Abstract
Oral administration of antibiotics to treat dental problems mostly yields slow actions due to slow onset and hepatic “first-pass.” Again, commonly used dental paints are generally washed out by saliva within few hours of application. To overcome the challenges, polymeric molds to be placed on an affected tooth (during carries and gum problems) were prepared and evaluated in vitro for sustained drug release for prolonged local action. Here, amoxicillin trihydrate and lidocaine hydrochloride were used as model drugs. Dental molds were prepared using corn zein, carbopol 934 P, gum karaya powder, and poloxamer 407 by mixing and solvent evaporation technique. Different physicochemical evaluation studies such as tooth adhesion test, surface pH, swelling index, and drug-distribution pattern were carried out. Percentage swelling varied from 56% to 93%. Average tooth adhesion strength and mean initial surface pH of the formulations were 50 g and 6.5, respectively. As assessed by scanning electron microscopy, drug distribution was uniform throughout the matrix. Cumulative percentage release of lidocaine hydrochloride and amoxicillin trihydrate in simulated saliva were 98% and 50%, respectively. In vitro drug-release studies revealed the sustained-release patterns of the drugs in simulated saliva at least for 24 h. The stability study shows that the drugs were stable in the formulations following the conditions as per ICH guideline. The formulation is a novel approach to deliver the drug(s) for a prolonged period for local action upon its application on an affected tooth.
Key words: amoxicillin trihydrate, dental molds, lidocaine hydrochloride, tooth adhesion test
INTRODUCTION
The most familiar symptom of a tooth problem is toothache. The severity of the pain depends on the level of which part of the tooth is affected and how deeply the decay extends (1). Tooth decay is a destruction of the tooth enamel (2). Each tooth has a coating of enamel, which protects the underlying dentine containing soft tissue and nerves. When foods are frequently left on the teeth, bacteria that live in the mouth thrive on these foods and produce acids over a period of time; these acids destroy tooth enamel, resulting in tooth decay (3). To treat dental problems such as pain due to dental caries, periodonitis, gingivitis, and other gum infections, painkillers along with antibiotics and some dental paints are the commonly prescribed drugs by dentists as initial mode of treatment (4). But the common side effects of most of the painkillers are hyperacidity and gastric irritation upon oral administration. On the other hand, most antibiotics due to slow onset of action and hepatic “first-pass” effect fail to produce prompt and prolong actions (5). Moreover, most of the dental formulations are washed out by saliva within a few hours of application. Hypothesis of the study is that if a soft moldable gummy material containing analgesic as well as antibiotic drugs is attached to an offending tooth and sustained drug release occurs from it, a prolonged local action of the drugs is achieved. The polymeric mold should have an appropriate adhesiveness so that it may be easily fixed on the affected tooth and can be removed easily whenever necessary. Amoxicillin trihydrate, a β-lactam antibiotic, inhibits the cross-linkage between the linear peptidoglycan polymer chains of the cell wall of Gram-positive and Gram-negative bacteria (6). The drug has been used to treat dental microbial plaques and periodontal diseases as bacterial infection (7,8). Lidocaine hydrochloride, an anesthetic agent, is available as dermal formulation, intravenous injection, intravenous infusion, nasal spray, oral gel, and topical gel (9). Lidocaine has been widely used as anesthetic agent for dental scaling, root planning, pain sensitivity, and early wound healing following nonsurgical periodontal therapy (10,11).
Sustained-release delivery systems allocate extended drug action to treat dental and periodontal diseases compared to the conventional dosage forms. Better patient compliance in terms of application frequency, better relief for longer period of time, and reduction in dose of drug leads to overcome the adverse reactions due to higher dose to achieve the same effectiveness when given orally. Besides faster local action as compared to slow onset of action by oral route and avoidance of hepatic “first-pass” effect are important advantages of this formulation. Search of sustained-release devices is a relatively new area in dentistry. Many researches are in progress to develop the similar formulations. Dental gels (12), mucoadhesive tablets (13), films (14), injectable semisolid systems (15), inserts (16), and sponges (17) are some of the sustain-release drug-delivery approaches for the treatment of periodontal diseases. However, they are mostly on experimental level.
Therefore, an effort was made here to develop and evaluate in vitro a dental mold “denticap” (since it is cap-like, we name it here as denticap) containing lidocaine hydrochloride and amoxicillin trihydrate as model drugs for sustained local action.
MATERIALS AND METHODS
Materials
Carbopol 934 P (Corel Pharma-Chem, Ahmedabad, India), gum karaya powder # 150 (Nutriroma, Hyderabad, India), lidocaine hydrochloride (Heer Pharmaceuticals Pvt. Ltd., Mumbai, India), and amoxicillin trihydrate (Unimerk Remedies, Birganj, Nepal) were obtained as gift samples. Corn zein (Sigma, St. Louis, MO, USA), disodium hydrogen orthophosphate anhydrous, potassium dihydrogen phosphate, sodium chloride (Process Chemical Industries, Kolkata, India), ethyl cellulose (S.D Fine Chemical Ltd., Boisar, India), poloxamer 407 (Sciencelab.com, Houston, TX, USA), and absolute ethanol (Changsu Yangyuan Chemical, Jiangsu, China) were purchased.
Development of Formulation
Initially several polymers mixed at different ratios (by weight) were screened for developing dental molds. The best formulation based on physicochemical properties and drug release has been reported here. Moldable “denticaps” were formulated with combination of polymers such as corn zein, carbopol 934 P, gum karaya, poloxamer 407, and ethyl cellulose (Table I). Here, corn zein was used to form drug matrix for sustained release of drugs (18). Carbopol 934 P and gum karaya were used for achieving adhesiveness in the formulations since they are known to possess mucoadhesive property (19,20). Poloxamer 407 was a wetting agent (21). Ethyl cellulose was used for coating purpose (22).
Table I.
Drug–Polymer Composition of Denticap Formulations
| Formulation | Ingredients (ratio by weight)a | Weight of drug (milligram) |
|---|---|---|
| Denticap-L | Corn zein, Carbopol 934 P, Gum karaya, and Poloxamer 407 (8:8:4:1; ethyl cellulose was used for coating only) | Lidocaine hydrochloride 50 mg |
| Denticap-A | Corn zein, Carbopol 934 P, Gum karaya, and Poloxamer 407 (8:8:4:1; ethyl cellulose was used for coating only) | Amoxicillin trihydrate 70 mg |
| Denticap-AL | Corn zein, Carbopol 934 P, Gum karaya, and Poloxamer 407 (8:8:4:1; ethyl cellulose was used for coating only) | 70 mg for amoxicillin trihydrate and 50 mg for lidocaine hydrochloride |
aTotal weight of the polymer blend in each formulation 262.5 mg
L lidocaine hydrochloride, A amoxicillin trihydrate, AL amoxicillin trihydrate–lidocaine hydrochloride
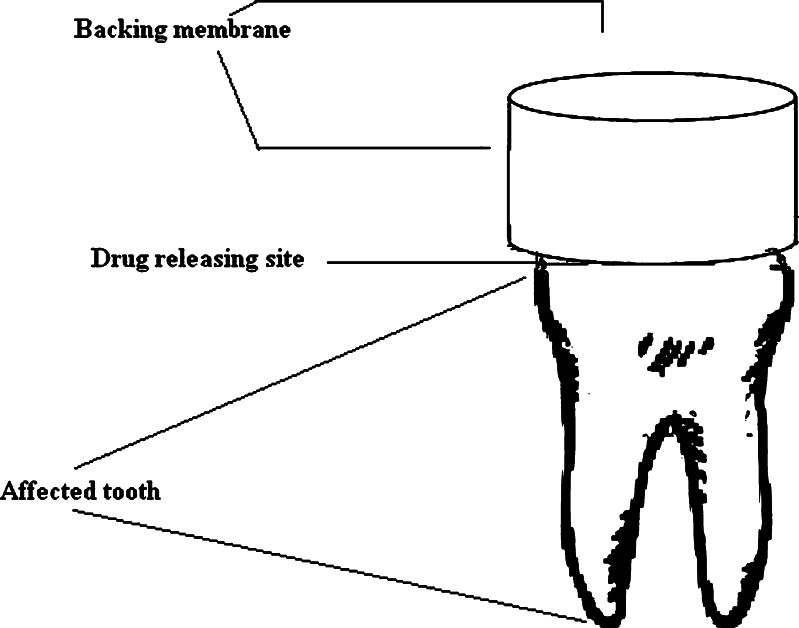
Drugs and polymers were mixed together in ethanol (95%), using homogenizer to form a paste. The mixture was poured in a cap-shaped ethanol-proof plastic molds (internal diameter 1 cm, height 1 cm) and was then subjected to evaporation of solvent at 37°C for 2 h. Finally, the formulations were coated from all the sides except the side for drug release on the affected tooth (Fig. 1). Prior to coating, the formulations were removed from the plastic molds. The coating was done using 5% ethanolic solution of ethylcellulose following the method available (23) keeping drug release side-covered.
Fig. 1.
Outline diagram of a denticap on an affected tooth
Drug-Excipient Interaction Study Using FTIR Spectroscopy
Drug-excipient interaction study is a preformulation experiment (24) to confirm whether the excipients of a formulation interact chemically among themselves as well as with drug(s) present in the formulation. FTIR spectroscopy was used here for the purpose. Pure lidocaine hydrochloride and amoxicillin trihydrate separately; mixture of them; mixture of lidocaine hydrochloride and amoxicillin trihydrate with polymeric combinations; and polymeric combinations alone were mixed separately with IR grade KBr in the ratio 1:100 and corresponding pellets were prepared by applying 5.5 metric ton of pressure in a hydraulic press. The pellets were scanned over a wave number range of 4,000–400 cm−1 in Magna IR 750 Series II (Nicolet, USA) FTIR spectroscope.
Tooth Adhesion Test
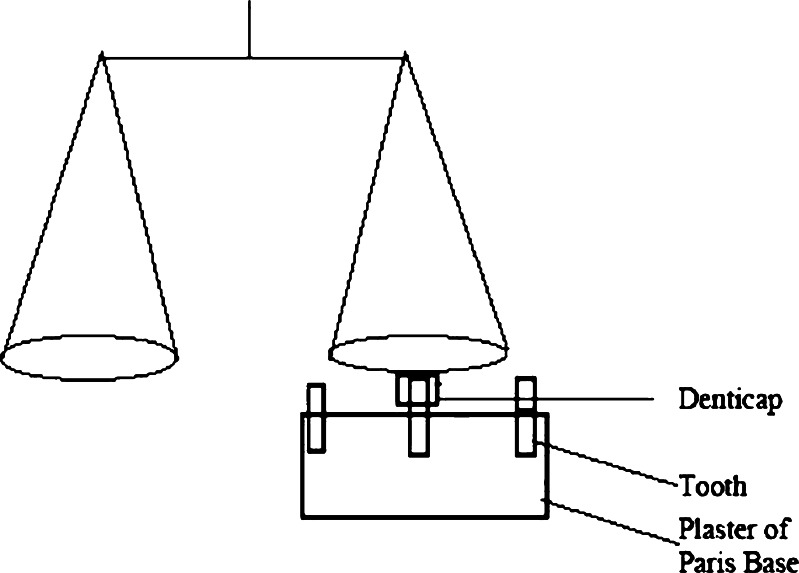
Teeth of goats collected from a slaughterhouse were fixed on a plaster of Paris (CaSO4, 0.5H2O) base to prepare tooth model. Teeth were cleaned with distilled water before fixing. The individual experimental formulation was attached (at the surface which had not been coated with ethyl cellulose) to the tooth model after being made wet with simulated saliva (pH 6.8, viscosity 0.740 cp at 37 ± 0.5°C) for 2 min. A physical balance with two circular pans, hanged from a rod which was balanced with a fulcrum on a stand (Fig. 2), was used as a modified tooth adhesion test assembly (25). Lower surface of a pan was fixed to the denticap attached to the tooth model as described above, by both-side adhesive tape. Weights were given on the other pan continuously until the denticap was detached from the tooth model. Simulated saliva was prepared by dissolving 2.38 g Na2HPO4, 0.19 g KH2PO4, and 8 g of NaCl in a liter of distilled water (26).
Fig. 2.
Mucoadhesive strength test assembly
Percent Swelling
The original weights of the denticaps (Wo) were determined and allowed to swell on a Petri dish in simulated saliva, pH 6.8 at 37 ± 0.5°C. At predetermined time intervals (1–5 h), increase in weights (wet weight) was reported after removal of excess saliva with filter paper. When the weight became constant (Wt), percent swelling was calculated in terms of water uptake (27,28).
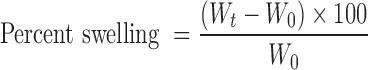 |
Surface pH
The denticaps were incubated in a Petri dish in simulated saliva, pH 6.8 at 37 ± 0.5°C for 2 h. Then, the surface pH was determined by touching the electrode of a pH meter (Toshniwal Instruments, Ajmer, India) in the excess simulated saliva present at the surface of the denticaps (29).
Scanning Electron Microscopy
The drug-distribution patterns of denticaps (before and after drug release) were studied using scanning electron microscope (JSM-6700F, JEOL, Tokyo, Japan). Experimental samples were cut and mounted onto stubs, and then platinum was sputtered under vacuum. They were visualized at an acceleration voltage of 5 KV.
In Vitro Drug-Release Study
Release of lidocaine hydrochloride–amoxicillin trihydrate from denticaps was carried out in a USP apparatus I. In this apparatus, denticaps were placed inside the basket and the drug release occurs from only one side of the formulation, which remains open towards the reservoir containing 100 mL of simulated saliva, pH 6.8 as a dissolution media at 37 ± 0.5°C, and 50 rpm (16). The flow rate of saliva was maintained by replacing the dissolution medium from time to time at a predetermined time intervals (30). Amoxicillin trihydrate and lidocaine hydrochloride were assayed by simultaneous equation UV method (31) at their λmax, respectively, using Cary 50 UV-VIS spectrophotometer (Varian, Palo Alto, CA, USA). Both the drugs obeyed linearity at the concentration of 10–100 µg/mL, and the correlation coefficient (R2) was less than 1 in both the cases (for lidocaine hydrochloride, 0.9996 and for amoxicillin trihydrate, 0.9994). The calculated molar absorptivities (E1%, 1 cm) of lidocaine hydrochloride were 297.88 and 460.36 M−1 cm−1 at 273 and 263 nm, respectively, and for amoxicillin trihydrate, the values were 1,048.62 and 796.95 M−1 cm−1 at 273 and 263 nm, respectively.
Drug Content Analysis
Dental mold was taken in 100 mL simulated saliva, pH 6.8 in a volumetric flask, and the mixture was stirred for 48 h at room temperature using a magnetic stirrer (Remi Equipments, Mumbai, India). The drug content analysis for amoxicillin trihydrate and lidocaine hydrochloride was done by simultaneous equation UV method as described above.
Initially, the time of analysis of the method was standardized by taking formulation with measured amount of drug in simulated saliva and determination of amount of drug released with the duration. It was found that 100% release of both the drugs was achieved in 48 h. Therefore, the time of drug content analysis was chosen up to 48 h.
Temperature-Dependent Stability Study
Denticaps containing the drugs were stored at elevated temperatures and relative humidity (30 ± 2°C/60% RH, 45 ± 2 °C/75% RH) in a stability analysis chamber (Darwin Chambers Company, St. Louis, MO, USA) over a period of 3 months as per ICH guideline (32). Denticaps stored at 2–8°C were used as control. The surface pH, drug content, tooth adhesion strength, in vitro release profiles, and FTIR data of the samples kept for 1 and 3 months for stability analysis were compared with those of the control formulations (33).
Statistics
Data were assessed by one-way ANOVA followed by Tukey HSD Test using Vassar Stats software (USA). P < 0.01 has been considered as statistical significance.
RESULTS
Several combinations of various polymers were screened to obtain the required physicochemical properties and in vitro drug-release pattern. The denticap reported here consisted of corn zein, carbopol 934 P, gum karaya, and poloxamer 407 at a ratio of 8:8:4:1 (total amount of the polymer blend was 262.5 mg). The polymeric ratio 8:8:4:1 was selected through random screening procedure and selection of polymers, and their ratios in mixture were established experimentally and the best formulation has been reported in the study. The amounts of amoxicillin trihydrate and lidocaine hydrochloride in each formulation were 70 and 50 mg, respectively.
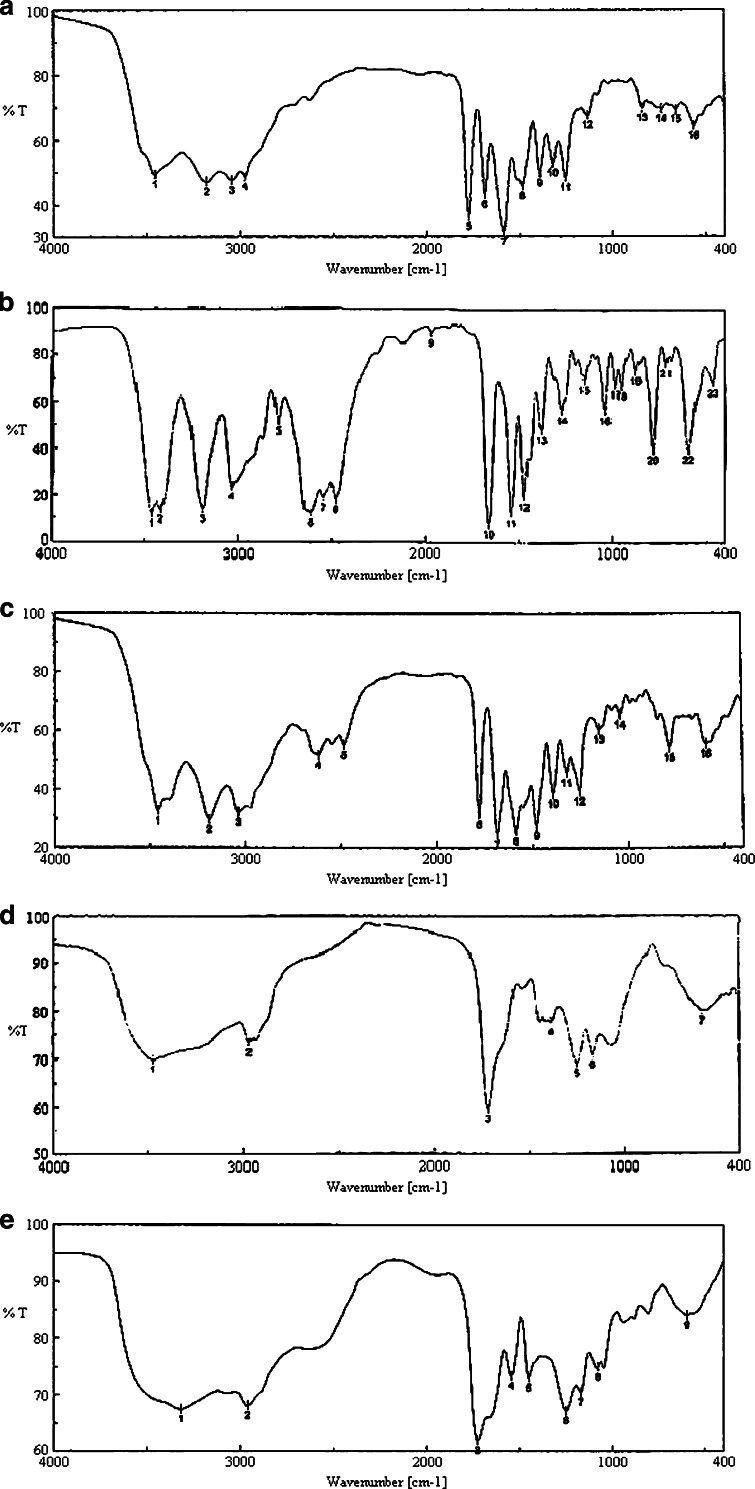
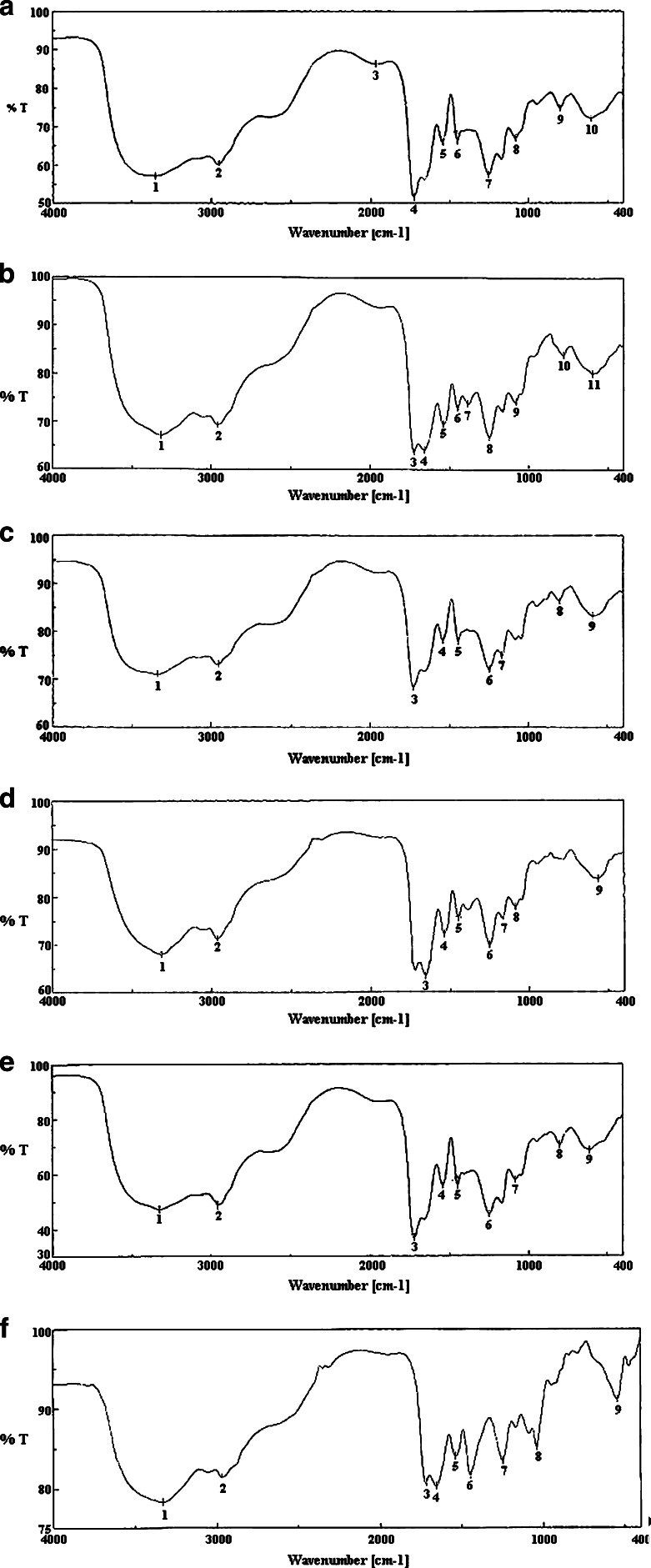
Here, drug–excipient interaction was studied using FTIR spectroscopy. Fig. 3a–e shows the FTIR spectra of amoxicillin trihydrate; lidocaine hydrochloride; a mixture of amoxicillin trihydrate and lidocaine hydrochloride; a mixture of polymers—corn zein, carbopol 934 P, gum karaya, poloxamer 407, and ethyl cellulose; and a mixture of amoxicillin trihydrate and lidocaine hydrochloride with those polymers, respectively. The FTIR spectra of pure amoxicillin trihydrate (Fig. 3a) and lidocaine hydrochloride (Fig. 3b) shows that all the characteristic peaks of amoxicillin trihydrate and lidocaine hydrochloride are present (34,35). Again, the FTIR spectra of the combination of both drugs (Fig. 3c) show the presence of their characteristic peaks. This indicates that the drugs do not interact chemically in their physical mixture. Fig. 3d provides FTIR spectra of the excipients alone. When the drugs were mixed with the excipients, variation of some peaks were noticed (Fig. 3e). When Fig. 3c and d were compared, between 3,200 and 2,800 cm−1 and between 1,800 and 1,000 cm−1 wave numbers, variations at transmission spectroscopy data were noted. Alkenyl (>C=C<; 3,020–3,100 cm−1), amide (>NH; 1,000–1,250 cm−1) ketonyl (>C=O; 1,710–1,720 cm−1), phenolic (–OH; 970–1,250 cm−1) stretches are mainly responsible functional groups for those regions. This suggests the formation of weak to medium intensity bonds due to the forces such as Vander Waal force, dipole moment, and electrostatic force between drugs, and the excipients in those regions since no major shifting of peaks was noted.
Fig. 3.
FTIR spectra of a amoxicillin trihydrate; b lidocaine hydrochloride; c a mixture of amoxicillin trihydrate and lidocaine hydrochloride in a ratio of 1:1; d a mixture of polymers corn zein, carbopol 934 p, gum karaya, poloxamer 407, and ethyl cellulose; e a mixture of amoxicillin trihydrate and lidocaine hydrochloride with those polymers
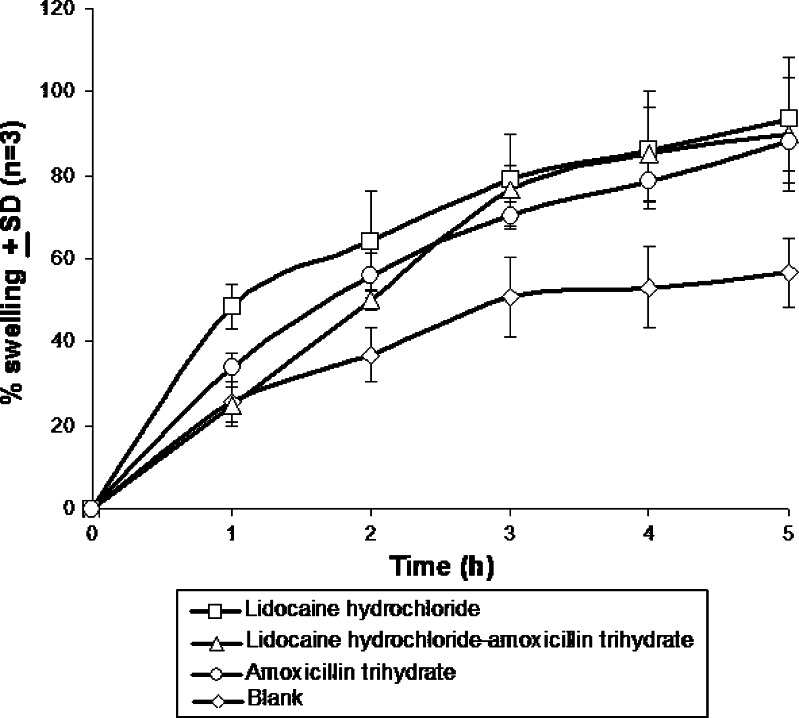
The percent swelling results were expressed in terms of percentage water uptake at 37°C (36). Figure 4 shows the swelling pattern of denticaps. The water uptake of the denticaps can be arranged as follows: blank (only polymers) < amoxicillin trihydrate denticap < amoxicillin trihydrate-lidocaine hydrochloride denticap < lidocaine hydrochloride denticap. The results show that the percentage swelling of the various denticaps varied from 56% to 93%.
Fig. 4.
Percent swelling in terms of water absorption capacity of denticaps. Data show mean ± SD (n = 3)
Table II represents the tooth adhesive strength of the experimental denticaps containing both amoxicillin trihydrate and lidocaine hydrochloride. The average initial tooth adhesive strength (measured in g) was found to be 50 g. Average mucoadhesive strength required for mucoadhesion was reported to be 30 g (37), whereas the mean adhesive strength obtained in our formulation was 50 g. This was advantageous because the surface of mucous layer of buccal cavity is uneven compared to tooth surface; hence, more adhesion strength is required to attach the formulation on tooth surface (38,39). Moreover, tooth adhesive strength was found to be good enough to hold the formulations to the tooth, and again, the adhesiveness in our formulations was such that the formulations were easily removable from the tooth with a little effort.
Table II.
Surface pH and Tooth Adhesive Strength of Denticaps (Containing both Lidocaine Hydrochloride and Amoxicillin Trihydrate) Stored at Different Temperature and Humidity Conditions
| Storage condition | Surface pH ± SD (n = 3) | Tooth adhesive strength (g) ± SD (n = 3) | ||||
|---|---|---|---|---|---|---|
| Initial | 1 months | 3 months | Initial | 1 months | 3 months | |
| 30 ± 2°C/60% RH | 6.5 ± 0.75 | 6.97 ± 0.43 | 6.9 ± 0.58 | 50 ± 4.56 | 25 ± 2.21 | – |
| 45 ± 2°C/75% RH | 7.3 ± 0.36 | 6.95 ± 0.44 | 10 ± 3.75 | – | ||
| 2–8°C | 6.69 ± 0.19 | 6.77 ± 0.32 | 30 ± 2.25 | 20 ± 4.89 | ||
The surface pH obtained (Table II) in this study were within the limits and showed hardly any variation from time to time which omits the chances of irritation in the mucosal membrane upon application.
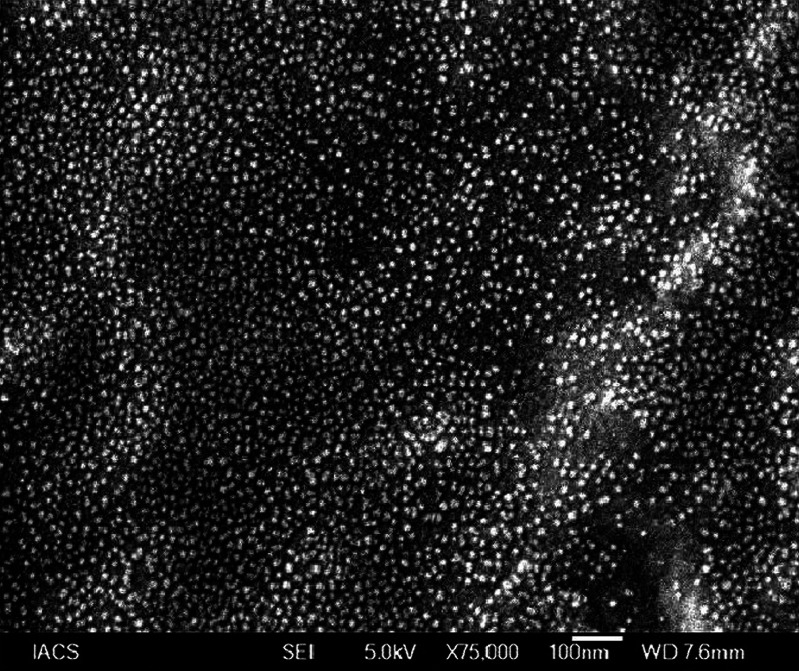
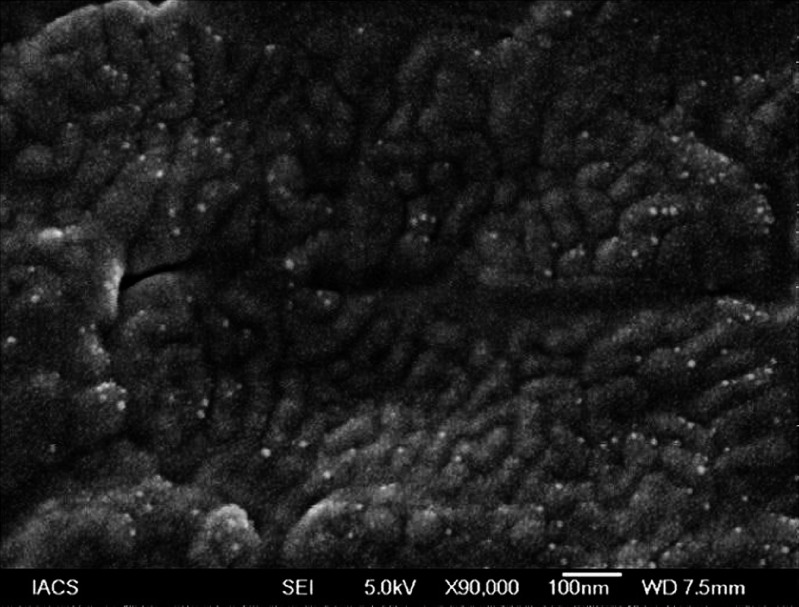
Figures 5, 6 show the distribution of amoxicillin trihydrate and lidocaine hydrochloride in the matrix before and after the release, respectively. Drug particles were distributed throughout the matrix, and particle sizes were about 10 nm. After drug-release study, most of the drug particles were found to diffuse out of the formulation. Further, the matrix (background) as shown in Fig. 6 was found to be different from that of Fig. 5. Upon the exposure of the matrix to the simulated saliva during drug-release study, penetration of liquid into the formulation eventually caused the matrix to swell as supported by the results of Fig. 4. This resulted in differences in appearance in polymer matrices in the scanning electron microscopy (SEM) microphotographs between Fig. 5 (without any appearance of swelling) and Fig. 6 (appearance of swelling).
Fig. 5.
SEM photograph of amoxicillin trihydrate–lidocaine hydrochloride denticap before drug release
Fig. 6.
SEM photograph of amoxicillin trihydrate–lidocaine hydrochloride denticap after drug release
The drug content analysis of denticaps containing both amoxicillin trihydrate and lidocaine hydrochloride shows that the initial recoveries of amoxicillin trihydrate and lidocaine hydrochloride were 98.85% and 99.89%, respectively (Table III). The data were reproducible.
Table III.
Percentage Drug content of Denticaps (Containing Both Lidocaine Hydrochloride and Amoxicillin Trihydrate) Stored at Different Temperature and Humidity Conditions
| Storage condition | % Drug content ± SD (n = 3) | ||
|---|---|---|---|
| Initial | 1 months | 3 months | |
| 30 ± 2°C/60% RH | Lidocaine hydrochloride 99.89 ± 3.53 Amoxicillin trihydrate 98.85 ± 0.84 | Lidocaine hydrochloride 99.51 ± 6.39 | Lidocaine hydrochloride 99.26 ± 1.57 |
| Amoxicillin trihydrate 99.01 ± 5.11 | Amoxicillin trihydrate 99.08 ± 3.07 | ||
| 45 ± 2°C/75% RH | Lidocaine hydrochloride 98.71 ± 5.81 | Lidocaine hydrochloride 95.25 ± 2.29 | |
| Amoxicillin trihydrate 99.44 ± 5.26 | Amoxicillin trihydrate 96.76 ± 3.05 | ||
| 2–8°C | Lidocaine hydrochloride 99.58 ± 0.76 | Lidocaine hydrochloride 99.84 ± 3.32 | |
| Amoxicillin trihydrate 99.56 ± 1.8 | Amoxicillin trihydrate 99.61 ± 1.87 | ||
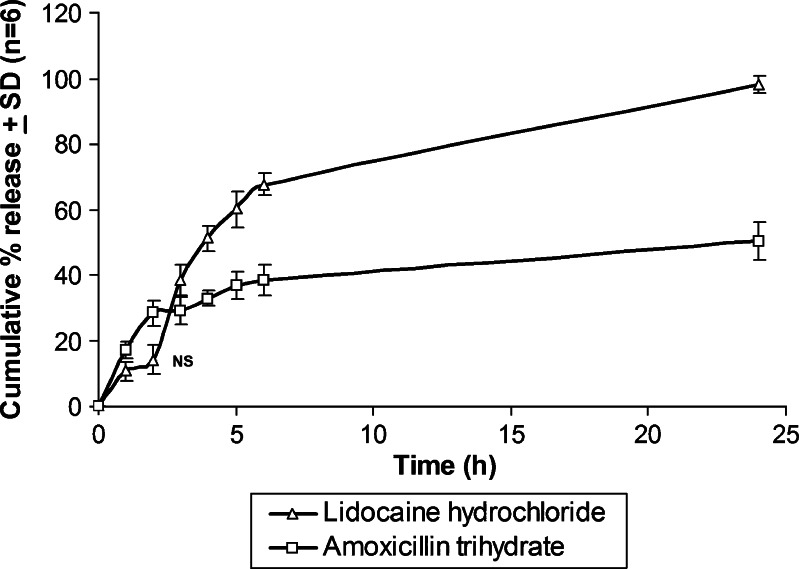
Release patterns of lidocaine hydrochloride and amoxicillin trihydrate from the prepared denticaps were studied in simulated saliva (pH 6.8). Figure 7 shows the release of amoxicillin trihydrate and lidocaine hydrochloride from the denticap for 24 h. The cumulative percentage release of lidocaine hydrochloride and amoxicillin trihydrate were about 98.18% and 50.46%, respectively, over a time period of 24 h.
Fig. 7.
In vitro release of lidocaine hydrochloride and amoxicillin trihydrate from the denticap having both amoxicillin trihydrate and lidocaine hydrochloride in simulated saliva (pH 6.8). Data shows mean (n = 6) ± SD. Values were significantly different as accessed by one-way ANOVA followed by Tukey HSD test (p < 0.01)
To investigate the drug-release kinetic pattern, drug-release data were checked using Zero Order, First Order, Higuchi, Korsmeyer–Peppas, and Hixson–Crowell kinetic models. R2 values for the studied kinetics have been included as Table IV. The release of lidocaine hydrochloride from the denticap showed to follow apparent zero-order kinetics (Table IV). Likewise, amoxicillin trihydrate release was found to obey apparent zero-order kinetics, too.
Table IV.
Drug-Release Kinetics of Denticaps
| Formulation | Zero-order kinetics | First-order kinetics | Higuchi kinetics | Korsmeyer et al. kinetics | Hixon–Crowell kinetics |
|---|---|---|---|---|---|
| Combined denticap (lidocaine hydrochloride release) | y = 1.8678x + 53.509 R 2 = 0.9796 | y = −0.0935x + 2.0739 R 2 = 0.9323 | y = 22.359x − 1.5838 R 2 = 0.8486 | y = 0.7573x + 1.1241 R 2 = 0.8244 | y = 0.1348x + 0.3062 R 2 = 0.9445 |
| Combined denticap (amoxicillin trihydrate release) | y = 0.7728x + 32.133 R 2 = 0.9455 | y = − 0.0068x + 1.8526 R 2 = 0.9156 | y = 7.594x + 16.249 R 2 = 0.8649 | y = 0.3239x + 1.3066 R 2 = 0.8997 | y = 0.0237x + 0.4462 R 2 = 0.7762 |
Tables II, III represent the various data of stability test samples stored at different temperature and humidity conditions as per ICH guideline (32). There was no predominant variation in surface pH values of the stored formulations. However, 2% to 3% degradation of both the drugs were noticed when the samples had been stored at 45 ± 2°C/75% RH. Adhesive strength was found to be reduced during storage. This could be due to the loss of moisture from the formulation during storage. Figure 8a–f shows the FTIR spectra of polymer mixture stored at 2–8°C; polymer mixture along with amoxicillin trihydrate and lidocaine hydrochloride stored at 2–8°C; polymer mixture stored at 30 ± 2°C/60% RH; polymer mixture along with amoxicillin trihydrate and lidocaine hydrochloride stored at 30 ± 2°C/60% RH; polymer mixture stored at 45 ± 2°C/75% RH; and polymer mixture along with amoxicillin trihydrate and lidocaine hydrochloride stored at 45 ± 2°C/75% RH, respectively. Figure 8f (denticaps with drugs stored at 45 ± 2°C/75% RH for 3 months) appeared to have some variations in peaks, in particular, between 1,500 and 1,000 cm−1 and between 800 and 400 cm−1. Wave numbers between 1,250 and 1,000 cm−1 is the stretching vibration zone of C–N and between 1,250 and 970 cm−1 is the stretching vibration zone of C–O. Wave numbers between 1,360 and 1,350 cm−1 and between 1,390 and 1,370 cm−1 are the bending vibration zone of –CH2 and –CH3 deformation and –OH bending. Further, the wave numbers between 900 and 660 cm−1 are the bending vibration zone of C–H bending and ring puckering, –OH bending (out of plane), C–H deformation, and NH2 and N–H wagging due to shifts on H-bonding. Since N–H, –OH, NH2, O=, C–H, N–H, –N=, –CH2 groups are present in drugs and excipients, there might be some physical interactions between drugs and excipients in those regions at high temperature and humidity condition (45 ± 2°C/75% RH) which differs the spectra of Fig. 8f from the rest (Fig. 8a–e). No different drug–excipient interactions as compared to control samples were noted in the formulations stored at the other mentioned conditions.
Fig. 8.
FTIR spectra a polymer mixture stored at 2–8°C; b polymer mixture along with amoxicillin trihydrate and lidocaine hydrochloride stored at 2–8°C; c polymer mixture stored at 30 ± 2°C/60% RH; d polymer mixture along with amoxicillin trihydrate and lidocaine hydrochloride stored at 30 ± 2°C/60% RH; e polymer mixture stored at 45 ± 2°C/75% RH; f polymer mixture along with amoxicillin trihydrate and lidocaine hydrochloride stored at 45 ± 2°C/75% RH
DISCUSSION
A cap-like, soft gummy dental mold (denticap) containing amoxicillin trihydrate and lidocaine hydrochloride was developed so that the drugs might release from the formulation for a prolonged period upon its application on an affected tooth. The doses of the drugs were selected as per the earlier reports (14,40).
One of the important preformulation studies to develop a new dosage form is drug–excipient interaction. Among the various methodologies available to evaluate drug–excipient interaction, the FTIR-spectroscopy is an easy and simple option (41). FTIR spectroscopy shows interaction between molecules at the level of functional groups (42). The FTIR spectra suggests that there may be physical interactions due to formation of weak to medium intensity bonds since no major shifting of peaks was noted (36). Blend of polymers is known to change the rate and pathway of diffusion of drug molecules by varying entanglement in polymeric network (43). Thus, the physical interactions might be helpful in sustaining the release of drug molecules from the experimental formulations.
Moisture uptake by polymers and subsequent swelling in polymer matrix are common phenomenon in case of most of the available polymers (44). Swelling depends on the polymer concentration and the moisture absorption capacity of the polymers (45). Flexibility of polymer chain from individual polymer is important for interpenetration and entanglement, and in presence of water molecules, polymers become cross-linked and the mobility of individual polymer chain decreases and swelling occurs (46). In the present study, drug–polymer matrix swelled more (40%) as compared to the polymeric matrix without drug (Fig. 4). Incorporation of drug shows more swelling of the matrix, and this may be because of the hygroscopic nature of lidocaine hydrochloride (47) and presence of water of crystallization in amoxicillin trihydrate. This might provide more water molecules deep inside of the polymeric matrix as compared to the blank formulation (without drug), where seepage of water molecules from the surface to the core might take more time, which ultimately caused less swelling at the same time points except at the first hour where the value was similar to that of denticaps containing both drugs (reason is unknown). Further presence of drug in the polymeric network structure might make more pathways for the seepage of water into the core of the polymers faster as compared to the polymer matrix without drug. The highest percentage of moisture uptake was obtained for lidocaine hydrochloride denticap. The reduced swelling ability of amoxicillin trihydrate denticap may be because of less water affinity of amoxicillin trihydrate than lidocaine hydrochloride (47,48) and presence of amoxicillin trihydrate in the matrix.
The tackiness of the formulations was determined by tooth adhesion test. Tooth adhesive strength was found to be good enough to hold the formulations to the tooth and again, the tackiness was such that the denticaps were easily removable from the tooth with a little effort.
SEM photographs show that drug particles were very small (approximately 10 nm) in size and were distributed uniformly throughout the matrix. Following drug-release study, most of both drugs were found to diffuse. However, presence of amoxicillin trihydrate in drug matrix at 24 h after drug release was predominantly more than that of lidocaine hydrochloride. This was further supported by studying the drug-release patterns from the formulations. Cumulative amount of lidocaine was found to be twice more than that of amoxicillin at 24 h in drug-release study. This could be because of the higher aqueous solubility of lidocaine hydrochloride as compared to amoxicillin trihydrate. However, the study suggests that the formulations would release the drugs for an extended period of time in the oral cavity and the drugs (amoxicillin trihydrate and lidocaine hydrochloride) would be available for permeation through gums and gingival mucosa. Available reports regarding the mucosal permeation of lidocaine hydrochloride (49) and amoxicillin trihydrate (50) suggest that the drugs would provide local action, and there may be possibility of systemic drug action form the formulations also. However, the present work was intended for local action only, and no study was performed here to evaluate systemic drug absorption from the formulation.
When drug-release patterns were investigated, data suggest that initially, faster diffusion of the drug molecules from the surface of the denticaps showed “first-order” drug release. But the drug-release patterns varied in 2 h for amoxicillin trihydrate and in 5 h in case of lidocaine hydrochloride. It was noted that drug release gradually changed from concentration dependent “first-order” release kinetics to concentration independent “zero-order” kinetic pattern. With the time, the swelling of the polymers varied the entanglement of polymeric pathways to control the diffusion of the drugs from the formulations (51). Again, amoxicillin trihydrate was found to release much slower than lidocaine hydrochloride with the duration. This could be due to the higher aqueous solubility of lidocaine hydrochloride than that of amoxicillin trihydrate (47,48).
Results of the stability study show that drug content and surface pH were almost the same as those of the control formulations, whereas the tooth adhesive strength decreased with time and elevated temperature conditions. The stability study was carried out following ICH guideline for stability testing of new drug substances and products (32) in a container-closure system. Since the study was conducted in a container-closure system, the product could have lost a percentage of moisture in the container-closure system during its storage for 3 months at 2–8°C temperature. This possibly resulted in a decrease of tooth adhesive strength value as compared to the fresh formulations (i.e., before storage). The release patterns (not presented here) for both amoxicillin trihydrate and lidocaine hydrochloride also followed the same pattern as the control denticaps. FTIR spectra of all the samples which have undergone the stability study had similarity with those of the control ones, and there was hardly any variation among the samples stored at different temperature and humidity conditions except that the samples stored at 45 ± 2°C/75% RH for 3 months where some variations in the spectra were noticed. Therefore, it is obvious from the results that the denticaps were stable both physically and chemically at those elevated temperature and humidity conditions other than 45 ± 2°C/75% RH. Our data regarding the stability of the drugs are also supported by the reported findings (52,53).
CONCLUSION
“Denticap” is a novel approach to local drug delivery for a prolonged period by applying it on the affected tooth. More patient compliance over the conventional dosage forms is the expected outcome of such approach. In the future, in root canal treatment and in different gum therapy, this type of dosage form may be very effective. Therefore, denticap may open a new era in dentistry in near endeavor.
Acknowledgements
This work was supported by grants from the Council of Scientific and Industrial Research (Grant No. 27(0149)/05/EMR-II) and the Indian Council of Medical Research (Grant No. 45/55/2004-PHA/BMS).
Notations
- cm
centimeter
- cp
centipoise
- °C
degree Celsius
- FTIR
Fourier transform infrared
- g
gram
- h
hour
- KV
kilo volt
- λ
lambda
- µg/mL
microgram/mililiter
- mL
mililiter
- M−1 cm−1
molar−1centimeter−1
- nm
nanometer
- rpm
rotation per minute
- SEM
scanning electron microscopy
- UV
ultraviolet
References
- 1.Feiglin B. Aspects of dentinal and pulpal pain. Diagnosing dental pain. Ann R Australas Coll Dent Surg. 1994;12:143–152. [PubMed] [Google Scholar]
- 2.Carvalho RM, Tay FR, Giannini M, Pashley DH. Effects of pre- and post-bonding hydration on bond strength to dentin. J Adhes Dent. 2004;6:13–17. [PubMed] [Google Scholar]
- 3.Marthaler TM. Changes in dental caries 1953-2003. Caries Res. 2004;38:173–181. doi: 10.1159/000077752. [DOI] [PubMed] [Google Scholar]
- 4.Vyas SP, Sihorkar V, Mishra V. Controlled and targeted drug delivery strategies towards intraperiodontal pocket diseases. J Clin Pharm Ther. 2000;25:21–42. doi: 10.1046/j.1365-2710.2000.00261.x. [DOI] [PubMed] [Google Scholar]
- 5.Letendre L, Scott M, Dobson G, Hidalgo I, Aungst B. Evaluating barriers to bioavailability in vivo: validation of a technique for separately assessing gastrointestinal absorption and hepatic extraction. Pharm Res. 2004;21:1457–1462. doi: 10.1023/B:PHAM.0000036921.87928.72. [DOI] [PubMed] [Google Scholar]
- 6.Orosz SE, Jones MP, Cox SK, Zagaya NK, Frazier DL. Pharmacokinetics of amoxicillin plus clavulanic acid in blue-fronted Amazon parrots (Amazona aestiva aestiva) J Avian Med Surg. 2000;14:107–112. doi: 10.1647/1082-6742(2000)014[0107:POAPCA]2.0.CO;2. [DOI] [Google Scholar]
- 7.Bascones-Martinez A, Fiquero-Ruiz E. Periodontal diseases as bacterial infection. Med Oral Patol Oral Cir Bucal. 2004;9:92–100. doi: 10.4321/s1699-65852005000300002. [DOI] [PubMed] [Google Scholar]
- 8.Akincibay H, Orsal SO, Sengün D, Tözüm TF. Systemic administration of doxycycline versus metronidazole plus amoxicillin in the treatment of localized aggressive periodontitis: a clinical and microbiologic study. Quintessence Int. 2008;39:e33–e39. [PubMed] [Google Scholar]
- 9.Khaliq W, Alam S, Puri N. Topical lidocaine hydrochloride for the treatment of postherpetic neuralgia. Cochrane Database Syst Rev. 2007;18:CD004846. doi: 10.1002/14651858.CD004846.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Stoltenberg JL, Osborn JB, Carlson JF, Hodges JS, Michalowicz BS. A preliminary study of intra-pocket topical versus injected anaesthetic for scaling and root planing. J Clin Periodontol. 2007;34:892–896. doi: 10.1111/j.1600-051X.2007.01128.x. [DOI] [PubMed] [Google Scholar]
- 11.Kasaj A, Heib A, Willershausen B. Effectiveness of a topical salve (Dynexan) on pain sensitivity and early wound healing following nonsurgical periodontal therapy. Eur J Med Res. 2007;12:196–199. [PubMed] [Google Scholar]
- 12.Stoltze K, Stellfeld M. Systemic absorption of metronidazole after application of a metronidazole 25% dental gel. J Clin Periodontol. 1992;19:693–697. doi: 10.1111/j.1600-051x.1992.tb02530.x. [DOI] [PubMed] [Google Scholar]
- 13.Perioli L, Ambrogi V, Rubini D, et al. Novel mucoadhesive buccal formulation containing metronidazole for the treatment of periodontal disease. J Control Release. 2004;95:521–533. doi: 10.1016/j.jconrel.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Ahuja A, Ali J, Rahman S. Biodegradable periodontal intrapocket device containing metronidazole and amoxycillin: formulation and characterisation. Pharmazie. 2006;61:25–29. [PubMed] [Google Scholar]
- 15.Schwach-Abdellaoui K, Vivien-Castioni N, Gurny R. Local delivery of antimicrobial agents for the treatment of periodontal diseases. Eur J Pharm Biopharm. 2000;50:83–99. doi: 10.1016/S0939-6411(00)00086-2. [DOI] [PubMed] [Google Scholar]
- 16.Barat R, Srinatha A, Pandit JK, Ridhurkar D, Balasubramaniam J, Mittal N, Mishra DN. Niridazole biodegradable inserts for local long-term treatment of perodontis: possible new life for an orphan drug. Drug Deliv. 2006;13:365–173. doi: 10.1080/10717540500398126. [DOI] [PubMed] [Google Scholar]
- 17.Ang CY, Samsudin AR, Karima AM, Nizam A. Locally produced bovine bone sponge as a haemostatic agent. Med J Malaysia. 2004;59:149–150. [PubMed] [Google Scholar]
- 18.Liu X, Sun Q, Wang H, Zhang L, Wang JY. Microspheres of corn protein, zein, for an ivermectin drug delivery system. Biomaterials. 2005;26:109–115. doi: 10.1016/j.biomaterials.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Patel VM, Prajapati BG, Patel MM. Effect of hydrophilic polymers on buccoadhesive Eudragit patches of propranolol hydrochloride using factorial design. AAPS PharmSciTech. 2007;8:45. doi: 10.1208/pt0804089. [DOI] [PubMed] [Google Scholar]
- 20.Collys KD, Roma de Sousa AC, Smeyers-Verbeke J. Soluble denture adhesives: pH and sodium content. Eur J Prosthodont Restor Dent. 1997;5:63–67. [PubMed] [Google Scholar]
- 21.Wong SM, Kellaway IW, Murdan S. Enhancement of the dissolution rate and oral absorption of a poorly water soluble drug by formation of surfactant-containing microparticles. Int J Pharm. 2006;317:61–68. doi: 10.1016/j.ijpharm.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Bussemer T, Bodmeier R. Formulation parameters affecting the performance of coated gelatin capsules with pulsatile release profiles. Int J Pharm. 2003;267:59–68. doi: 10.1016/j.ijpharm.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Oh E, Luner PE. Surface free energy of ethylcellulose films and the influence of plasticizers. Int J Pharm. 1999;188:203–119. doi: 10.1016/S0378-5173(99)00224-0. [DOI] [PubMed] [Google Scholar]
- 24.Bruni G, Amici L, Berbenni V, Marini A, Orlandi A. Drug-excipient compatibility studies. Search of interaction indicators. J Thermal Anal Cal. 2002;68:561–573. doi: 10.1023/A:1016052121973. [DOI] [Google Scholar]
- 25.Gupta A, Garg S, Khar RK. Measurement of bioadhesive buccal tablets: design of an in vitro assembly. Indian Drugs. 1993;30:152–155. [Google Scholar]
- 26.Yvonne TFT, Kok KP, Al-Hanbali O. Effect of carbopol and polyvinylpyrrolidone on the mechanical, rheological and release properties of bioadhesive polyethylene glycol gels. AAPS Pharm Sci Tech. 2000;1(3):24. doi: 10.1208/pt010324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semalty A, Bhojwani M, Bhatt GK, Gupta GD, Shrivastav AK. Design and evaluation of mucoadhesive buccal films of diltiazem hydrochloride. Indian J Pharm Sci. 2005;67:548–552. [Google Scholar]
- 28.Vishnu MP, Bhupendra GP, Harsha VP, Karshanbhi MP. Mucoadhesive bilayer tablets of propranolol hydrochloride. AAPS Pharm Sci Tech. 2007;8(3):77. doi: 10.1208/pt0803077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nafee NA, Ismail FA, Boraie NA, Mortada LM. Mucoadhesive buccal patches of miconazole nitrate:in vitro/in vivo performance and effect of ageing. Int J Pharm. 2003;264:1–14. doi: 10.1016/S0378-5173(03)00371-5. [DOI] [PubMed] [Google Scholar]
- 30.Colin D. Salivary flow patterns and the health of hard and soft oral tissues. J Am Dent Asso. 2008;139:18–24. doi: 10.14219/jada.archive.2008.0351. [DOI] [PubMed] [Google Scholar]
- 31.Devarajan S, Lakshmi S. Simulataneous spectrophotometric determination of valdecoxib and tizanidine in tablets. Indian J Pharm Sci. 2006;68:240–242. doi: 10.4103/0250-474X.25725. [DOI] [Google Scholar]
- 32.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Stability testing of new drug substances and products Q1A (R2). Current Step 4 version dated 6 February 2003.
- 33.Garg M, Dutta T, Jain NK. Stability study of stavudine-loaded O-palmitoyl-anchored carbohydrate-coated lyposomes. AAPS PharmSciTech. 2007;8:38. doi: 10.1208/pt0802038. [DOI] [PubMed] [Google Scholar]
- 34.Brittain HG. Solid-state fluorescence of the trihydrate phases of ampicillin and amoxicillin. AAPS PharmSciTech. 2005;6:E444–E448. doi: 10.1208/pt060355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abu-Huwaij R, Assaf S, Salem M, Sallam A. Mucoadhesive dosage form of lidocaine hydrochloride: I. Mucoadhesive and physicochemical characterization. Drug Dev Ind Pharm. 2007;33:855–864. doi: 10.1080/03639040701377516. [DOI] [PubMed] [Google Scholar]
- 36.Mukherjee B, Das S, Patra B, Layek B. Nefopam containing transdermal- matrix based on pressure-sensitive adhesive polymers. Pharm Tech (USA). 2006;30:146–163. [Google Scholar]
- 37.Singh B, Ahuja N. Development of controlled-release buccoadhesive hydrophilic matrices of diltiazem hydrochloride: optimization of bioadhesion, dissolution, and diffusion parameters. Drug Dev Ind Pharm. 2002;28:431–442. doi: 10.1081/DDC-120003004. [DOI] [PubMed] [Google Scholar]
- 38.Badran D, Soutar DS, Robertson AG, Payne AP, McDonald SW, Scothorne RJ. Scanning electron microscopy of the surface morphology of superficial cells of buccal mucosa is unlikely to be useful in monitoring radiotherapy. Clin Anat. 2005;7:34–41. doi: 10.1002/ca.980070107. [DOI] [Google Scholar]
- 39.Hamada S, Torii M, Kotani S, Tsuchitani Y. Adherence of Streptococcus sanguis clinical isolates to smooth surfaces and interactions of the isolates with Streptococcus mutans glucosyltransferase. Infect Immun. 1981;32:364–372. doi: 10.1128/iai.32.1.364-372.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leopold A, Wilson S, Weaver JS, Moursi AM. Pharmacokinetics of lidocaine delivered from a transmucosal patch in children. Anesth Prog. 2002;49:82–87. [PMC free article] [PubMed] [Google Scholar]
- 41.McCauley JA, Brittain HG. In physical characterizations of pharmaceutical solids. New York: Marcel Dekker; 1995. [Google Scholar]
- 42.Kötting C, Gerwert K. Monitoring protein-ligand interactions by time- resolved FTIR difference spectroscopy. Methods Mol Biol. 2005;305:261–286. doi: 10.1385/1-59259-912-5:261. [DOI] [PubMed] [Google Scholar]
- 43.Singh SK, Fan LT. A generalized model for swelling controlled release systems. Biotechnol Prog. 1986;2:145–156. doi: 10.1002/btpr.5420020309. [DOI] [PubMed] [Google Scholar]
- 44.Bagyalakshmi J, Vamsikrishna RP, Manavalan R, Ravi TK, Manna PK. Formulation development and in vitro and in vivo evaluation of membrane-moderated transdermal systems of ampicillin sodium in ethanol: pH 4.7 buffer solvent system. AAPS PharmSciTech. 2007;8:7. doi: 10.1208/pt0801007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moustafine RI, Margulis EB, Sibgatullina LF, Kemenova VA, Van den Mooter G. Comparative evaluation of interpolyelectrolyte complexes of chitosan with Eudragit L100 and Eudragit L100–55 as potential carriers for oral controlled drug delivery. Eur J Pharm Biopharm. 2008;70:215–225. doi: 10.1016/j.ejpb.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Narasimha B, Peppas NA. The role of modeling studies in the development of future control release devices. In: Park K, editor. Controlled drug delivery: challenges and strategies. Washington D.C.: American Chemical Society; 1997. pp. 529–557. [Google Scholar]
- 47.Gulsen D, Chauhan A. Effect of water content on transparency, swelling, lidocaine diffusion in p-HEMA gels. J Membr Sci. 2006;269:35–48. doi: 10.1016/j.memsci.2005.06.024. [DOI] [Google Scholar]
- 48.Song M, Li N, Sun S, Tiedt LR, Liebenberg W, de Villiers MM. Effect of viscosity and concentration of wall former, emulsifier and pore-inducer on the properties of Amoxicillin microcapsules prepared by emulsion solvent evaporation. Farmaco. 2005;60:261–267. doi: 10.1016/j.farmac.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 49.Perry DA, Gansky SA, Loomer PM. Effectiveness of a transmucosal Lidocaine delivery and root planning. J Clin Periodontal. 2005;32:590–594. doi: 10.1111/j.1600-051X.2005.00717.x. [DOI] [PubMed] [Google Scholar]
- 50.Ahuja A, Rahman S, Ali J, Chaudhry R. Effect of dental films containing amoxicillin and metronidazole on periodontal pathogens: microbiological response. Pharmazie. 2003;58:716–720. [PubMed] [Google Scholar]
- 51.Mukherjee B, Mahapatra S, Gupta R, Patra B, Tiwari A, Arora P. A comparison between povidone-ethyl cellulose and povidone-eudragit transdermal dexamethasone matrix patches based on in vitro skin permeation. Eur J Pharm Biopharm. 2005;59:475–483. doi: 10.1016/j.ejpb.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 52.Kayumba PC, Risha PG, Shewiyo D, Msami A, Masuki G, Ameye D, Vergote G, Ntawukuliryayo JD, Remon JP, Vervaet C. The quality of essential antimicrobial and antimalarial drugs marketed in Rwanda and Tanzania: influence of tropical storage conditions on in vitro dissolution. J Clin Pharm Ther. 2004;29:331–338. doi: 10.1111/j.1365-2710.2004.00568.x. [DOI] [PubMed] [Google Scholar]
- 53.Abdelmageed R, Labyad N, Watson DG, Pournamdari M, Cable CG, Stanley E. Evaluation of the stability of morphine sulphate in combination with InstillagelR. J Clin Pharm Ther. 2008;33:263–271. doi: 10.1111/j.1365-2710.2008.00914.x. [DOI] [PubMed] [Google Scholar]