZS, Najib NM. In vitro and in vivo evaluation of glibenclamide in solid dispersion systems. Drug Dev Ind Pharm. 2004;30:601–607. doi: 10.1081/DDC-120037491. [DOI] [PubMed] [Google Scholar]
- 9.Sanghavi NM, Venkatesh H, Tandel V. Solubilizaiton of glibenclamide with β-cyclodextrin and its derivative. Drug Dev Ind Pharm. 1994;20:1275–1283. doi: 10.3109/03639049409038367. [DOI] [Google Scholar]
- 10.Zerrouk N, Corti G, Ancillotti S, Maestrelli F, Cirri M, Mura P. Influence of cyclodextrins and chitosan, separately or in combination, on glyburide solubility and permeability. Eur J Pharm Biopharm. 2006;62:241–246. doi: 10.1016/j.ejpb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2000;45:89–121. doi: 10.1016/S0169-409X(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 12.Date AA, Patravale VB. Microemulsions: applications in transdermal and dermal delivery. Crit Rev Ther Drug Carrier Syst. 2007;24:547–596. doi: 10.1615/critrevtherdrugcarriersyst.v24.i6.20. [DOI] [PubMed] [Google Scholar]
- 13.Yang SC, Gursoy RN, Lambert G, Benita S. Enhanced oral absorption of paclitaxel in a novel self-microemulsifying drug delivery system with or without concomitant use of P-glycoprotein inhibitors. Pharm Res. 2004;21:261–270. doi: 10.1023/B:PHAM.0000016238.44452.f1. [DOI] [PubMed] [Google Scholar]
- 14.Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58:173–182. doi: 10.1016/j.biopha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Li P, Ghosh A, Wagner RF, Krill S, Joshi YM, Serajuddin ATM. Effect of combined use of nonionic surfactant on formation of oil-in-water microemulsions. Int J Pharm. 2005;288:27–34. doi: 10.1016/j.ijpharm.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Piclin PS, Homar M, Valant AZ, Perlin MG. Sodium ascorbyl phosphate in topical microemulsions. Int J Pharm. 2003;256(1–2):65–73. doi: 10.1016/S0378-5173(03)00063-2. [DOI] [PubMed] [Google Scholar]
- 17.Kongan A, Garti N. Microemulsions as transdermal drug delivery vehicles. Adv Colloid Interface Sci. 2006;123–126:369–385. doi: 10.1016/j.cis.2006.05.014. [DOI] [PubMed] [Google Scholar]