Abstract
The present investigation aims at developing microemulsion-based formulations for topical delivery of acyclovir. Various microemulsions were developed using isopropyl myristate/Captex 355/Labrafac as an oil phase, Tween 20 as surfactant, Span 20 as cosurfactant, and water/dimethylsulfoxide (1:3) as an aqueous phase. Transcutol, eucalyptus oil, and peppermint oil were used as permeation enhancers. In vitro permeation studies through laca mice skin were performed using Franz diffusion cells. The optimum formulation containing 2.5% Transcutol as the penetration enhancer showed 1.7-fold enhancement in flux and permeation coefficient as compared to marketed cream and ointment formulation. In vivo antiviral studies were performed in female Balb/c mice against induced herpes simplex virus I infection. A single application of microemulsion formulation containing 2.5% Transcutol given 24 h post-injection resulted in complete suppression of development of herpetic skin lesions.
KEY WORDS: acyclovir, antiviral, herpes simplex virus, microemulsion, topical
INTRODUCTION
Acyclovir (ACV), a guanine analogue, is a first-line antiviral drug for the treatment of infections caused by the herpes viruses, including herpes simplex 1 and 2 (cold sores and genital herpes), varicella zoster (shingles and chicken pox), and the Epstein–Barr virus (mononucleosis). Recurrent herpes labialis and herpes genitalis represent the most common clinical manifestations associated with herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2). Topical formulations for the treatment of herpetic mucocutaneous infections have several potential benefits over oral and intravenous administration, including targeting of drug to the specific sites of infection, higher tissue drug levels, reduced side effects, lower treatment costs, and better patient compliance and convenience. The low efficacy of currently available topical ACV cream formulation may be attributed to poor penetration of ACV through the stratum corneum and thus lack of sufficient drug at the basal epidermis target site requiring five times a day application (1–3).
Our aim is to develop a therapeutically effective topical delivery system, which will enable sufficient penetration of the active drug to the inner skin layers with no significant systemic absorption. Improved penetration allows formation of a reservoir of active drug within the skin (4), thus maintaining persistent high levels of ACV at the infection site. Among the many options available in novel colloidal carriers, microemulsions offer advantage of enhanced drug solubility, good thermodynamic stability, and enhanced effects on skin penetration ability over conventional formulations (5). Microemulsions have been visualized as superior drug carriers because of its several permeation enhancement mechanisms such as an increased concentration gradient and thermodynamic activity toward skin and the permeation enhancement activity of components of microemulsions (6).
In this study, we have developed microemulsion-based topical formulations of ACV and evaluated their efficacy by in vitro skin permeation studies through mice skin, and finally, the optimized formulation was evaluated for its antiviral activity in murine model of cutaneous HSV-I infection.
MATERIALS
ACV was obtained as gift sample from Arochem Industries, Mumbai, India and Captex 355 EP/NF (glycerol caprylate caprate) was received as a gift from Abitec Corporation, UK. Labrafac CC (caprylic/capric triglycerides) and Transcutol (ethoxy diglycol) were gifted by Colorcon Asia Pvt. Ltd., India. Eucalyptus and peppermint oil were purchased from Ajanta Chemicals Co., Gurgaon, India. ACV Cream B.P. (Herpex, 5%®) manufactured by Torrent Pharmaceuticals Ltd., India was used as control in the studies. All other reagents/chemicals used were of AR grade.
METHODS
Solubility Studies
Solubility studies were carried out in water, water/dimethyl sulfoxide DMSO (1:3), phosphate buffer pH 6.4, and water/propylene glycol (PG; 1:1) using the standard method of solubility determination (7). An excess of drug was added to a fixed volume of each solvent (10 mL) in different flasks, which were kept at 37 ± 1°C in a thermostatic water shaker bath for 24 h. The flasks were removed after 24 h, contents were filtered through 0.22 μ filter, and filtrates were analyzed spectrophotometrically at λmax 254 nm after appropriate dilution.
Construction of Pseudoternary Phase Diagrams
To investigate the microemulsion region, pseudoternary phase diagrams were constructed by titration of varied concentrations of oil [isopropyl myristate (IPM)/Captex 355/Labrafac CC], water/DMSO (1:3), Tween 20, and Span 20 with a fixed ratio of Tween 20 and Span 20 (3:1). For each point, the required quantities of Tween 20, Span 20, and oil were gently mixed to form a monophasic mixture that was slowly titrated with aliquots of water/DMSO and stirred at 25°C for a sufficient time to attain equilibrium. After equilibrium was reached, the mixtures were checked visually for transparency. Clear and isotropic water-rich samples of low viscosity were deemed to be within the oil-in-water microemulsion region. Again Tween 20, Span 20, and water/DMSO mixture was mixed and titrated with oil and checked for transparency. Clear region was water-in-oil microemulsion region.
Preparation of Microemulsion Formulations
After identification of the microemulsion regions in the phase diagram, the microemulsion formulations were prepared (Table I). ACV (0.3% w/w) was incorporated into the aqueous phase (water/DMSO, 1:3) followed by the addition of Tween 20. Span 20 was dissolved in the oil followed by the addition of penetration enhancer, and then, the aqueous phase was added slowly to the oily phase under continuous stirring using magnetic stirrer at ambient temperature. Homogenous and stable microemulsions were formed spontaneously.
Table I.
Composition of Various Microemulsion Formulations of ACV
| Ingredients (quantities, % w/w) | ME | E-1 | E-2 | E-3 | T-1 | T-2 | T-3 | P-1 | P-2 | P-3 |
|---|---|---|---|---|---|---|---|---|---|---|
| Isopropyl Myristate | 38 | 36.63 | 35.13 | 32.63 | 37.61 | 36.11 | 33.6 | 36.63 | 35.13 | 32.63 |
| Tween 20 | 30 | 32.08 | 32.08 | 32.08 | 27.43 | 27.43 | 27.43 | 32.08 | 32.08 | 32.08 |
| Span 20 | 10 | 8.49 | 8.49 | 8.49 | 11.6 | 11.6 | 11.6 | 8.49 | 8.49 | 8.49 |
| Water: Dimethyl sulfoxide (1:3) | 22 | 21.8 | 21.8 | 21.8 | 22.36 | 22.36 | 22.36 | 21.8 | 21.8 | 21.8 |
| Eucalyptol | – | 1.0 | 2.5 | 5.0 | – | – | – | – | – | – |
| Transcutol | – | – | – | – | 1.0 | 2.5 | 5.0 | – | – | – |
| Peppermint | – | – | – | – | – | – | – | 1.0 | 2.5 | 5.0 |
Preparation of PEG Ointment
For comparative studies, ACV (5% w/w) was formulated in a conventional ointment system containing PEG 400 (37.5% w/w) and PEG 4000 (57.5% w/w). It was taken as control II, and ACV Cream B.P. (Herpex, 5%®) was taken as control I.
Characterization of Microemulsions
Drug content was determined using method given in British Pharmacopoeia (8). pH of the prepared formulation was determined using pH meter. Morphology and structure of the microstructures of drug-loaded microemulsion was determined with the aid of transmission electron microscopy. Droplet size and size distribution of prepared microemulsion were analyzed using laser light scattering technique (Malvern Zetasizer™). The developed formulations were studied for their rheological behavior using Brookfield digital viscometer equipped with small adaptor and spindle number 21. The microemulsions were submitted to up and down cycles (0–100 rpm spindle rotation speed) at 29 ± 1°C, and the rheological behavior of each disperse system was evaluated by plotting shear rate against shear stress.
Stability Studies
Three batches of each formulation were stored in sealed glass containers at 4°C, 25°C, and 40°C for 45 days. The stability of the microemulsions was checked periodically with respect to transparency, phase separation, color change, and drug content of ACV at three different temperature conditions.
In vitro Skin Permeation Study
In vitro permeation studies of microemulsion formulations of ACV were performed through skin of Laca mice using Franz diffusion cell assembly. The animals were killed by spinal dislocation method. The hair on the dorsal side of the animal was removed with the help of surgical blade number 23 in the direction of tail to head. The shaven part of the animal skin was separated. Hypodermis, including the blood vessels, was removed using surgical blade number 23. The excised skin was washed with normal saline and subsequently used. The skin was mounted on the diffusion cell assembly, with an effective diffusion area of 2.84 cm2, keeping the stratum corneum toward donor compartment. The receptor compartment consisted of 30 mL phosphate buffer, pH 6.4, maintained at 37°C. The prepared formulations containing an amount equivalent to 3 mg of ACV were applied on the membrane in the donor compartment. An aliquot of 1 mL sample was withdrawn at suitable time intervals and replaced immediately with an equal volume sof fresh diffusion medium. The samples were analyzed spectrophotometrically at 254 nm after suitable dilutions. All experiments were performed in triplicate. The cumulative amount of ACV permeated through mice skin was plotted as a function of time.
Skin Retention Study
At the end of the permeation experiments (after 24 h), the remaining formulation in the donor phase was scrapped off the skin, and the exposed skin surface was rinsed with water/DMSO (1:3) to remove excess drug from the surface. The receptor media was then replaced with fresh water/DMSO (1:3). Receptor contents were allowed to stir for the next 24 h. After 24 h, the media was analyzed for the amount of drug retained in skin (9).
Statistical analysis
All the data were statistically analyzed by one-way analysis of variance followed by multiple comparison Tukey test. P value of 0.05 was considered to be significant.
Histopathological Examination
Laca mice weighing around 20–25 g were used for the study. The hair on the dorsal side of animals was removed with surgical blade number 23 in the direction of tail to head without damaging the skin. Selected formulation T-2 was applied uniformly on the dorsal region. The microemulsion was kept in contact with the skin for 4 h. After that, the animals were killed with overdose inhalation of chloroform, and exposed dorsal surface was cut. Then, the specimens were fixed in 10% buffered formalin, embedded in paraffin, and microtoned. The sections were stained with hematoxylin and eosin. Finally, the specimens were observed under a high power light microscope.
Skin Sensitivity Testing
Selected formulations were applied on the forearm skin of 20 healthy human volunteers (age 20–25 years) and observed for any irritation, erythema, skin rash, and edema for 24 h. A prior written informed consent was obtained from all the volunteers, and they were apprised of any possible ill effects of the study.
In vivo Pharmacodynamics Study
Murine model of cutaneous HSV-I infection was used to study the antiviral effect of ACV microemulsion. HSV-I propagated in Vero cells (African green monkey kidney cells) in minimum essential medium and female BALB/c mice (5–7 weeks old) were used in this study (10). The plaque assay was performed to check the viral infectivity (11). Animals were anesthetized by intraperitoneal injection of a mixture containing 70 mg of ketamine hydrochloride and 11.5 mg of xylazine per kilogram of body weight. Mice were then cutaneously infected with HSV-1 after scarification (scratching the skin six times in a crossed-hatched pattern) of the shaved right mid-flank region with a 27-gauze needle held vertically (10,12). Treatment was initiated 24 h post-infection (i.e. before the appearance of zostiform rash). Mice in three groups of four each were treated topically five times daily with 15 mg of marketed cream (control I), 15 mg of PEG ointment (control II), and once daily with 250 µl microemulsion formulation (T-2) containing 0.75 mg ACV. The fourth untreated group served as control. The efficacy of different formulations was evaluated in terms of evolution of lesions and reduction in number of lesions.
RESULTS AND DISCUSSION
Solubility Studies
Solubility studies were carried out in different media to assess the solubility behavior so as to select the appropriate medium for in vitro permeation studies and optimal aqueous medium for developing water in oil microemulsion formulations of hydrophilic ACV. In water/DMSO (1:3), solubility of ACV was found to be highest (13.7 ± 0.3 mg/mL), followed by water/PG (1:1), and water and phosphate buffer pH 6.4 (Table II). Accordingly, water/DMSO (1:3) was chosen as the most appropriate aqueous phase for the development of microemulsion. In phosphate buffer, pH 6.4, the drug was found to be sufficiently soluble for being used as a receptor medium and for maintaining sink conditions during in vitro permeation studies.
Table II.
Solubility of ACV in Various Solvents
| Medium | Solubility (mg/mL) |
|---|---|
| Phosphate buffer, pH 6.4 | 1.4 ± 0.2 |
| Water | 2.5 ± 0.1 |
| Water/propylene glycol (1:1) | 3.5 ± 0.2 |
| Water/dimethyl sulfoxide (1:3) | 13.7 ± 0.3 |
Phase Studies
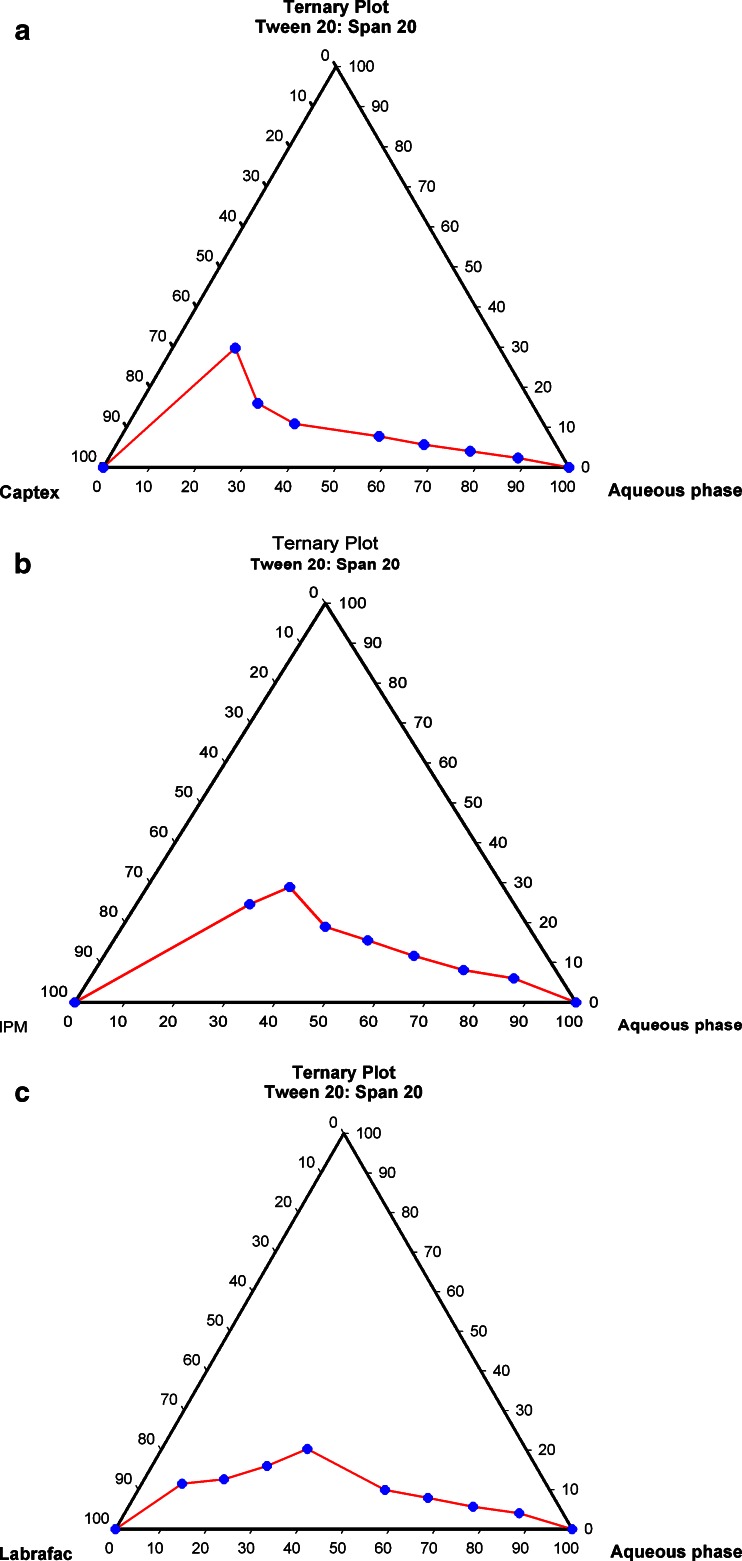
Phase studies were carried out to find the area of microemulsion existence and to investigate the effect of different surfactant/cosurfactant weight ratios on the extent of stable microemulsion region. The psuedoternary phase diagrams with various oils (isopropyl myristate, Captex, and Labrafac) are depicted in Fig. 1a–c. The weight ratio of 3:1 of Tween 20 and Span 20 as surfactant/cosurfactant mixture resulted in most stable microemulsion. Water/DMSO (1:3) was used as the aqueous phase. The largest area of microemulsion existence was observed in phase diagram employing isopropyl myristate as an oil phase. Hence, IPM (Fig. 1b) was selected as oil phase.
Fig. 1.
Pseudoternary phase diagram of the system containing Tween 20, Span 20, aqueous phase and a IPM, b Captex, and c Labrafac
Characterization of Microemulsion
Results of our study indicate development of successful microemulsion formulations of ACV with optimum characteristics. The drug content of different formulations was found to be in the range of 98 ± 0.15 to 99.89 ± 0.52%. The pH was found to vary between 6.32 and 6.72, a near neutral range ideal for topical applications. The formulations were clear and transparent. The transmission electron microscopic picture revealed spherical nature of the microemulsion droplets. The mean diameter was found to be 400 nm. The zeta potential of the prepared microemulsion was found to be −22 mV. Rheological studies showed Newtonian nature of the microemulsions. The viscosities of different formulations were found to be in the range of 182.4–372.2 mPa s at 29°C, suggesting suitability for topical application.
Stability Studies
Stability studies data at three different temperatures showed that all the selected microemulsion formulations were stable for drug content and other macroscopic characteristics like phase separation and color change. All the batches of microemulsion remained clear even after a period of 45 days at temperature 25°C and 40°C, but turbidity occurred in the formulations stored at temperature 4°C. However, when brought to room temperature, all the formulations returned to their initial states.
In vitro Skin Permeation Studies
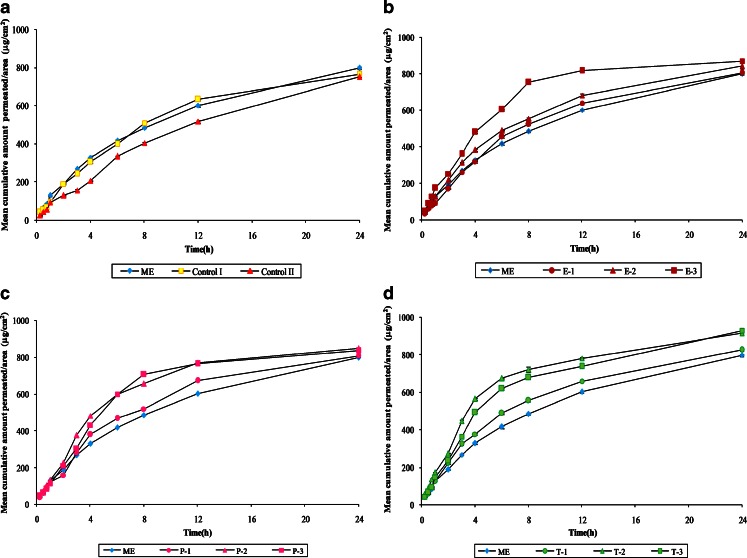
The permeation profiles of ACV through mice skin from various microemulsions are shown in Fig. 2a–c. As expected, the mean cumulative amount permeated per unit area of ME in 24 h (Fig. 2a) was found to be 798.48 ± 6.07 µg/cm2, which was significantly greater (P < 0.05) than control I, the marketed cream (763.18 ± 2.25 µg/cm2), and control II, the conventional PEG ointment (751.12 ± 3.1 µg/cm2). The lesser release from ointment can be explained on the basis of the drug–vehicle interaction that results in a lower thermodynamic activity of the drug (13,14). Other researchers have suggested that the retardant effect of PEG is due to its inability to hydrate the stratum corneum or to a relative osmotic effect, which tends to dehydrate the stratum corneum (15). Microemulsions and creams show better drug release because of the penetration-enhancing properties of their components. The steady-state flux (Fig. 3) followed the same suite with ME having the maximum flux followed by marketed cream (control I) and PEG ointment (control II).
Fig. 2.
Comparison of mean cumulative amount of ACV released per unit area of mice skin through a formulation ME, control I, and control II, b formulations ME, E-1, E-2, and E-3, c formulations ME, P-1, P-2, and P-3, d formulations ME, T-1, T-2, and T-3
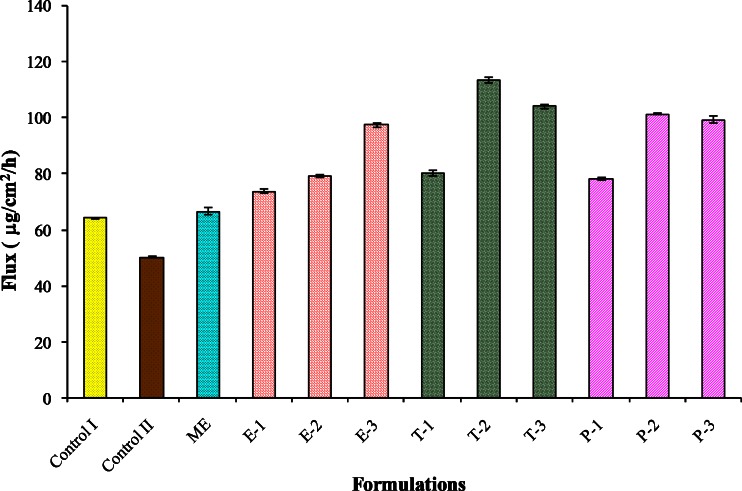
Fig. 3.
Comparison of flux of ACV from different formulations. Mean Flux values for all the microemulsions were significantly different from control II at P < 0.05. Mean flux values for all the microemulsions except ME were significantly different from control I at P < 0.05
In order to further improve the permeation rate of ACV from the microemulsion, various enhancers like eucalyptus oil, peppermint oil, and Transcutol in the concentration ranging from 1% to 5% were employed, and their effects were determined. Results indicate significant improvement in the permeation pattern of ACV with the incorporation of enhancers (Fig. 2b–d).
Upon increase in the concentration of eucalyptus oil from 1.0% (E-1) to 5.0% (E-3), there was a significant improvement in the permeation characteristics of the drug (804.57 ± 4.53 to 869.29 ± 2.29 µg/cm2 amount after 24 h; Fig. 2b). A 1.46-fold enhancement was seen in the flux of formulation E-3 containing 5% eucalyptol. The enhancement achieved with eucalyptus oil can be the result of combined process of partition and diffusion and the latter being dominant (16).
Peppermint oil also proved to be a good permeation enhancer. Formulation P-2 containing 2.5% peppermint oil showed highest mean cumulative amount permeated per unit area in 24 h among formulations P-1, P-2, and P-3. A 6.5% raise was seen in amount permeated in P-2 when compared with ME. No further significant improvement was seen in the permeation on increasing the amount of peppermint oil from 2.5% to 5% (Fig. 2c). Same trend was observed in the flux with P-2 formulation having an enhancement ratio of 1.52 (Fig. 3). Peppermint oil containing high proportion of l-menthol has been reported to alter skin permeation by a dual mechanism of forming a eutectic mixture with the penetrating compound, thereby increasing its solubility and by altering the barrier properties of the stratum corneum (17).
The presence of Transcutol in the formulations also resulted in an increase in the mean cumulative amount permeated per unit area in 24 h, indicating that Transcutol also acts as good penetration enhancer for ACV (Fig. 2d). Formulation T-2 containing 2.5% Transcutol resulted in maximum enhancement ratio (1.70) as well as the maximum flux (113.58 ± 0.85 µg cm−2 h−1; Fig. 3). The enhancing ability of Transcutol has been attributed to its ability to pass through the skin (18) and get incorporated into the multiple-lipid bilayers, thereby swelling the intercellular lipids (19).
Skin Retention Studies
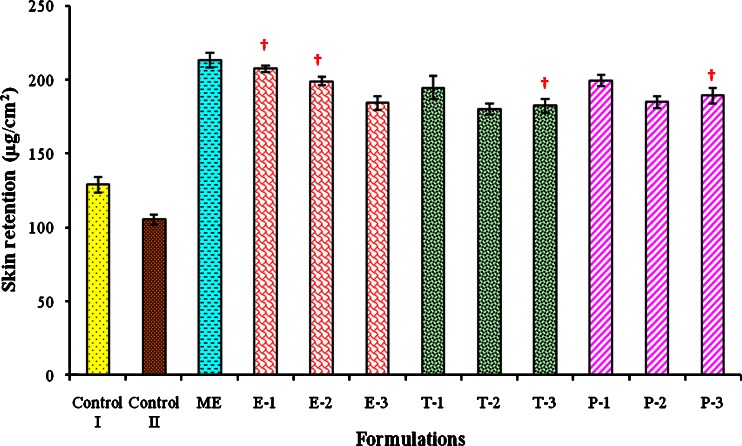
The amount of ACV retained in the skin was observed to be significantly higher (at P < 0.05) in all the microemulsion formulations (with or without enhancer) as compared to retention observed with the conventional cream (control I) or ointment (control II). These results indicate that the microemulsion formulations penetrate into the skin and form microdrug reservoirs that can hold the sufficient drug for a longer period of time and may help in the suppression of the viral growth more effectively. It was also observed that, as the concentration of permeation enhancers was increased, the amount permeated and also the flux was increased, resulting in the reduction in skin retention of ACV (Fig. 4).
Fig. 4.
Comparison of skin retention of ACV from different formulations. Dagger Mean value is not significantly different from preceding mean value at P < 0.05
Histopathological Studies
Dermal tolerance of microemulsion formulation T-2 (containing Transcutol as enhancer) was compared with untreated mouse skin. No anatomical or pathological changes were observed.
Skin Sensitivity Studies
The results of skin sensitivity testing using open patch test showed absence of any redness, irritation, and swelling, indicating the non-sensitizing characteristics of the tested microemulsion formulations.
In vivo Pharmacodynamic Studies
Exposure to HSV at mucosal surfaces or abraded skin sites permits the entry of virus and initiation of its replication in cells of epidermis and dermis (20). The pharmacodynamic evidence of the prepared ACV microemulsion formulations was carried out by employing the murine model of cutaneous HSV-1 infection. The efficacy of the optimized formulation containing 2.5% Transcutol as enhancer (T-2) was tested after single application given 24 h post-infection in mice. In the untreated infected animals, no pathological signs of cutaneous infection were visible during the first 5 days following the infection. This can be explained as when the virus is inoculated in the skin, where the primary infection occurs; it starts spreading from this site, probably by retrograde axonal flow, to sensory ganglia and the central nervous system. Thereafter, the virus reaches axons that innervate skin within the same dermatome as the inoculation site, spreads via orthograde flow into the skin, and produces herpetic lesions within the affected dermatome (10). On the sixth day, herpetic skin lesions appeared in the form of small vesicles distant from inoculation site. On the seventh day, ulcerations developed in the infected mice in local region. However, infected mice treated with all the three drug formulations (T-2, control I, and control II) developed no lesions, thus suggesting that the prepared microemulsion containing 2.5% Transcutol (T-2), which was applied once daily, was as effective as conventional marketed cream (control I) and PEG ointment (control II) that were applied five times daily, in the treatment of herpetic cutaneous lesions.
CONCLUSION
Results of in vitro permeation studies through mice skin suggest that, using microemulsion-based topical delivery system, ACV penetrates the skin well, retains in the skin, and may have efficacy superior to that of topical ointment and cream. Furthermore, the results of in vivo investigations using murine model of cutaneous HSV-1 confirms the efficacy of this formulation against experimental HSV-I infection. These encouraging outcomes of preliminary investigations strongly warrant further clinical investigations to examine the efficacy of these formulations in the treatment of human mucocutaneous herpes simplex virus disease.
Acknowledgments
The authors gratefully acknowledge the gift sample of ACV supplied by Arochem Industries (Mumbai, India), Captex 355 EP/NF (Abitec Corporation, UK), and Labrafac CC and Transcutol (Colorcon Asia Pvt. Ltd., India). Authors also thank Prof. Radha Kanta Ratho (Head) and Assistant Prof. Mini P. Singh, Department of Virology, Postgraduate Institute of Medical Education and Research, Chandigarh, India for their help and guidance and for providing laboratory facilities for antiviral studies.
References
- 1.Fiddian P, Yeo JM, Clark AE. Treatment of herpes labialis. J Infect. 1983;6:41–47. doi: 10.1016/S0163-4453(83)94118-X. [DOI] [PubMed] [Google Scholar]
- 2.Freeman DJ, Sheth NV, Spruance SL. Failure of topical acyclovir in ointment to penetrate human skin. Antimicrob Agents Chemother. 1986;29:730–732. doi: 10.1128/aac.29.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parry GE, Dunn P, Shah VP, Pershing LK. Acyclovir bioavailability in human skin. J Invest Dermatol. 1992;98:856–863. doi: 10.1111/1523-1747.ep12456948. [DOI] [PubMed] [Google Scholar]
- 4.Chikhale P, Bodor N. Improved delivery of acyclovir to the skin using a dihydrotrigonelline trigonelline REDOX carrier. J Pharm Sci. 1991;80:801–802. doi: 10.1002/jps.2600800425. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence MJ, Rees GD. Microemulsion based media as novel drug delivery systems. Adv Drug Del Rev. 2000;45:89–121. doi: 10.1016/S0169-409X(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 6.Peltola S, Savolainen P, Keisvaara J, Suhonen TM, Urtti A. Microemulsions for topical delivery of estradiol. Int J Pharm. 2003;254:99–107. doi: 10.1016/S0378-5173(02)00632-4. [DOI] [PubMed] [Google Scholar]
- 7.Aulton ME. Dissolution and solubility. In: Aulton ME, editor. Pharmaceutics. Edinburgh, UK: Churchill Livingstone; 2002. pp. 15–32. [Google Scholar]
- 8.British Pharmacopoeia, Vol. II. The stationary Office, London; 1999, p. A-87.
- 9.Jain S, Jain P, Jain NK, Umamaheshwari RB. Transfersomes—a novel vesicular carrier for enhanced transdermal delivery: development, characterization, and performance evaluation. Drug Dev Ind Pharm. 2003;29:1013–1026. doi: 10.1081/DDC-120025458. [DOI] [PubMed] [Google Scholar]
- 10.Piret J, Desormeaux A, Gourde AP, Juhasz J, Bergeron MG. Efficacies of topical formulations of foscarnet and acyclovir and of 5-percent acyclovir ointment (Zovirax) in a murine model of cutaneous herpes simplex virus type 1 infection. Antimicrob Agents Chemother. 2000;44:30–38. doi: 10.1128/AAC.44.1.30-38.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schimdt NJ. Cell culture techniques for diagnostics virology. In: Lennette EH, Schimdt NJ, editors. Diagnostic procedure for viral, rickettisial and chlamydial infections. Washington: American Public Health Association; 1979. pp. 100–104. [Google Scholar]
- 12.Kurokawa M, Basnet P, Ohsugi M, Hozumi T, Kadota S, Namba T, Kawana T, Shiraki K. Anti-herpes simplex virus activity of moronic acid purified from Rhus javanica in vitro and in vivo. J Pharmacol Exp Ther. 1999;289:72–78. [PubMed] [Google Scholar]
- 13.Davis SS, Hadgraft J, Al-Khamis K. Percutaneous absorption of methyl salicylate from polyethylene glycol vehicles. J Pharm Pharmacol. 1981;33:97P. [Google Scholar]
- 14.Hadgraft J. Percutaneous absorption: Possibilities and problems. Int J Pharm. 1983;16:255–270. doi: 10.1016/0378-5173(83)90145-X. [DOI] [Google Scholar]
- 15.Barret W, Hadgraft JW, Sarnaky I. The influence of vehicles on skin penetration. J Pharm Pharmacol. 1964;16:104T–107T. doi: 10.1111/j.2042-7158.1964.tb07545.x. [DOI] [PubMed] [Google Scholar]
- 16.Abdullah, Ping QN, Liu GH. Enhancing effect of essential oils on the penetration of 5-fluorouracil through rat skin. Yao Xue Xue Bao. 1996;31:214–221. [PubMed] [Google Scholar]
- 17.Kaplun-Frischoff Y, Touitou E. Testosterone skin permeation enhancement by menthol through formation of eutectic with drug and interaction with skin lipids. J Pharm Sci. 1997;86:1394–1399. doi: 10.1021/js9701465. [DOI] [PubMed] [Google Scholar]
- 18.Ganem-Quintanar, Lafforgue AC, Falson-Rieg F, Buri P. Evaluation of the transepidermal permeation of diethyleneglycol monoethylether and skin water loss. Int J Pharm. 1997;147:165–172. doi: 10.1016/S0378-5173(96)04809-0. [DOI] [Google Scholar]
- 19.Godwin A, Kim NH, Felton LA. Influence of Transcutol CG on the skin accumulation and transepidermal permeation of ultraviolet absorbers. Eur J Pharm Biopharm. 2002;53:23–27. doi: 10.1016/S0939-6411(01)00215-6. [DOI] [PubMed] [Google Scholar]
- 20.Wang F, Kief E. Viral diseases. In: Fauci AS, Braunwald E, Isselbacher KJ, Wilson JD, Martin JB, Kasper DL, Hauser SL, Longo DL, editors. Harrison’s principles of internal medicine. Singapore: McGraw-Hill; 1998. pp. 1076–1085. [Google Scholar]