Abstract
Porous calcium phosphate pellets were produced according to two granulation processes (low and high shear wet granulations) and drug loaded with five ibuprofen contents (1.75%, 7%, 12.5%, 22%, and 36%) in order to ensure both bone defect filling and local drug delivery. The drug-release kinetics from the two types of pellets was studied using three dissolution apparatuses: paddle apparatus, reciprocating cylinder, and flow-through cell. The paper compared the three dissolution methods and considered the effect of the granulation process on the ibuprofen-release kinetics. Dissolution data were analyzed using the Weibull function as well as the difference (f1) and similarity (f2) factors. Dissolution kinetics was not influenced by the granulation process, regardless of the dissolution apparatus and of the drug content. The comparison of the three dissolution devices indicated that ibuprofen was released faster from granules loaded with 36% of drug content with the reciprocating apparatus, due to the disintegration of the granules occurring during the dissolution test. For the other drug contents, dissolution profiles were not significantly different from one apparatus to another. However, the flow-through cell seemed to be more suitable for the drug-release study of implantable materials.
Key words: calcium phosphate pellets, flow-through cell, ibuprofen delivery system, in vitro drug release, paddle apparatus, reciprocating cylinder
INTRODUCTION
In pharmaceutical industry, in vitro dissolution test is performed early in order to validate initial screening among potential formulations to detect the influence of critical manufacturing variables and to help in the selection of the candidate formulation (1,2). The use of dissolution test can speed up the formulation development, enabling a prompt identification of potential problems in drug release (3). In vitro release testing is also a very important tool for batch to batch quality control (1,2,4). In Europe as well as in the USA, more than 30 years of research have been devoted to the characterization of the biopharmaceutical product properties (5–15). Several guidelines have been then published (16–18) and all pharmacopeias include recommendations concerning dissolution tests (19,20). Moreover, dissolution tests have become one of the primary pharmacopeial tests performed to ensure the dosage form compliance to quality standards (21). Furthermore, in vitro studies are the latest tests performed before the biological evaluations (18). However, the selection of the appropriate method and data interpretation are not easily affordable due to the influence of technological differences especially inducing varied hydrodynamic conditions (22,23).
From the last decades, various medical devices have been developed. Among these products, bone implants are common due to people aging all around the world. In fact, surgeons are confronted to more and more traumatic and degenerative bone diseases. Because of the drawbacks of autologous grafts, calcium phosphate substitutes, such as hydroxyapatite or tricalcium phosphate, have been increasingly used for bone-defect filling due to their chemical composition close to bone mineral phase (24–28). Indeed, these bioceramics are biocompatible, bioactive, osteoconductive, and resorbable (25,26,29,30). Moreover, they can offer great interest as drug delivery systems (31–34), achieving a therapeutic drug concentration directly at the site to be treated, while maintaining a low systemic drug level (35–38). In order to evaluate the drug-substance release as well as the biocompatibility and the osteoconductivity of such delivery systems, in vivo studies must be carried out. These tests require animals and cell cultures (39) and are consequently often long and expensive. Therefore, in vitro dissolution tests could be used in the first development stages prior to the in vivo experiments, as for drug products. However, dissolution devices described in the pharmacopeias relate to pharmaceutical dosage forms, but no standard apparatus has been defined so far for the study of drug release from biomaterials. Considering the variety of methods described in the literature to try to simulate the implanted conditions and characterize the drug delivery systems (40–44), it is proposed, in this paper, to investigate the three dissolution apparatuses described for testing oral dosage forms in US and European Pharmacopeias (19,20) because of the lack of biomaterial dissolution test harmonization.
The most common device is the paddle apparatus (apparatus 2). This assembly consists of a 1000-mL capacity glass vessel, which may be covered to limit evaporation phenomenon, and a paddle made up of a blade and a shaft, used as the stirring element. The vessel is immersed in a suitable water-bath maintained at 37°C during the test (19). The dosage unit form is poured into the vessel containing a fixed volume of dissolution medium. Samples are withdrawn at regular intervals in order to assay the drug dissolved.
The second apparatus is the reciprocating cylinder, introduced in the United States Pharmacopoeia in 1991 as USP Apparatus 3 (45). Its design is based on the disintegration tester (46). The apparatus is composed as follows:
a set of cylindrical, flat-bottomed glass outer vessels;
a set of glass reciprocating inner cylinders;
screens designed to fit the tops and the bottoms of the reciprocating cylinders.
The outer vessels are immersed in a water-bath maintaining the 250 mL of dissolution medium at 37°C during the run (19). The operation involves programming the agitation rate (in dip per minute, dpm) of the ups and downs for the inner tube inside the outer tube. At the upstroke, the bottom mesh in the inner tube moves upward to contact the tested form and at the downstroke, the sample leaves the mesh and floats freely within the inner tube, allowing the tested form to be studied through a moving medium (3,46). Defined volumes of dissolution medium are withdrawn at regular intervals prior to drug substance dosage.
The last dissolution method tested in this paper is the flow-through cell. The assembly consists of (19):
a reservoir and a pump ensuring a constant flow rate of the dissolution medium;
a flow-through cell adapted to the dosage unit form and mounted vertically. A 5-mm diameter ruby bead and 1-mm glass beads are respectively positioned at the apex and at the bottom cone of the cell in order to ensure a laminar flow of the dissolution medium entering the cell;
a water-bath maintaining the dissolution medium at 37°C.
One significant advantage of the flow-through cell is that sink conditions can be maintained, whatever the drug solubility, using an open loop. However, the run can also be performed in a closed-loop mode, in which a small volume of medium circulates through the system to provide sample concentration levels sufficient for the assay.
This paper describes the ability of the three dissolution apparatuses mentioned in US and European Pharmacopeias to study the release of ibuprofen, an anti-inflammatory agent, from calcium phosphate granules. These implantable bioceramics were elaborated by two granulation processes (low and high shear wet granulation) and loaded with five drug contents. The effect of granulation process and drug content on the dissolution profiles is also considered for the three dissolution apparatuses.
MATERIALS AND METHODS
Materials
Two types of phosphocalcic granules (710–1,000 µm) were produced, from calcium phosphate (CaP, batch number G8138/3, Cooper, France) with pregelatinized starch (Sepistab ST 200, batch number 80551, Seppic, France) as a binder. Either high shear wet granulation followed by spheronization in a Mi-Pro granulator (Pro-C-epT, Zelzate, Belgium) (47) or low shear wet granulation in a Kenwood mixer (model KM201, Kenwood Ltd, England) was used. Granules, thus, prepared were, respectively, called Mi-Pro pellets and Kenwood granules thereafter. Granules were then submitted to a heat treatment (up to 900°C) (48) in order to create porosity by the removal of the binder also used as a pore former. After that, granules were drug-loaded with 17.5, 67, 125, 222, and 364 mg of ibuprofen per gram of granules, corresponding, respectively, to 1.75%, 7%, 12.5%, 22%, and 36% of ibuprofen, by a solvent evaporation technique from an ethanolic ibuprofen solution (Ibuprofen 50, batch number IB1M738, BASF, Ludwigshafen, Germany) in a Rotavapor (Büchi, Switzerland).
In vitro Release of Ibuprofen
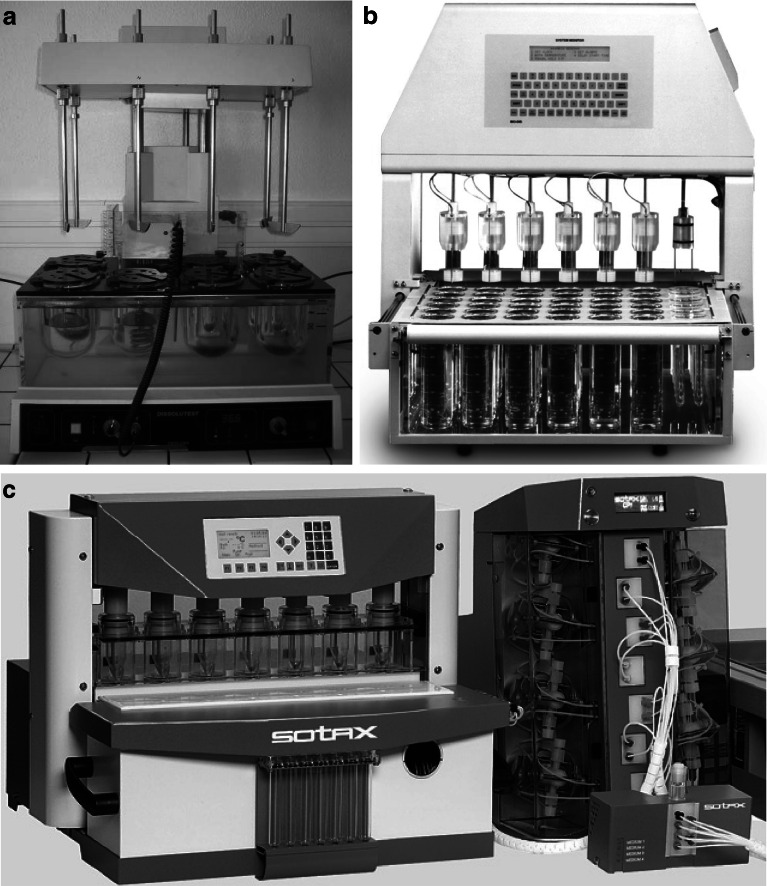
Dissolution tests were performed on loaded granules previously described, corresponding to the two granulation processes and the five ibuprofen contents. Unloaded granules were also tested and used as reference. Trials were carried out, in triplicate, up to the total release of ibuprofen (49), using a phosphate buffer solution (pH 7.48) at 37°C as the dissolution medium. Ibuprofen quantity was determined, at regular intervals, by UV absorption spectrophotometry at 264 nm, corresponding to the wavelength of maximal absorption. Three dissolution apparatuses (Fig. 1) were used (19,20):
rotating paddle apparatus (apparatus 2, Prolabo Dissolution Tester, France) equipped with a paddle stirrer rotating at 100 rpm (Fig. 1a). The vessels contained 500 mL of dissolution medium and about 550 mg accurately weighted of loaded granules. Three milliliters of dissolution medium were withdrawn and filtered before ibuprofen quantity was determined with an offline UV-Vis spectrophotometer (Uvikon 930, Kontron Instrument, UK);
reciprocating cylinder (apparatus 3, Bio-Dis, Varian, Cary, CA, USA) equipped with outer tubes containing 250 mL of medium (Fig. 1b). The top and the bottom of the inner tubes were polypropylene sieves of 405 meshes. About 275 mg accurately weighted of loaded granules were placed on the sieve of the inner tube, agitated at 15 dpm. Three milliliters of dissolution medium were withdrawn and filtered before ibuprofen dosage was performed offline with a UV-Vis spectrophotometer (Uvikon 930, Kontron Instrument, UK);
flow-through cell (apparatus 4) equipped with tablet cells of 12 mm (Fig. 1c). A ruby bead of 5 mm diameter and glass beads of 1 mm diameter were placed in the apex of the flow-through cell in order to ensure laminar flow of the 250 mL of dissolution medium, previously deaerated by ultrasonic waves, entering into the cell with a flow rate of 8 mL min−1. About 275 mg accurately weighted of granules were placed on the glass bead bed. The automated system CE 7smart (Sotax, Basel, Switzerland) was linked to a piston pump CP7–35 (Sotax, Basel, Switzerland) and a UV-VIS spectrophotometer (Lambda 20, Elmer Perkin, USA) for a direct online analysis of the filtered sample.
Fig. 1.
Dissolution apparatuses a paddle apparatus (dissolution tester, Prolabo) b reciprocating cylinder (Bio-Dis, Varian), and c flow-through cell (CE 7smart, Sotax)
Dissolution Data Treatment
Dissolution profiles, i.e., cumulative percentage of drug release (Q, %) versus time (t, min), obtained with the three configurations were plotted for Mi-Pro pellets and Kenwood granules for the five tested drug contents.
They were analyzed using the Weibull function (50) in order to determine the characteristic time TW 80% (min), corresponding to the time necessary to dissolve 80% of the drug.
Then, release kinetics were compared one to one using the difference (f1) and similarity factors (f2), as indicated by the FDA Center of Drug Evaluation and Research (16,17) in order to evaluate, for each drug content:
the influence of the dissolution method on ibuprofen release;
the effect of the granulation process on the release kinetics.
Two dissolution profiles are considered similar when the f1 value varies from 0 to 15 and f2 is greater than or equal to 50. It should be noted that at least three points are necessary to compare dissolution profiles with the f1f2 procedure (51,52). Moreover, when 85% or more of the drug substance is dissolved in 15 min or less, the profile comparison with f1f2 is unnecessary (53), and the drug-release profiles are considered as similar.
Results were then modeled according to two mathematical equations, respectively characterizing diffusion or erosion prevalence, Higuchi’s square root of time equation (54):
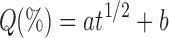 |
1 |
where a is the release rate (% min−1/2) and b a constant; and Hixson–Crowell’s cube root of time equation (55):
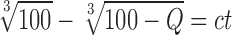 |
2 |
where c is the release rate.
Finally, dissolution data were fitted to the Kopcha’s empirical equation (56) in order to quantify, when both mechanisms occur, the respective diffusion (A) and erosion (B) contributions:
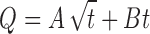 |
3 |
According to this equation, if diffusion/erosion ratio A/B = 1, release mechanism is equally controlled by diffusion and erosion. If A/B > 1, then diffusion prevails and if A/B < 1, then erosion predominates.
The initial rate of release was also calculated considering the quantity of ibuprofen (Q, mg) effectively dissolved during the first 5 min.
RESULTS AND DISCUSSION
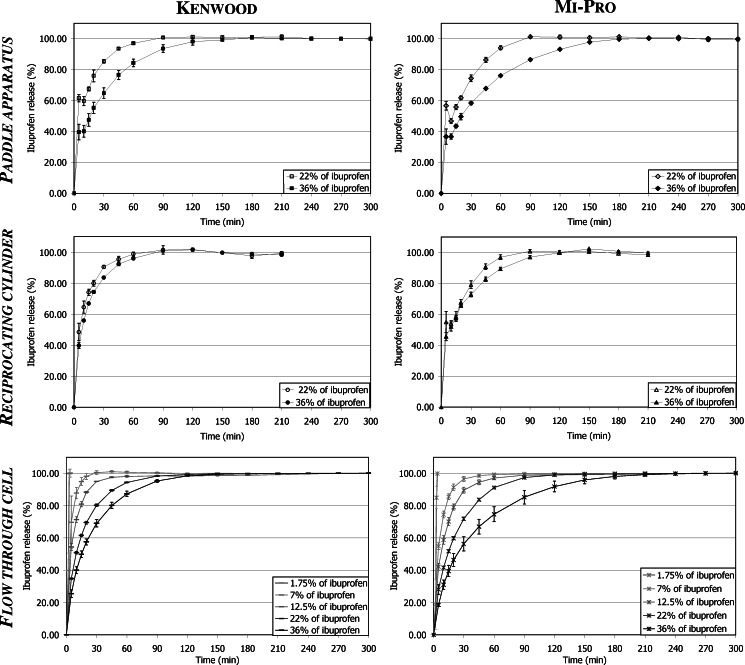
The dissolution profiles obtained with each dissolution apparatus for the two types of granules are presented in Fig. 2. They highlight the ability of the three dissolution methods to allow the total release of the ibuprofen quantity deposited during the loading procedure.
Fig. 2.
Ibuprofen dissolution profiles from the two types of granules in each apparatus
Results indicated that only the flow-through cell could be used to test granules loaded with the lower ibuprofen contents (drug contents smaller than 22%). In fact, in the case of the paddle apparatus and of the reciprocating cylinder, the ibuprofen concentrations were too low to be accurately quantified by UV spectroscopy due to the device configurations and to the experimental conditions (vessel sizes, volumes of dissolution medium). To limit these drawbacks, two kinds of adjustment might be considered:
decreasing the dissolution volume, but smaller equipments would be required (vessel/paddle and tube, respectively);
increasing the sample quantity, but this could be difficult in development stages
Consequently, it should be noticed that for dissolution data analyses:
TW 80% could not be calculated in all cases, either because dissolution data were scattered, with a coefficient of variation larger than 15% (low drug contents in paddle apparatus and reciprocating cylinder as seen previously), or because the release was too fast to be modeled (granules loaded with 1.75% of ibuprofen, tested in flow-through cell);
f1f2 comparison could not be achieved for the lower drug contents (1.75% and 7%) as they did not satisfy the requirements mentioned in “Dissolution Data Treatment.”
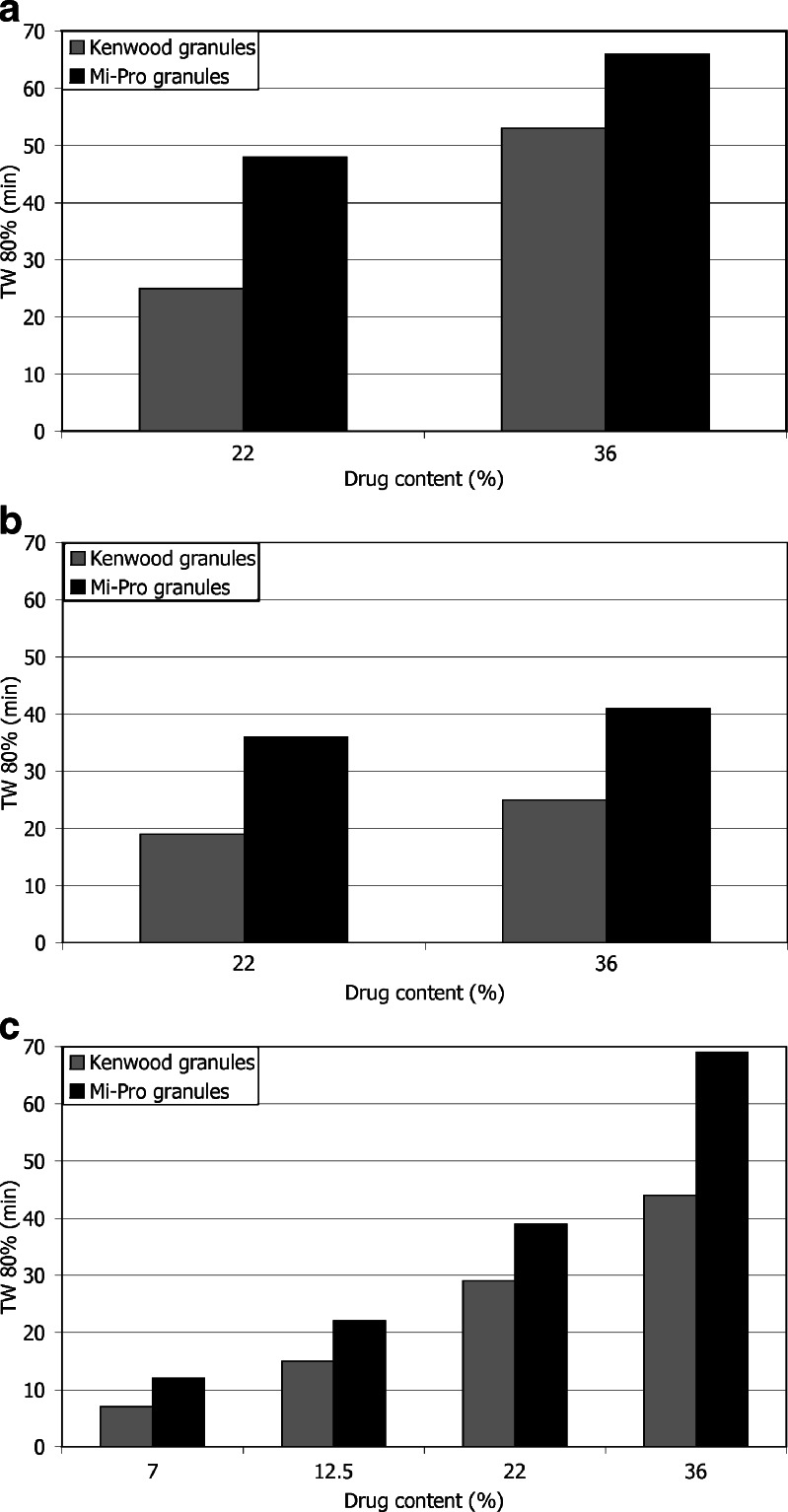
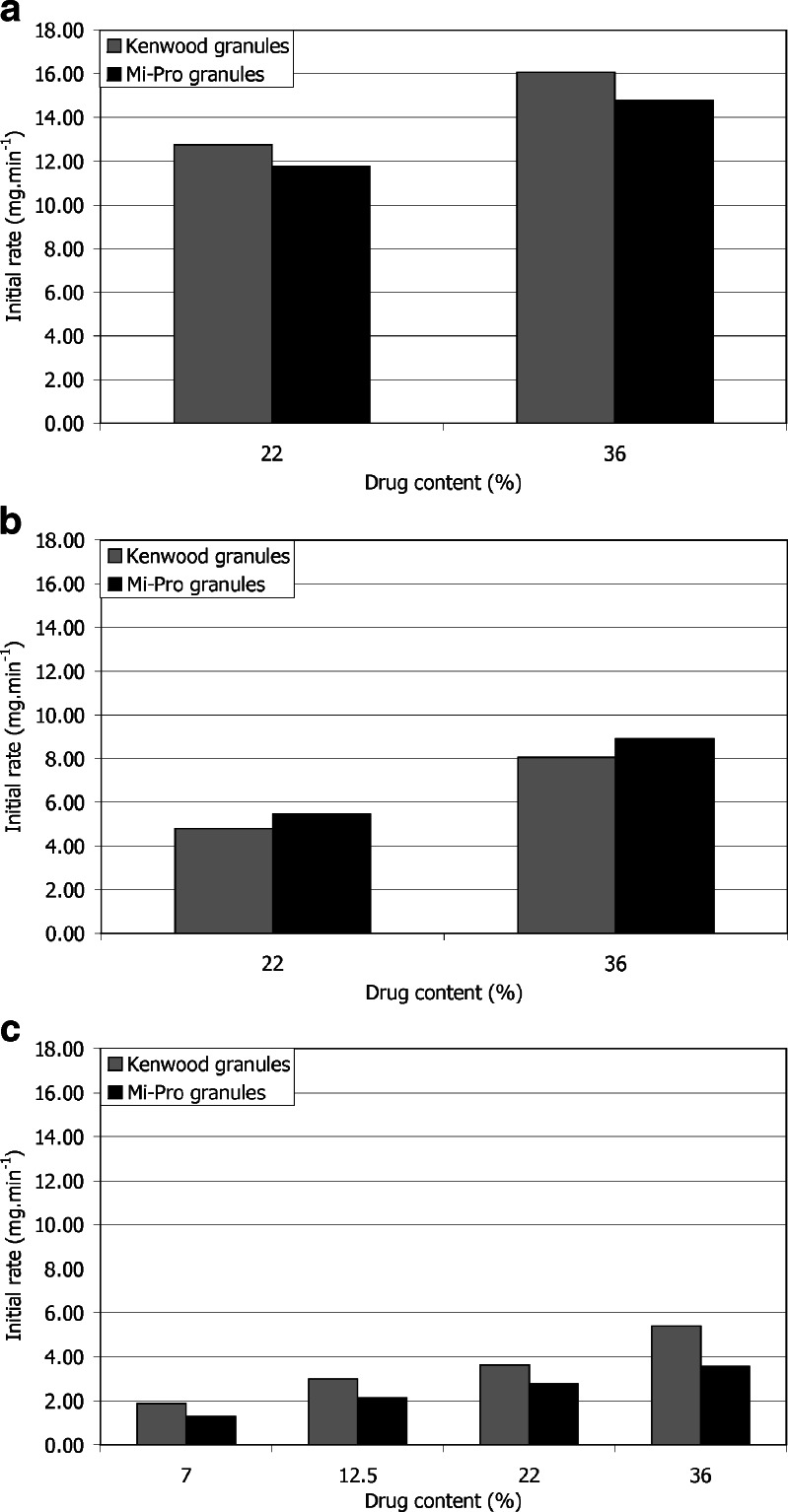
Ibuprofen appeared to be faster released in the reciprocating cylinder (Fig. 2) as indicated by the lower TW 80% values (Fig. 3). Nevertheless, despite these lower TW 80% values, the pairwise procedure indicated that the dissolution kinetics was significantly faster with the reciprocating cylinder, only for 36% of ibuprofen content (Table I). It should be noted that TW 80% value increased, for the three apparatuses, when the drug content increased (Fig. 3), but this phenomenon was less obvious in the case of the reciprocating cylinder. In fact, the vertical up-and-down motion of the inner tube favored ibuprofen dissolution by shaking the granules. Furthermore, the design of the apparatus, based on the disintegration tester, also induced granule degradation as highlighted by the clouding of the dissolution medium occurring during the dissolution test. This required to take into account the absorbance due to the partial disintegration of the CaP granules (see “In vitro release of Ibuprofen”). Considering the specific application as a bone implant, hydrodynamic conditions involved in the reciprocating cylinder were not well-adapted. Moreover, these particular agitation conditions were less discriminating to study the influence of drug content. Indeed, f1f2 procedure indicated no significant difference between the dissolution kinetics corresponding to 22% and 36% drug content, as opposed to the results obtained with the two other apparatuses (Table II). Figure 4 presents the initial rate of drug release for the three apparatuses. First, it appeared that both flow-through cell and paddle apparatus exhibited slower initial dissolution for Mi-Pro pellets than for Kenwood granules, which was in accordance with the discussion concerning TW 80% values. The reciprocating cylinder reversed the granulation process effect, probably due to its specific hydrodynamic conditions which submitted the granules to up-and-down motions. Such a way, the outer ibuprofen layer would be easier to be reached. The ibuprofen surface quantity was higher in the case of Mi-Pro pellets, explaining this higher initial rate (Chevalier et al. Ibuprofen loaded calcium phosphate granules: combination of innovative characterization methods to rely mechanical strength to drug location. Acta Biomater, submitted). Secondly, it could also be noticed that the initial kinetics decreased from paddle apparatus to reciprocating cylinder and finally flow-through cell. However, a downside should be pointed out for the paddle apparatus which involved large dissolution volumes compared to physiological conditions. Therefore, all the results promoted the use of the flow-through cell that appeared to be more suitable to evaluate drug release over a long period.
Fig. 3.
Evolution of the characteristic time TW 80% as a function of granule ibuprofen content in a paddle apparatus, b reciprocating cylinder, and c flow-through cell
Table I.
Comparison of Dissolution Profiles Obtained with the Three Dissolution Apparatuses
| Granulation process | Kenwood | Mi-Pro | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug content (%) | 22 | 36 | 22 | 36 | ||||||||||||||||||||
| Dissolution apparatus | P | RC | P | F | RC | F | P | RC | P | F | RC | F | P | RC | P | F | RC | F | P | RC | P | F | RC | F |
| f1 difference factor | 9.1 | 6.8 | 10.2 | 21.7 | 6.6 | 19.0 | 5.5 | 4.2 | 9.8 | 21.3 | 10.3 | 26.0 | ||||||||||||
| f2 similarity factor | 55.3 | 60.6 | 49.8 | 41.1 | 60.3 | 42.0 | 68.4 | 73.1 | 53.9 | 44.6 | 54.4 | 36.1 | ||||||||||||
| Statistical significance | No difference | No difference | No difference | Difference | No difference | Difference | No difference | No difference | No difference | Difference | No difference | Difference | ||||||||||||
P paddle apparatus, RC reciprocating cylinder, F flow-through cell
Table II.
Comparison of Dissolution Profiles Obtained from Granules Loaded with Various Ibuprofen Contents
| Dissolution apparatus | Paddle apparatus | Reciprocating cylinder | Flow through cell | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Granulation process | Kenwood | Mi-Pro | Kenwood | Mi-Pro | Kenwood | Mi-Pro | ||||||||||||||
| Drug content (%) | 22 | 36 | 22 | 36 | 22 | 36 | 22 | 36 | 12.5 | 22 | 12.5 | 36 | 22 | 36 | 12.5 | 22 | 12.5 | 36 | 22 | 36 |
| f1 difference factor | 21.1 | 17.4 | 7.5 | 5.5 | 16.6 | 25.5 | 13.8 | 15.9 | 32.8 | 17.4 | ||||||||||
| f2 similarity factor | 36.8 | 42.9 | 59.0 | 62.3 | 40.0 | 30.6 | 49.8 | 43.7 | 25.0 | 47.1 | ||||||||||
| Statistical significance | Difference | Difference | No difference | No difference | Difference | Difference | Difference | Difference | Difference | Difference | ||||||||||
Fig. 4.
Initial rate of the drug release as a function of granule ibuprofen content in a paddle apparatus, b reciprocating cylinder, and c flow-through cell
Dissolution data were modeled in order to determine the release mechanism occurring in the three dissolution devices. The correlation coefficients (r2) for Higuchi’s and Hixson–Crowell’s equations, given in Table III, were similar in all cases, preventing diffusion or erosion prevalence to be assumed. A strong correlation with Kopcha’s equation (r2 > 0.99) confirmed the coexistence of diffusion and erosion. Furthermore, the A/B ratio, higher than 1, indicated that diffusion prevailed (56), regardless of the granulation process and of the drug content. Thus, even in the case of the reciprocating cylinder where granules were partially disintegrated during the test, release mechanism was preserved, confirming its relation with the granule formulation. As diffusion prevalence was better highlighted by flow-through cell compared to paddle apparatus and reciprocating cylinder (Table III), the flow-through cell was recommended for this specific application.
Table III.
Modeled Dissolution Characteristics
| Dissolution apparatus | Paddle apparatus | Reciprocating cylinder | Flow through cell | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug content (%) | 22 | 36 | 22 | 36 | 22 | 36 | |||||||
| Granulation process | K | MP | K | MP | K | MP | K | MP | K | MP | K | MP | |
| Higuchi | r 2 | 0.994 | 0.998 | 0.995 | 0.992 | 0.986 | 0.983 | 0.980 | 0.995 | 0.972 | 0.992 | 0.981 | 0.981 |
| Hixson–Crowell | r 2 | 0.996 | 0.999 | 0.995 | 0.992 | 0.984 | 0.989 | 0.980 | 0.996 | 0.980 | 0.991 | 0.982 | 0.977 |
| Kopcha | R 2 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.997 | 0.999 | 0.992 | 0.999 | 0.999 | 0.998 | 0.995 |
| A/B | 1.60 | 2.94 | 5.12 | 4.01 | 2.32 | 1.99 | 6.95 | 1.42 | 15.36 | 5.35 | 15.41 | 33.47 | |
K Kenwood, MP Mi-Pro
Based on the previous considerations concerning dissolution tests, some conclusions may be drawn about the influence of the granulation process. TW 80% values were always higher for Mi-Pro granules (Fig. 3); this might be related to their higher rupture strength, their lower porosity, and their higher sphericity, previously studied (data not published). Nevertheless, the dissolution kinetics of ibuprofen from granules obtained by the two granulation processes were not statistically different, whatever the drug content and the dissolution apparatus (Table IV). In fact, even in the reciprocating cylinder, where samples were submitted to a significant motion, no difference was observed in the behavior between the two types of granules.
Table IV.
Comparison of Dissolution Profiles of the Two Types of Granules
| Dissolution apparatus | Paddle apparatus | Reciprocating cylinder | Flow through cell | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug content (%) | 22 | 36 | 22 | 36 | 12.5 | 22 | 36 | |||||||
| Granulation process | K | MP | K | MP | K | MP | K | MP | K | MP | K | MP | K | MP |
| f1 difference factor | 10.4 | 6.5 | 10.3 | 9.5 | 9.7 | 11.0 | 14.7 | |||||||
| f2 similarity factor | 50.9 | 67.9 | 50.4 | 56.3 | 51.3 | 54.6 | 51.4 | |||||||
| Statistical significance | No difference | No difference | No difference | No difference | No difference | No difference | No difference | |||||||
K Kenwood, MP Mi-Pro
CONCLUSION
Although discriminating dissolution conditions are interesting to develop drug delivery systems, difficulties may arise in routine quality control as well as in bioequivalence studies when dosage forms present high sensitivity to external dissolution conditions (2). The present work compared ibuprofen release from two types of granules prepared either by low shear or by high shear granulation process, intended for bone implantation. In vitro dissolution studies were performed with three compendial apparatuses used in pharmaceutical field (19,20). Release kinetics was not influenced by the granulation process, regardless of the dissolution apparatus. The three devices were able to exhibit how dissolution time increased with the ibuprofen content. This paper demonstrated the importance of the apparatus selection, as dissolution method and conditions influenced ibuprofen release from the two types of granules. In the case of the reciprocating cylinder, dissolution kinetics was faster, and the effect of the granulation process could hardly be taken into account. This dissolution method was less discriminating, probably due to the specific design and motion of the reciprocating cylinder, inducing an undesirable disintegration of the granules. Therefore, despite the short time and the small volume required for dissolution test in the reciprocating cylinder, both interesting for routine tests, the paddle apparatus and the flow-through cell should be preferred in this specific application. Nevertheless, the volume of dissolution medium used in the paddle apparatus, even though reduced in this study, was still too important in comparison with the in vivo conditions. Therefore, to develop bone implantable materials used as drug delivery systems, the compendial flow-through cell seems to be more suitable. Furthermore, the dissolution medium flow rate could be adjusted in order to better mimic bone fluid hydrodynamic conditions. In this purpose, it would be also interesting to test either the dissolution apparatus 7 which is a compendial small volume apparatus recently developed for testing medical devices (57) and the T apparatus which was designed from the flow-through cell to control the convection and diffusion processes all around the dosage form (58,59).
However, in order to support the in vitro dissolution data obtained, in vivo experiments have to be performed to establish in vitro/in vivo correlations and to conclude to the relevance of the dissolution test.
Acknowledgments
The authors thank the Région Limousin for its financial support.
References
- 1.Pillay V, Fassihi R. Evaluation and comparison of dissolution data derived from different modified release dosage forms: an alternative method. J Contr Rel. 1998;55:45–55. doi: 10.1016/S0168-3659(98)00022-4. [DOI] [PubMed] [Google Scholar]
- 2.Missaghi S, Fassihi R. Release characterization of dimehydrinate from an eroding and swelling matrix: selection of appropriate dissolution apparatus. Int J Pharm. 2005;293:35–42. doi: 10.1016/j.ijpharm.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 3.Joshi A, Pund S, Nivsarkar M, Vasu K, Shishoo C. Dissolution test for site-specific release isomiazid pellets in USP apparatus 3 (reciprocating cylinder): optimization using response surface methodology. Eur J Pharm Biopharm. 2008;69:769–75. doi: 10.1016/j.ejpb.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 4.Siewert M, Dressman J, Brown CK, Shah VP. FIP/AAPS Guidelines to dissolution in vitro release testing of novel/special dosage forms. AAPS PharmSciTech. 2003;4:1–10. doi: 10.1208/pt040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattok GL, McGilveray IJ, Hossie RD. Technical problems of the USP/NF dissolution test. J Pham Sci. 1971;61:460–2. doi: 10.1002/jps.2600610331. [DOI] [PubMed] [Google Scholar]
- 6.Smolen VF, Weigand WA. Optimally predictive in vitro drug dissolution testing for in vivo bioavailability. J Pharm Sci. 1976;65:1718–24. doi: 10.1002/jps.2600651207. [DOI] [PubMed] [Google Scholar]
- 7.Nasir SS, Wilken Jr LO, Nasir SM. New in vitro dissolution test apparatus. J Pharm Sci. 1978;68:177–81. doi: 10.1002/jps.2600680215. [DOI] [PubMed] [Google Scholar]
- 8.Roseman TJ, Derr GR, Nelson KG, Lieberman BL, Butler SS. Continuous flow bead-bed dissolution apparatus for suppositories. J Pharm Sci. 1980;70:646–51. doi: 10.1002/jps.2600700618. [DOI] [PubMed] [Google Scholar]
- 9.Brossard C, Lefort des Ylouses D, Duchêne D, Puisieux F, Carstensen JT. Dissolution of a soluble drug substance from vinyl polymer matrices. J Pharm Sci. 1982;72:162–9. doi: 10.1002/jps.2600720217. [DOI] [PubMed] [Google Scholar]
- 10.Aiache JM, Islasse M, Beyssac E, Aiache S, Renoux R, Kantelip JP. Kinetics of indomethacin release from suppositories. In vitro-in vivo correlation. Int J Pharm. 1987;39:235–42. doi: 10.1016/0378-5173(87)90221-3. [DOI] [Google Scholar]
- 11.Aiache JM. French and /or European perspectives on biopharmaceutical characterization of drug dosage forms. J Pharm Biomed Anal. 1990;8:499–506. doi: 10.1016/0731-7085(90)80059-X. [DOI] [PubMed] [Google Scholar]
- 12.Mehta AC. Dissolution testing of tablet and capsule dosage forms. J Clin Pharm Ther. 1993;18:415–20. doi: 10.1111/j.1365-2710.1993.tb00880.x. [DOI] [Google Scholar]
- 13.Jorgensen E, Bhagwat D. Development of dissolution tests for oral extended-release products. PSTT. 1998;1:128–35. [Google Scholar]
- 14.Beyssac E, Lavigne J. Dissolution study of active pharmaceutical ingredients using the flow through apparatus USP 4. Dissolution Technologies. 2005;12:23–5. [Google Scholar]
- 15.Graffner C. Regulatory aspects of drug dissolution from a European perspective. Eur J Pharm Sci. 2006;29:288–93. doi: 10.1016/j.ejps.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 16.US Department of Health and Human Services, Food and Drug Administration, Center of Drug Evaluation and Research (CDER) Guidance for industry: Dissolution testing of immediate release solid dosage forms. Rockville: FDA; 1997. [Google Scholar]
- 17.US Department of Health and Human Services, Food and Drug Administration, Center of Drug Evaluation and Research (CDER) Guidance for industry: Extended release oral dosage forms: development, evaluation and application of in vitro/in vivo correlations. Rockville: FDA; 1997. [Google Scholar]
- 18.International Conference of Harmonization, ICH Q8 Pharmaceutical Development, www.ich.org; 2 September 2008.
- 19.Council of Europe . European Pharmacopoeia. 6. Strasbourg: Council of Europe; 2006. [Google Scholar]
- 20.The United States Pharmacopoeia 29th ed, United States Pharmacopoeial Convention, Rockville, 2006.
- 21.Maggio RM, Castellano PM, Kaufman TS. A new principle component analysis-based approach for testing “similarity” of drug dissolution profiles. Eur J Pharm Sci. 2008;34:66–77. doi: 10.1016/j.ejps.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Dürig T, Fassihi R. Evaluation of floating and sticking extended release delivery systems: an unconventional dissolution test. J Contr Release. 2000;67:37–44. doi: 10.1016/S0168-3659(00)00194-2. [DOI] [PubMed] [Google Scholar]
- 23.Kukura J, Baxter JL, Muzzio FJ. Shear distribution and variability in the USP Apparatus 2 under turbulent conditions. Int J Pharm. 2004;279:9–17. doi: 10.1016/j.ijpharm.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 24.De Groot K. Bioceramics consisting of calcium phosphate salts. Biomaterials. 1980;1:47–50. doi: 10.1016/0142-9612(80)90059-9. [DOI] [PubMed] [Google Scholar]
- 25.LeGeros RZ. Calcium phosphate materials in restorative dentistry: a review. Adv Dent Res. 1988;2:164–80. doi: 10.1177/08959374880020011101. [DOI] [PubMed] [Google Scholar]
- 26.Daculsi G, LeGeros RZ, Nery E, Lynch K, Kerebel B. Transformation of biphasic calcium phosphate ceramics in vivo: ultrastructural and physicochemical characterization. J Biomed Mat Res. 1989;23:883–94. doi: 10.1002/jbm.820230806. [DOI] [PubMed] [Google Scholar]
- 27.Passuti N, Daculsi G, Martin S, Basle M, Roher S. In Heimke G, Soltesz U, Lee AJC (eds). Macroporous calcium phosphate ceramics for long bone surgery in human and dogs—Clinical and histological studies. Clinical Implants Materials. Advances in Biomaterials. BV, Amsterdam: Elsevier Sciences Publishers, 1990, pp. 255–258.
- 28.Vallet-Regi M, Gonzalez-Calbet JM. Calcium phosphates as substitution of bone tissues. Prog Solid State Chem. 2004;32:1–31. doi: 10.1016/j.progsolidstchem.2004.07.001. [DOI] [Google Scholar]
- 29.Daculsi G, LeGeros RZ, Heughebaert M, Barbieux I. Formation of carbonate-apatite crystals after implantation of calcium phosphate ceramics. Calcif Tissue Int. 1990;46:20–7. doi: 10.1007/BF02555820. [DOI] [PubMed] [Google Scholar]
- 30.LeGeros RZ, Daculsi G, Orly I, Gregoire M, Heughebaert M, Gineste M, Kijkowska R. In Ducheyne, Kokubo, Van Blitterswijk (eds). Formation of carbonate apatite on calcium phosphate materials: Dissolution/precipitation processes. Bone-bonding. Reed Healthcare Communications, 1992, pp. 201–212.
- 31.Arcos D, Ragel CV, Vallet-Regi M. Bioactivity in glass/PMMA composites used as drug delivery system. Biomaterials. 2001;22:701–8. doi: 10.1016/S0142-9612(00)00233-7. [DOI] [PubMed] [Google Scholar]
- 32.Meseguer-Olmo L, Ros-Nicolas MJ, Clavel-Sainz M, Vicente-Ortega V, Alcaraz-Banos M, Lax-Perez A, Arcos D, Ragel CV, Vallet-Regi M. Biocompatibility and in vivo gentamicin release from bioactive sol-gel glass implants. J Biomed Mater Res Part A. 2002;61:458–65. doi: 10.1002/jbm.10188. [DOI] [PubMed] [Google Scholar]
- 33.Ginebra MP, Traykova T, Planell JA. Calcium phosphate cements as drug delivery systems: a review. J Control Release. 2006;113:102–10. doi: 10.1016/j.jconrel.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Balcik C, Tokdemir T, Senköylü A, Koc N, Timucin M, Akin S, Korkusuz P, Korusuz F. Early weight bearing of porous HA/TCP (60/40) ceramics in vivo: a longitudinal study in a segmental bone defect model of rabbit. Acta Biomaterialia. 2007;3:985–96. doi: 10.1016/j.actbio.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Corry D, Moran J. Assessment of acrylic bone cement as a local delivery vehicle for the application of non-steroidal anti-inflammatory drugs. Biomaterials. 1998;19:1295–301. doi: 10.1016/S0142-9612(98)00012-X. [DOI] [PubMed] [Google Scholar]
- 36.Mendez JA, Fernandez M, Gonzalez-Corchon A, Salvado M, Collia F, De Pedro JA, Levenfeld BL, Lopez-Bravo A, Vazquez B, San Roman J. Injectable self-curing bioactive acrylic-glass composites charged with specific anti-inflammatory/analgesic agent. Biomaterials. 2004;25:2381–92. doi: 10.1016/j.biomaterials.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez-Corchon MA, Salvado M, De la Torre BJ, Collia F, De Pedro JA, Vazquez B, San Roman J. Injectable and self-curing composites of acrylic/bioactive glass and drug systems. A histomorphometric analysis of the behaviour in rabbits. Biomaterials. 2006;27:1778–87. doi: 10.1016/j.biomaterials.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 38.Vallet-Regi M, Balas F, Arcos D. Mesoporous materials for drug delivery. Angew Chem Int. 2007;46:7548–58. doi: 10.1002/anie.200604488. [DOI] [PubMed] [Google Scholar]
- 39.Detsch R, Mayr H, Ziegler G. Formation of osteoclast-like cells on HA and TCP ceramics. Acta Biomaterialia. 2008;4:139–48. doi: 10.1016/j.actbio.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 40.Guicheux J, Grimandi G, Trécant M, Faivre A, Takahashi S, Daculsi G. Apatite as carrier for growth hormone: in vitro characterization of loading and release. J Biomed Mater Res. 1997;34:165–70. doi: 10.1002/(SICI)1097-4636(199702)34:2<165::AID-JBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 41.Pillay V, Fassihi R. Unconventional dissolution methodologies. J Pharm Sci. 1999;88:843–51. doi: 10.1021/js990139b. [DOI] [PubMed] [Google Scholar]
- 42.Krajewski A, Ravaglioli A, Roncari E, Pinasco P. Porous ceramic bodies for drug delivery. J Mater Sci Mater Med. 2000;12:763–71. doi: 10.1023/A:1008988127294. [DOI] [PubMed] [Google Scholar]
- 43.Sunder M, Babu NR, Victor SP, Kumar KR, Sampath Kumar TS. Biphasic calcium phosphates for antibiotic release. Trends Biomater Artif Organs. 2005;18:213–8. [Google Scholar]
- 44.Melville AJ, Rodriguez-Lorenzo LM, Forsythe JS. Effects of calcination temperature on the drug delivery behaviour of ibuprofen from hydroxyapatite powders. J Mater Sci Mater Med. 2008;19:1187–95. doi: 10.1007/s10856-007-3185-4. [DOI] [PubMed] [Google Scholar]
- 45.The United States Pharmacopoeia 22nd ed, United States Pharmacopoeial Convention, Rockville, 1991.
- 46.Yu LX, Wang JT, Hussain AS. Evaluation of USP Apparatus 3 for dissolution testing of immediate release products. AAPS Pharm Sci. 2002;4:1–5. doi: 10.1208/ps040101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chevalier E, Viana M, Pouget C, Chulia D. Influence of process parameters on pellets elaborated in a Mi-Pro high-shear granulator. Pharm Dev Technol. 2007;12:133–44. doi: 10.1080/10837450701212461. [DOI] [PubMed] [Google Scholar]
- 48.Chevalier E, Viana M, Pouget C, Cazalbou S, Champion E, Chulia D. In Bioceramics: Properties, Preparations and Application. From porous pellet fabrication to drug loading and release: the case of calcium phosphate matrix loaded with ibuprofen. 2009; in press.
- 49.Chevalier E, Viana M, Cazalbou S, Chulia D. Validation of a fabrication process of pellets for bone filling and drug delivery. J Drug Deliv Sci Technol. 2008;18(6):438–444. [Google Scholar]
- 50.Gibassier D, Sado P, Le Verge R, Devissaguet JP. Test de dissolution et fonction de Weibull. Labo Pharma Pro Tech. 1982;30:250–5. [Google Scholar]
- 51.Moore JW, Flanner HH. Mathematical comparison of curves with an emphasis on in vitro dissolution profiles. Pharm Tech. 1996;20:64–74. [Google Scholar]
- 52.Shah VP, Tsong Y, Sathe P. In vitro dissolution profile comparison-statistics and analysis of the similarity factor, f2. Pharm Res. 1998;15:889–96. doi: 10.1023/A:1011976615750. [DOI] [PubMed] [Google Scholar]
- 53.Costa P, Sousa Lobo JM. Modelling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13:123–33. doi: 10.1016/S0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
- 54.Higuchi T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52:1145–9. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- 55.Hixson AW, Crowell JH. Dependence of reaction velocity upon surface and agitation. Ind Eng Chem. 1931;23:923–31. doi: 10.1021/ie50260a018. [DOI] [Google Scholar]
- 56.Kopcha M, Lordi N, Tojo KJ. Evaluation of release from selected thermosoftening vehicles. J Pharm Pharmacol. 1991;43:382–7. doi: 10.1111/j.2042-7158.1991.tb03493.x. [DOI] [PubMed] [Google Scholar]
- 57.Zhou MX, Shoudt D, Calderon G, Feng M. Application of USP Apparatus 7 to in vitro drug release in scopolamine transdermal systems. Dissolution Technol. 2007;14:25–9. [Google Scholar]
- 58.Amyot F, Boudy V, Jurski K, Counord JL, Guiffant G, Dufaux J, Chaumeil JC. A new experimental method for the evaluation of the release profiles of drug-loaded microbeads designed for embolisation. ITBM-RBM. 2002;23:285–9. doi: 10.1016/S1297-9562(02)80054-7. [DOI] [Google Scholar]
- 59.Borovac T, Pelage JP, Kasselouri A, Prognon P, Guiffant G, Laurent A. Release of ibuprofen from beads for embolization: in vitro and in vivo studies. J Control Rel. 2006;115:266–74. doi: 10.1016/j.jconrel.2006.08.008. [DOI] [PubMed] [Google Scholar]