Abstract
Poly(d,l-lactic acid) nanoparticles were freeze-dried in this study. With respect to drying, effect of protective excipients and purification from excess surfactant were evaluated. The nanoparticles were prepared by the nanoprecipitation method with or without a surfactant, poloxamer 188. The particles with the surfactant were used as such or purified by tangential flow filtration. The protective excipients tested were trehalose, sucrose, lactose, glucose, poloxamer 188, and some of their combinations. The best freeze-drying results in terms of nanoparticle survival were achieved with trehalose or sucrose at concentrations 5% and 2% and, on the other hand, with a combination of lactose and glucose. Purification of the nanoparticle dispersion from the excess surfactant prior to the freeze-drying by tangential flow filtration ensured better drying outcome and enabled reduction of the amount of the protective excipients used in the process. The excess surfactant, if not removed, was assumed to interact with the protective excipients decreasing their protective mechanism towards the nanoparticles.
Key words: freeze-drying, nanoparticles, poly(lactic acid), protective excipients, tangential flow filtration
INTRODUCTION
Polymeric nanoparticles, poly(lactic acid) (PLA) and its derivates being recognized examples, are a high-potential platform for efficient exploitation of different drug delivery formulations and routes because of the properties provided by their small size (1). These possible benefits include controlled release, protection of the encapsulated drug, and drug targeting. Nanoparticles are generally applied as injectable dispersions, but due to the stability problems encountered in the liquid state, they have to be dehydrated for storage (2). Freeze-drying is one of the most convenient methods enabling liquid removal and further reconstitution of nanoparticles for therapeutical use (3).
Freeze-drying is a complicated process involving changes in temperature and physical state of materials as well as concentrations of different substances in the liquid environment, which easily disturb the stability of nanoparticle dispersion. Therefore, appropriate protective excipients are generally regarded as indispensable for successful freeze-drying cycles, and their choice needs to be evaluated for different nanoparticulate formulations. Approved protective sugars are, e.g., trehalose (2–12), sucrose (6,10–13), lactose (6,7,14,15), glucose (2,4,7,14,15), saccharose and maltose (10), and mannitol (7,11,12). The protective mechanism of these excipients originates from the amorphous matrix of the frozen sugar in water (16). The amorphous matrix forms hydrogen bonds with the nanoparticles acting as a water substitute inhibiting the destructive effect of ice crystals.
Certain potentially toxic residues may remain in the nanoparticulate dispersion after the preparation process including organic solvents, surfactants/stabilizers, coating agents, and, possibly, monomers/initiators from the polymerization process. Tangential flow filtration (TFF), or cross-flow filtration, has been found as a very practical method to purify nanoparticulate dispersions from these impurities (5,13,17–20). It is a more gentle and continuous process and more easily scaled up than, e.g., the traditional dead-end ultrafiltration (21) or ultracentrifugation (22). In TFF, the dispersion is pumped tangentially along the surface of the membrane. Filtrate is formed when an applied pressure forces part of the dispersion medium through the membrane. The retained particles do not block the membrane because they are swept away with the tangential flow. TFF is still a less used method in the nanoparticle purification, probably due to the very small volumes used in the nanoparticle research in laboratory scale and limited selection of commercial products available for the small volumes.
The aim of this study was to evaluate protective excipients, i.e., different sugars (trehalose, sucrose, glucose and lactose) and a surfactant (poloxamer 188), on the outcome (quality parameters like redispersibility and maintained size distribution) of freeze-dried poly(d,l-lactic acid) nanoparticles. The protective excipients were tested on surfactant-free nanoparticles and nanoparticles prepared with the surfactant. Additionally, suitability of the tangential flow filtration was assessed in the purification of the PLA nanoparticles (removal of excess surfactant) prior to the freeze-drying. Functionality of the different excipients was evaluated after the drying by visual inspection, electron microscopy, and size determinations (photon correlation spectroscopy).
MATERIALS AND METHODS
Materials
PURASORB® PDL 02A poly(d,l-lactic acid) (a donation from PURAC Biomaterials, Gorinchem, The Netherlands; IV 0.20 dl/g) was used in the nanoparticle preparation. Other excipients used in the preparation and drying were acetone (Riedel-de Haën, Seelze, Germany), poloxamer 188 (Lutrol® F 68, BASF, Ludwigshafen, Germany), d-trehalose, sucrose, d-glucose (Sigma-Aldrich Chemie, Steinheim, Germany), lactose (DMV International, Veghel, The Netherlands), and ultrapurified water (Millipore, Molsheim, France). Eleven-micrometer paper filters (Whatman, Brentford, UK) were used for the raw purification of the nanoparticle dispersions. 0.2-μm Isopore™ membrane filters (Millipore, Molsheim, France) aided in the visualization by electron microscopy.
Nanoparticle Preparation and Purification
PLA nanoparticles were prepared by the nanoprecipitation method (23). Twenty-five milligrams of PLA were dissolved in 2 ml of acetone. The polymeric solution was added with a syringe and a gauge (0.45 mm) directly into 4 ml of the outer phase (water or water with the surfactant, 20 mg of Poloxamer 188) under mild stirring. The organic solvent was evaporated for 40 min in fume hood and the nanoparticle dispersion was diluted with water and filtered (paper filter) to remove possible undesired aggregates formed during the nanoprecipitation.
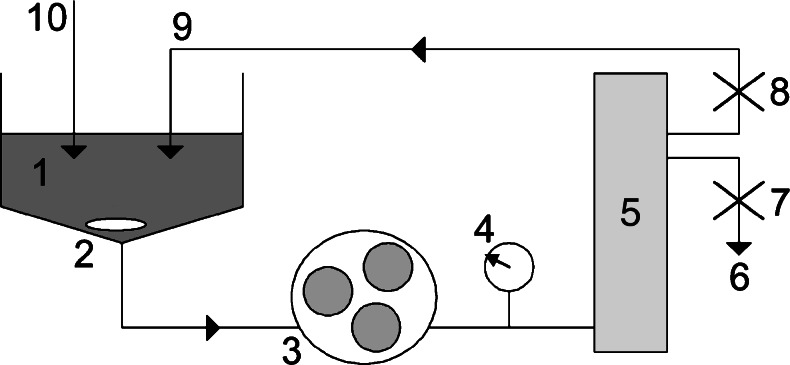
Nanoparticle batches prepared with poloxamer 188 were dried as such (with excipients), or the excess surfactant was first removed by TFF. The filtration was performed with a continuous diafiltration mode using a Minimate™ TFF System and a Capsule with Omega TI300K Membrane (Pall Corporation, Ann Arbor, USA) run by a peristaltic pump (Fig. 1). Briefly, the nanoparticle dispersion circulated through the filtration capsule with the help of a peristaltic pump. The flow rate was 40 ml/min, filtration rate 1–2 ml/min, and operating pressure 10 psi. The operating pressure could be adjusted by the clamps (7 and 8 in Fig. 1). The nanoparticle dispersion volume was 20 ml and the volume of water used in purification was 120 ml.
Fig. 1.
Schematic illustration of the TFF setup. (1) nanoparticle dispersion; (2) magnetic stirrer; (3) peristaltic pump; (4) pressure gauge; (5) filtration capsule; (6) filtrate; (7) a clamp to control the filtrate rate; (8) a clamp to control the pressure; (9) retentate; (10) diafiltration medium (water)
Freeze-drying
Nanoparticle dispersions were dispersed in vials (1 ml of nanoparticles + 250 μl of sugars/surfactant) and freeze-dried with a Lyostar II (FTS, Stone Ridge, USA). The freeze-drying cycle was as follows: freezing at −40°C (240 min); primary drying at −35°C and 150 mTorr (1,020 min); secondary drying at 20°C and 50 mTorr (180 min).
The nanoparticles were freeze-dried with different protective excipients at two concentrations, namely 0.4% or 0.2% (w/v nanoparticles in water). When single excipients (sugars and poloxamer 188 added after preparation) were used, their concentrations in the dispersions were 5%, 2%, and 1%. Lactose and glucose were used also in combination with mass ratio of 2:1 (lactose–glucose), at three different concentrations (lactose and glucose 4% and 2%, 1.6% and 0.8% and 0.8% and 0.4%), based on our previous positive experiences (15). For comparison purposes, lactose and glucose were used with poloxamer 188 in such manner that the sugar concentration was kept constant (2.5%) and the poloxamer 188 concentration was 2.5%, 1%, or 0.5%. All the concentrations are percent w/v.
Nanoparticle Characterization
Size distributions of the nanoparticles were determined with Malvern Zetasizer 3000HS (Malvern, Worcestershire, UK). Particle sizing was based on photon correlation spectroscopy; the results were analyzed by CONTIN algorithm and the sizes presented are based on the intensity distributions.
Freeze-dried samples were characterized by the appearance of the cake, redispersibility in water, Tyndall effect, presence of visual aggregates (AS = aggregation scale), polydispersity index (PI, a dimensionless number from 0 to 1 describing the homogeneity of the sample), and size relative to the initial size (Sf/Si). These parameters are commonly used in the characterization of the freeze-dried nanoparticles (3).
Appearance of the nanoparticle populations was visualized by scanning electron microscopy (SEM). Nanoparticulate samples, collected by ultrafiltration on membrane surfaces, were attached by two-sided tape on metal plates, sputtered for 20 s by platinum with an Agar Sputter Coater (Agar Scientific Ltd., Essex, UK) and analyzed with a DSM 962 SEM (Zeiss, Jena, Germany).
RESULTS AND DISCUSSION
Role of a surfactant is rarely studied in the freeze-drying process because it is usually included in the nanoparticle formulation as an indispensable stabilizer. Polymer selection of this study allowed preparation of surfactant-free nanoparticles: the d,l-PLA used was relatively short-chained (IV 0.20 dl/g corresponds to MW of ∼20,000 g/mol) and possessed free carboxylic acid chain ends. These properties provide water miscibility and charge for the hydrophobic polymer, which enables the surfactant-free preparation and stable nanoparticle dispersion.
Therefore, different PLA nanoparticles were prepared for the freeze-drying experiments: particles without the surfactant and particles with the surfactant (poloxamer 188). The surfactant-containing particles were used as such or purified by the TFF. Average diameters of all the nanoparticles were approximately 200 nm, which showed that neither the surfactant nor its removal affected the particle size. More precisely, the measured particle sizes and PI, respectively, were as follows: nanoparticles without the surfactant, 202 nm and 0.03; nanoparticles with the surfactant, 201 nm and 0.01; surfactant-containing nanoparticles purified by the TFF, 199 nm and 0.03. Polydispersity indices of all types of the particles were low, indicating very homogeneous size distributions. Particle size of each batch was measured before the freeze-drying to ensure precise comparison with the particle size after the drying (Sf/Si).
The freeze-drying cycle was a generic one providing sufficient conditions for successful drying: the freezing was performed with low-temperature shelves (−40°C), and the primary drying was performed in a relatively low temperature (−35°C) for an extensively long period (1,020 min). A one-for-all cycle was used because of the different properties of the different excipients (see later).
Most of the studied nanoparticle–protectant combinations expressed the Tyndall effect (opalescence due to the light scattering of the colloidal nanoparticle dispersion). These batches were characterized to possess the AS “0” or “1” (Table I). However, the batches with AS “1” could not be considered as successfully freeze-dried ones because of the presence of some visible particles in the dispersion. In addition to aggregation, other typical signs of poor freeze-drying processes are increased size (increase in Sf/Si) and increase in polydispersity index indicating a broader size distribution. Therefore, a freeze-drying process of nanoparticles can be considered successful, if the particles are easily redispersed in liquid without aggregation, the Sf/Si remains close to 1.0 and the PI does not change.
Table I.
Freeze-drying of the PLA Nanoparticles Dried at Concentrations of 0.2% (w/v)
| S f/S i | PI | AS | S f/S i | PI | AS | S f/S i | PI | AS | |
|---|---|---|---|---|---|---|---|---|---|
| 5% of protectant | 2% of protectant | 1% of protectant | |||||||
| Trehalosea | 1.0 | < 0.1 | 0 | 1.0 | < 0.1 | 0 | nd | nd | 2 |
| Trehaloseb | 1.2 | < 0.1 | 0 | 1.2 | 0.2 | 0 | nd | nd | 1 |
| Trehalosec | 1.1 | < 0.1 | 0 | 1.2 | < 0.1 | 0 | 1.2 | 0.8 | 0 |
| Sucrosea | 1.0 | < 0.1 | 0 | 1.0 | < 0.1 | 0 | nd | nd | 2 |
| Sucroseb | 1.0 | < 0.1 | 0 | 1.0 | < 0.1 | 0 | nd | nd | 1 |
| Sucrosec | 1.0 | < 0.1 | 0 | 1.0 | < 0.1 | 0 | 1.1 | 0.2 | 0 |
| Lactosea | nd | nd | 1 | nd | nd | 1 | nd | nd | 1 |
| Lactoseb | nd | nd | 1 | nd | nd | 1 | nd | nd | 1 |
| Lactosec | 1.3 | < 0.1 | 0 | nd | nd | 1 | nd | nd | 1 |
| Glucosea | nd | nd | 2 | nd | nd | 2 | nd | nd | 2 |
| Glucoseb | 1.1 | < 0.1 | 0 | nd | nd | 2 | nd | nd | 2 |
| Glucosec | 1.0 | < 0.1 | 0 | nd | nd | 2 | nd | nd | 2 |
| Poloxamerb | 1.1 | < 0.1 | 0 | nd | nd | 1 | nd | nd | 1 |
| Lactose 4% and glucose 2% | Lactose 1.6% and glucose 0.8% | Lactose 0.8% and glucose 0.4% | |||||||
| Lactose and glucosea | 1.0 | < 0.1 | 0 | 1.0 | < 0.1 | 0 | nd | nd | 1 |
| Lactose and glucoseb | 1.2 | < 0.1 | 0 | 1.3 | < 0.1 | 0 | nd | nd | 1 |
| Lactose and glucosec | 1.0 | < 0.1 | 0 | 1.1 | 0.1 | 0 | nd | nd | 1 |
| Sugar 2.5% and poloxamer 2.5% | Sugar 2.5% and poloxamer 1% | Sugar 2.5% and poloxamer 0.5% | |||||||
| Lactose and poloxamerb | 1.1 | <0.1 | 0 | 1.1 | <0.1 | 0 | 1.2 | <0.1 | 0 |
| Glucose and poloxamerb | 1.0 | <0.1 | 0 | 1.0 | <0.1 | 0 | 1.0 | 0.1 | 0 |
Concentrations of the additives are expressed as percent w/v
S f/S i ratio of nanoparticle size after and before freeze-drying, PI polydispersity index, AS aggregation scale (0 no aggregation, 1 uniform visible small aggregates, 2 strong aggregation), nd not determined due to observed aggregation
aNanoparticles prepared without the surfactant
bNanoparticles prepared with poloxamer 188, no TFF purification
cNanoparticles prepared with poloxamer 188, purified by TFF
Effect of Nanoparticle Concentration
Increase in the nanoparticle concentration has been reported to favor the freeze-drying (20). However, drying of these PLA nanoparticles at higher concentration (0.4%) was not very successful. Proper cakes were formed, but generally the redispersed nanoparticle dispersions were highly aggregated. The only acceptable aggregate-free drying results were achieved with the nanoparticles prepared with the poloxamer 188 and protected by 5% sucrose (Sf/Si 1.3; PI < 0.1) and a combination of lactose 4% and glucose 2% (Sf/Si 1.0; PI < 0.1). Interestingly, this high nanoparticle concentration was aggregated instantly once it was mixed with the highest tested trehalose concentration (5%) before drying. PLA nanoparticles have been found to aggregate when the electrolyte concentration of the dispersion medium increases to a certain level (24). This disturbs the interparticle electrostatic repulsion, which is the most important stabilization mechanism of these PLA nanoparticles. Trehalose molecules as well as other sugars are not charged but very polar ones instead. In conditions of extreme dehydration (such as freeze-drying), e.g., trehalose and sucrose have similar protective action, but in an aqueous environment trehalose is bound (hydrogen bonds) much more effectively to biomaterials (25). This is due to the different orientation of the hydroxyl groups of the sugars. Probably, in the cases of high nanoparticle and trehalose concentrations, the concentration increase (although not caused by electrolytes) and the strong dehydration effect of the sugar towards the nanoparticles led to the destruction of dispersion stability.
Using a nanoparticle concentration of 0.2%, freeze-drying could be performed successfully with the different combinations and concentrations of excipients. Results of those PLA nanoparticles freeze-dried with the different protective excipients are presented in Table I and are the topic of the following discussion. Concentrations in the low range are frequently encountered in the nanoparticle studies (20,26), especially when the nanoprecipitation has been used as a nanoparticle preparation method (6,10).
Effect of the Protective Excipients
The three kinds of nanoparticles were freeze-dried without any excipients for comparison purposes. These batches produced appropriate cakes during drying, but the formulations were highly aggregated upon redispersion in water.
When the nanoparticles were dried at a concentration of 0.2% with a single protectant, the higher the protectant concentration was the better were the quality parameters of the nanoparticles. This is in good agreement with previous data, as 5% sugar concentrations have often provided enough material for cake formation and nanoparticle protection (2,4,26). In this case, 5% resulted in 25:1 sugar to nanoparticle mass ratio, which is relatively high in terms of the protectant amount. The high ratio might be one reason for the positive freeze-drying outcomes. However, considering the amount of the sugars, concentrations up to 30% have been frequently studied and reported (2,13,14). In this study, on the other hand, the ranges of mass ratios of the sugars to nanoparticles of 10:1 and 5:1 (originating from 2% and 1% of the sugar, respectively) are common (4,6,10,14,20,26).
The best protective sugars were trehalose and sucrose used as concentrations of 5% and 2% (Sf/Si close to 1.0 and PI around 0.1). These concentrations protected adequately all the three kinds of nanoparticles. Slight increase in size (Sf/Si), typical for the freeze-drying of nanoparticles, was observed in some samples with trehalose, but the polydispersity indices still remained low. Instead, 1% of sugar was not enough to protect the nanoparticles leading to aggregation or at least increase in polydispersity. The slightly better results with sucrose compared to trehalose originate from the different orientations of the hydroxyl groups of the sugars, as discussed earlier. As expected based on our previous study, lactose alone could not provide protection for the nanoparticles due to its poor protective action during the freezing step (15).
Generally, all the tested formulations produced decent cakes except the ones with glucose as the only protectant. Collapse of a product can take place if sublimation (primary drying) takes place above the collapse temperature (Tc) of the given composition (27). Characteristics for collapsed products are high residual water contents and long redispersion times. Indeed, the glucose batches were not immediately redispersed in water after the freeze-drying but required a longer time to be reconstituted. Tc of glucose is around −42°C (4), which was below the primary drying temperature (−35°C). Tcs of trehalose, sucrose, and lactose are approximately −29°C, −31°C, and −31°C, respectively (28). Naturally, the primary drying could be performed at very low temperatures, but that might not be relevant in terms of process duration and energy consumption.
Despite the poor appearance of the glucose-protected formulations, the nanoparticles could be recovered in the cases of surfactant-containing nanoparticles with 5% of glucose. When glucose and poloxamer 188 were added in the nanoparticle formulations as a combination, high-quality nanoparticles could be reconstituted without aggregation. The amount of glucose was kept constant (2.5%), while the amount of poloxamer 188 varied from 2.5% to 0.5%. Even the lowest amount of poloxamer 188 enhanced the appearance of the cake and enabled successful drying. On the other hand, if poloxamer 188 was used as the only added protectant, a concentration of 5% was needed for good reconstitution results. As glucose is known to be an efficient cryoprotectant during the freezing step (15), these results emphasize the role of freezing as the crucial part of the freeze-drying process.
In our previous study, we have used lactose and glucose successfully together compensating each other’s limitations in the freeze-drying process (15): lactose has provided good appearance for the dried product whereas glucose has protected the formulation during the freezing step. Therefore, in this study, lactose and glucose were tested as a combination using three different sugar concentrations. The two higher concentrations gave positive results (Sf/Si close to 1.0 and PI around 0.1), but the combination lactose 0.8% and glucose 0.4% did not help in the drying. Taking into account the results with single sugars, it is obvious that a total concentration of at least 2% of protectants is needed to ensure successful freeze-drying of these PLA nanoparticles. This means a 10:1 mass ratio of sugar(s) to nanoparticles.
As observed, poloxamer 188 enhanced the protective effect of glucose. Even higher enhancement occurred when poloxamer 188 was used with lactose: the nanoparticles could be reconstituted with Sf/Si slightly above 1.0 and PI around 0.1. Although the lowest combination concentration of the sugar and poloxamer 188 in samples was 3% (higher than the lowest concentration of single sugars and lactose and glucose), it can be assumed that poloxamer 188, used at a high concentration relative to the nanoparticle concentration, acts as a coprotectant during the freeze-drying. As the surfactant enhanced the effect of poor cryoprotectant lactose, it can be considered as a protectant for the freezing step. On the contrary to our findings, poloxamer 188 and similar surfactants are reported to be susceptible to crystallization during freeze-drying, which leads to aggregation of nanoparticles (13,29). Another explanation is that poloxamer is more soluble in cold water (which is encountered during freezing) due to increased hydrogen bonding leading to destabilization (26). However, in these cases, poloxamer is an indispensable part of the nanoparticle structure (aggregation occurs if the surfactant is removed from the surface) compared to the nanoparticles in our current study (the surfactant is not necessary). At the same time, combination of a sugar and poloxamer in freeze-drying has been reported to give positive results because the sugar dehydrates the surfactant in the bulk solution forcing it to the particle surface (to stabilize the particles) (3). It is also agreed that poloxamer 188 forms a hydrophilic layer at the particle surface helping redispersion after the freeze-drying (26). Probably, in our current study, the added surfactant remains on the nanoparticle surface providing protection during freeze-drying. Similar results have been reported recently considering poloxamer 188 as an indispensable protectant used together with sugars (10). However, in the current study, because satisfactory results can be achieved using the single sugars, use of the poloxamer 188 as an additional protectant is not relevant.
Effect of Tangential Flow Filtration
Poloxamers are widely used as nanoparticle stabilizers and they are generally considered as safe excipients in human use (30). However, removal of the excess surfactant material remaining in the nanoparticle formulations after preparation should be considered because it may modify the physiological, physicochemical, and drug release properties of the system(s). Therefore, the TFF was applied as a purification method in this study.
The volume used in the purification, diavolume, was 6: 120 ml of water on 20 ml of nanoparticle dispersion. This diavolume should reduce the amount of the excess poloxamer 188 remaining in the dispersion close to 0.1% of the initial amount (31).
Freeze-drying results of the tangential-flow-filtrated batches were better than those of the nonfiltrated. Aggregate-free dispersions were observed after redispersion even when 1% of trehalose or sucrose was used. Also, in this case, the outcome with sucrose (PI 0.2) was better than with trehalose (PI 0.8). Among the tested lactose concentrations, the only satisfactory result was achieved with a tangential-flow-filtrated batch protected with 5% of lactose. The origin for the better freeze-drying results after TFF may be the following: in the cases of nonfiltrated batches, the excess poloxamer 188 forms hydrogen bonds with the protective sugars (32) thus disturbing their protective mechanism. The amount of poloxamer 188 used in the nanoparticle preparation was 20 mg. On the other hand, 1% of a protective sugar in the dispersion was 12.5 mg (250 μl of 5% sugar solution added in 1 ml of nanoparticle dispersion). Although a part of the poloxamer 188 was included in the nanoparticle structure, excess surfactant still existed capable of forming the hydrogen bonds with the sugars (i.e., the amounts of poloxamer 188 and the sugar were in the same range). This obviously competed with the hydrogen bonding between the sugar and the nanoparticle surface, which is the assumed prerequisite for the protection during the freeze-drying process (16). This effect could be avoided by removing the excess surfactant by TFF. It should be remembered, however, that both the TFF-purified nanoparticles and the surfactant-containing nonfiltrated nanoparticles survived better during the drying than the surfactant-free nanoparticles.
Summarizing the three different types of PLA nanoparticles, the best freeze-drying results were achieved with the tangential-flow-filtrated batches, then with the poloxamer-188-containing nonfiltrated batches and, finally, the poorest results with the surfactant-free nanoparticles. Thus, these results are in agreement with the current understanding that the use of surfactant provides extra stability for the prepared nanoparticles towards further processing and resulting changes in the dispersion state (10,24,26).
Imaging by Electron Microscopy
Reconstituted nanoparticle dispersions after freeze-drying are usually deposited (and let to dry) to microscopy plates for electron microscopy visualization. Because the protective excipients are not removed, the nanoparticles are covered by a sheet formed by the excipients, which makes observations of the particles difficult (4,15,33). To avoid this, the nanoparticle dispersion can be deposited on an ultrafiltration membrane of proper size: the freeze-drying excipients are removed by the filtration and the nanoparticles remaining on the membrane surface can be visualized by a conventional electron microscopy.
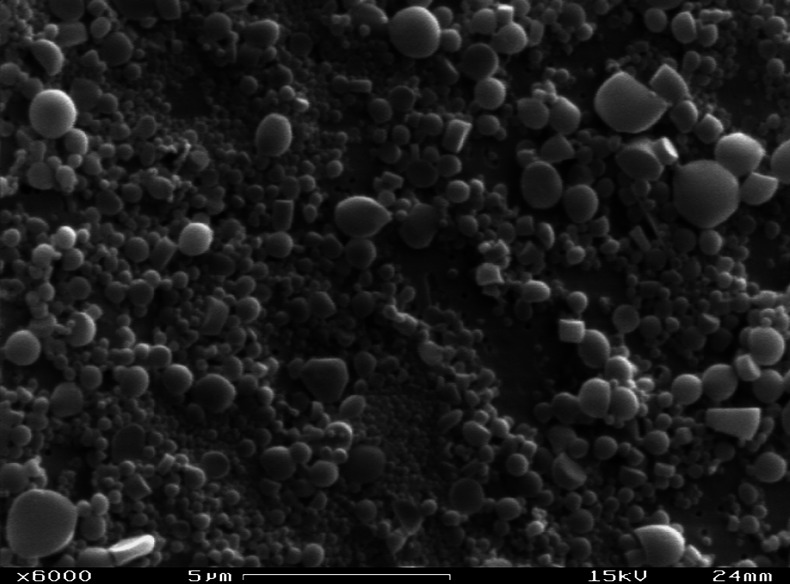
An example of a successfully freeze-dried nanoparticle population is presented in Fig. 2. For comparison, Fig. 3 shows a typical failure of the freeze-drying cycle: Tyndall effect could be observed, but with some visible aggregation and increases in particle size and polydispersity.
Fig. 2.
Example of successful freeze-drying process: TFF-filtrated PLA nanoparticles were freeze-dried with 2% of sucrose
Fig. 3.
Example of a poor freeze-drying process: PLA nanoparticles were freeze-dried with 2% of lactose
CONCLUSIONS
Poly(d,l-lactic acid) nanoparticles could be successfully freeze-dried when the concentrations of the nanoparticles and the protective excipients were optimized. A sugar concentration of at least 2% was needed for the successful drying, sucrose being the best choice for protection. Also, the combination of lactose and glucose gave positive results. In the case of these poly(lactic acid) nanoparticles, the use of poloxamer 188 surfactant was not necessary during the formulation and preparation steps, but it clearly enhanced the readiness of the nanoparticles for the freeze-drying. Furthermore, purification of the nanoparticles from the excess surfactant using tangential flow filtration enabled even better drying results when the different sugars were studied.
Acknowledgements
The Electron Microscopy Unit of the Institute of Biotechnology (University of Helsinki) is acknowledged for providing laboratory facilities and analytical equipment.
References
- 1.Couvreur P, Vauthier C. Nanotechnology: intelligent design to treat complex disease. Pharm Res. 2006;23:1417–1450. doi: 10.1007/s11095-006-0284-8. [DOI] [PubMed] [Google Scholar]
- 2.Chacón M, Molpeceres J, Berges L, Guzmán M, Aberturas MR. Stability and freeze-drying of cyclosporine loaded poly(d,l-lactide-glycolide) carriers. Eur J Pharm Sci. 1999;8:99–107. doi: 10.1016/S0928-0987(98)00066-9. [DOI] [PubMed] [Google Scholar]
- 3.Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Freeze-drying of nanoparticles: formulation, process and storage considerations. Adv Drug Deliv Rev. 2006;58:1688–1713. doi: 10.1016/j.addr.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Abdelwahed W, Degobert G, Fessi H. A pilot study of freeze drying of poly(ɛ-caprolactone) nanocapsules stabilized by poly(vinyl alcohol): formulation and process optimization. Int J Pharm. 2006;309:178–188. doi: 10.1016/j.ijpharm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 5.de Jaeghere F, Allémann E, Leroux J-C, Stevels W, Feijen J, Doelker E, et al. Formulation and lyoprotection of poly(lactic acid-co-ethylene oxide) nanoparticles: influence on physical stability and in vitro cell uptake. Pharm Res. 1999;16:859–866. doi: 10.1023/A:1018826103261. [DOI] [PubMed] [Google Scholar]
- 6.Jeong Y-I, Shim Y-H, Kim C, Lim G-T, Choi K-C, Yoon C. Effect of cryoprotectants on the reconstitution properties of surfactant-free nanoparticles of poly(d,l-lactide-co-glycolide) J Microencapsul. 2005;22:593–601. doi: 10.1080/02652040500162659. [DOI] [PubMed] [Google Scholar]
- 7.Konan YN, Gurny R, Allémann E. Preparation and characterization of sterile and freeze-dried sub-200 nm nanoparticles. Int J Pharm. 2002;233:239–252. doi: 10.1016/S0378-5173(01)00944-9. [DOI] [PubMed] [Google Scholar]
- 8.Cavalli R, Caputo O, Carlotti ME, Trotta M, Scarnecchia C, Gasco MR. Sterilization and freeze-drying of drug-free and drug-loaded solid lipid nanoparticles. Int J Pharm. 1997;148:47–54. doi: 10.1016/S0378-5173(96)04822-3. [DOI] [Google Scholar]
- 9.Schwarz C, Mehnert W. Freeze-drying of drug-free and drug-loaded solid lipid nanoparticles (SLN) Int J Pharm. 1997;157:171–179. doi: 10.1016/S0378-5173(97)00222-6. [DOI] [PubMed] [Google Scholar]
- 10.Layre A-M, Couvreur P, Richard J, Requier D, Ghermani NE, Gref R. Freeze-drying of composite core-shell nanoparticles. Drug Dev Ind Pharm. 2006;32:839–846. doi: 10.1080/03639040600685134. [DOI] [PubMed] [Google Scholar]
- 11.Anhorn MG, Mahler H-C, Langer K. Freeze drying of human serum albumin (HSA) nanoparticles with different excipients. Int J Pharm. 2008;363:162–169. doi: 10.1016/j.ijpharm.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Zillies JC, Zwiorek K, Hoffmann F, Vollmar A, Anchordoquy TJ, Winter G, et al. Formulation development of freeze-dried oligonucleotide-loaded gelatin nanoparticles. Eur J Pharm Biopharm. 2008;70:514–521. doi: 10.1016/j.ejpb.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 13.Saez A, Guzmán M, Molpeceres J, Aberturas MR. Freeze-drying of polycaprolactone and poly(d,l-lactic-glycolic) nanoparticles induce minor particle size changes affecting the oral pharmacokinetics of loaded drugs. Eur J Pharm Biopharm. 2000;50:379–387. doi: 10.1016/S0939-6411(00)00125-9. [DOI] [PubMed] [Google Scholar]
- 14.de Chasteigner S, Fessi H, Cavé G, Devissaguet JP, Puisieux F. Gastro-intestinal tolerance study of a freeze-dried oral dosage form of indometacin-loaded nanocapsules. STP Pharma Sci. 1995;5:242–246. [Google Scholar]
- 15.Hirsjärvi S, Peltonen L, Kainu L, Hirvonen J. Freeze-drying of low molecular weight poly(l-lactic acid) nanoparticles: effect of cryo- and lyoprotectants. J Nanosci Nanotechnol. 2006;6:3110–3117. doi: 10.1166/jnn.2006.439. [DOI] [PubMed] [Google Scholar]
- 16.Abdelwahed W, Degobert G, Fessi H. Investigation of nanocapsules stabilization by amorphous excipients during freeze-drying and storage. Eur J Pharm Biopharm. 2006;63:87–94. doi: 10.1016/j.ejpb.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Dalwadi G, Sunderland VB. Purification of PEGylated nanoparticles using tangential flow filtration (TFF) Drug Dev Ind Pharm. 2007;33:1030–1039. doi: 10.1080/03639040601180143. [DOI] [PubMed] [Google Scholar]
- 18.Dalwadi G, Benson H, Chen Y. Comparison of diafiltration and tangential flow filtration for purification of nanoparticle suspensions. Pharm Res. 2005;22:2152–2162. doi: 10.1007/s11095-005-7781-z. [DOI] [PubMed] [Google Scholar]
- 19.Limayem I, Charcosset C, Fessi H. Purification of nanoparticle suspensions by a concentration/diafiltration process. Sep Purif Technol. 2004;38:1–9. doi: 10.1016/j.seppur.2003.10.002. [DOI] [Google Scholar]
- 20.de Jaeghere F, Allémann E, Feijen J, Kissel T, Doelker E, Gurny R. Freeze-drying and lyopreservation of diblock and triblock poly(lactic acid)-poly(ethylene oxide) (PLA-PEO) copolymer nanoparticles. Pharm Dev Technol. 2000;5:473–483. doi: 10.1081/PDT-100102031. [DOI] [PubMed] [Google Scholar]
- 21.Hirsjärvi S, Peltonen L, Hirvonen J. Layer-by-layer polyelectrolyte coating of low molecular weight poly(lactic acid) nanoparticles. Colloid Surf B. 2006;49:93–99. doi: 10.1016/j.colsurfb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Sukhorukov GB, Donath E, Lichtenfeld H, Knippel E, Knippel M, Budde A, et al. Layer-by-layer self assembly of polyelectrolytes on colloidal particles. Colloid Surf A. 1998;137:253–266. doi: 10.1016/S0927-7757(98)00213-1. [DOI] [Google Scholar]
- 23.Fessi H, Puisieux F, Devissaguet JP, Ammoury N, Benita S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int J Pharm. 1989;55:R1–R4. doi: 10.1016/0378-5173(89)90281-0. [DOI] [Google Scholar]
- 24.Hirsjärvi S, Peltonen L, Hirvonen J. Surface pressure measurements in particle interaction and stability studies of poly(lactic acid) nanoparticles. Int J Pharm. 2008;348:153–160. doi: 10.1016/j.ijpharm.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Luzardo MdC, Amalfa F, Nuñez AM, Díaz S, Biondi de Lopez AC, Disalvo EA. Effect of trehalose and sucrose on the hydration and dipole potential of lipid bilayers. Biophys J. 2000;78:2452–2458. doi: 10.1016/S0006-3495(00)76789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quintanar-Guerrero D, Ganem-Quintanar A, Allémann E, Fessi H, Doelker E. Influence of the stabilizer coating layer on the purification and freeze-drying of poly(d,l-lactic acid) nanoparticles prepared by an emulsion–diffusion technique. J Microencapsul. 1998;15:107–119. doi: 10.3109/02652049809006840. [DOI] [PubMed] [Google Scholar]
- 27.Carpenter JF, Pikal MJ, Chang BS, Randolph TW. Rational design of stable lyophilized protein formulations: some practical advice. Pharm Res. 1997;14:969–975. doi: 10.1023/A:1012180707283. [DOI] [PubMed] [Google Scholar]
- 28.Adams GDJ, Ramsay JR. Optimizing the lyophilization cycle and the consequences of collapse on the pharmaceutical acceptability of Erwinial-asparaginase. J Pharm Sci. 1996;85:1301–1305. doi: 10.1021/js960146p. [DOI] [PubMed] [Google Scholar]
- 29.Zambaux MF, Bonneaux F, Gref R, Dellacherie E, Vigneron C. MPEO-PLA nanoparticles: effect of MPEO content on some of their surface properties. J Biomed Mater Res. 1999;44:109–115. doi: 10.1002/(SICI)1097-4636(199901)44:1<109::AID-JBM12>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 30.Singh-Joy SD, McLain VC. Safety assessment of poloxamers 101, 105, 108, 122, 123, 124, 181, 182, 183, 184, 185, 188, 212, 215, 217, 231, 234, 235, 237, 238, 282, 284, 288, 331, 333, 334, 335, 338, 401, 402, 403, and 407, poloxamer 105 benzoate, and poloxamer 182 dibenzoate as used in cosmetics. Int J Toxicol. 2008;27:93–128. doi: 10.1080/10915810802244595. [DOI] [PubMed] [Google Scholar]
- 31.Millipore Corporation . Technical brief: protein concentration and diafiltration by tangential flow filtration. Billerica: Millipore Corporation; 2003. [Google Scholar]
- 32.Kokol V. Interactions between polysaccharide polymer thickener and bifunctional bireactive dye in the presence of nonionic surfactants. Part 1: surface tension and rheological behavior of different polysaccharide solutions. Carbohydr Polym. 2002;50:227–236. doi: 10.1016/S0144-8617(02)00035-8. [DOI] [Google Scholar]
- 33.Choi MJ, Briancon S, Andrieu J, Min SG, Fessi H. Effect of freeze-drying process conditions on the stability of nanoparticles. Dry Technol. 2004;22:335–346. doi: 10.1081/DRT-120028238. [DOI] [Google Scholar]