Abstract
The present investigation was undertaken to fabricate modified transport fluconazole transdermal spray using ethyl cellulose and Eudragit® RS100 as film-forming polymers. Eudragit® RS100 (X1) and ethyl cellulose (X2) were selected as independent variables in 32 full factorial design, whereas drug transport in first hour (Y1) and the time required for 50% drug transport (Y2) were selected as dependent variables. Eutectic blend of camphor and menthol was used as permeation enhancer cum solvent for film-forming polymers. The pH, viscosity, volume of solution delivered upon each actuation, spray angle, ex–in vivo physical evaluation and in vitro drug transport of the formulated products were evaluated. The optimized batch B16 containing 5.25% w/w ethyl cellulose and 10.6% w/w Eudragit® RS100 was formulated by overlapping the contour plots of Y1 and Y2. The pH, viscosity, volume of solution sprayed upon each actuation and spray angle of the batch B16 was 6.3, 52.9 cPs, 0.24 ml and 82.6° respectively. The film of optimized batch was flexible and dermal-adhesive. The responses Y1 and Y2 of batch B16 were 7.91 μg/ml and 347 min respectively. The kinetics of drug transport was best explained by the Korsmeyer and Peppas model. The eutectic mixture consisting of equal parts of camphor and menthol showed improved drug permeation through shed snake skin. Short-term stability study demonstrated insignificant changes in performance characteristics.
Keywords: fluconazole, modified transport, transdermal spray, factorial design and short term stability study
INTRODUCTION
Tinea infections are caused by three species of fungi collectively known as dermatophytes. Tinea infections are named considering the affected area of human body, i.e., Tinea corporis (general skin), Tinea cruris (groin), and Tinea pedis (feet). Topical therapy is generally successful unless the infection covers an extensive area or is resistant to initial therapy. In these cases, systemic therapy may be required.
Fluconazole, a novel triazole antifungal drug, is used in the treatment of superficial and systemic fungal (Tinea) infection. The drug has moderate (8 mg/ml) solubility in water (1,2). Like other imidazole- and triazole-class antifungals, fluconazole inhibits the fungal cytochrome P450 enzyme 14α-demethylase (3). Fluconazole is primarily fungistatic, however may be fungicidal against certain organisms in a dose-dependent manner. Ayub et al. investigated in vitro skin penetration and permeation of fluconazole from emulsions containing propylene glycol and isopropyl myristate as penetration enhancers (4). El-Laithy and El-Shaboury evaluated influence of vehicle on the release and permeation of fluconazole dissolved in jojoba oil (5). Rivera et al. prepared fluconazole-loaded poly(d,l-lactic-co-glycolic) acid microspheres by spray-drying process (6). Kavitha et al. formulated and characterized topical drug delivery systems of fluconazole in form of ointment, cream and gel using water-soluble and water-insoluble base in absence of penetration enhancer (7).
The aim of the present research work was to develop patient-friendly modified transport transdermal spray of fluconazole using ethyl cellulose and Eudragit® RS100 as film-forming polymers. A 32 full factorial design was employed for optimization. The formulation contained eutectic mixture of menthol and camphor (dermal penetration enhancers) that readily partition into the stratum corneum (8–14). Eutectic mixture of camphor and menthol also acted as a solvent for film-forming polymers, imparted cooling effect to the skin and possessed antifungal activity (15,16).
MATERIALS AND METHODS
Materials
Fluconazole USP and ethyl cellulose (Ethocel standard 10FP premium) were received as gift samples from Zydus Cadila (Ahmedabad, India). Eudragit® RS100 was received as a gift sample from Degussa Pvt. Ltd. (Mumbai, India). Camphor and menthol were purchased from Gem Corporation (Ahmedabad, India) and Shreeji Pharma International (Ahmedabad, India), respectively. Polyethylene glycol (PEG 400) and acetone were purchased from S. D. Fine Chemical Pvt. Ltd. (Ahmedabad, India). Alcohol I.P was purchased from Baroda Chemicals Industries Ltd. (Baroda, India). The other chemicals and reagents were of analytical grade.
Method
Determination of Solubility of Fluconazole and Polymer in Various Solvents
The solubility of fluconazole was determined in a mixture containing 80 parts of alcohol (80% v/v) and 20 parts of acetone. The drug solubility was also determined in a eutectic mixture of camphor and menthol (1:1). The solubility of ethyl cellulose and Eudragit® RS100 was determined in the eutectic mixture. An excess amount of sample was added to 35 ml of solvent and stirred at 100 rpm on a magnetic stirrer (Remi Electronics, Ahmedabad, India) for 30 min at room temperature (35 ± 2°C) in a closed vessel. The mixtures were then filtered through 0.22 μm millipore filters and the weight of undissolved solid was recorded. The filtrate was carefully observed for clarity.
Preliminary Studies
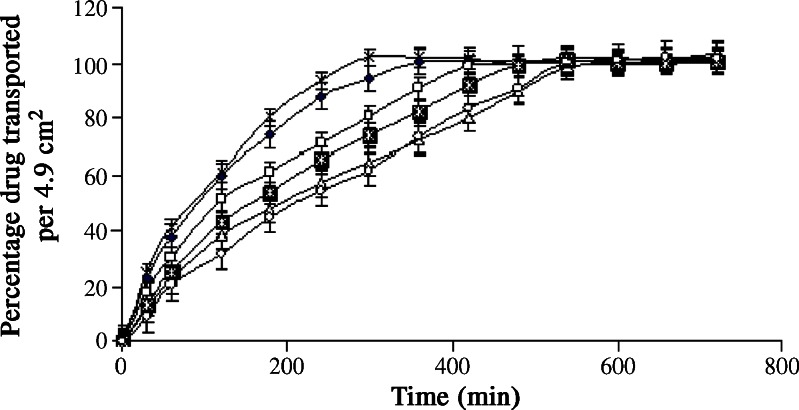
Film formers (ethyl cellulose and Eudragit® RS100) and plasticizer (polyethylene glycol) were sequentially dissolved in the eutectic mixture consisting of equal proportion of camphor and menthol. Fluconazole was separately dissolved in vehicle blend consisting of 80 parts of alcohol (80% v/v) and 20 parts of acetone. The solution of ethyl cellulose/Eudragit® RS100 in eutectic mixture was gradually added to the solution of fluconazole and mixed for 15 min at 80–100 rpm. The resulting solution was filled in a refillable container containing plastic dip tube of 75 mm length and 1.2 mm internal diameter. The aperture size of the tube was 0.3 mm. Table I displays the composition of the formulated batches (B1–B6). The sprays were analyzed for pH, viscosity, volume of solution delivered upon each actuation, spray angle, ex–in vivo physical characteristics and in vitro drug transport. The results of drug transport are expressed in Fig. 1.
Table I.
Composition and Evaluation of Fluconazole Sprays
| Ingredient (% w/w) | Batch code | |||||
|---|---|---|---|---|---|---|
| B1 | B2 | B3 | B4 | B5 | B6 | |
| Fluconazole | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Ethyl cellulose | 3 | 5 | 7.5 | – | – | – |
| Eudragit® RS100 | – | – | – | 5 | 10 | 15 |
| PEG 400 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Eutectic blend | 10 | 10 | 10 | 10 | 10 | 10 |
| Alcohol and acetone blend (q.s.) | 100 | 100 | 100 | 100 | 100 | 100 |
| Tests | Average results (n = 3) | |||||
| Viscosity (a ± 2.7 cPs) | 15.0 | 26.4 | 43.5 | 7.6 | 20.2 | 51.6 |
| Volume of solution delivered upon each actuation (b ± 0.03 ml) | 0.32 | 0.30 | 0.26 | 0.39 | 0.31 | 0.24 |
| Spray angle (c ± 0.4°) | 78.0 | 79.1 | 81.0 | 76.1 | 78.3 | 82.2 |
| Y a1 | 21.07 | 17.07 | 12.55 | 23.44 | 14.12 | 11.73 |
| Y b2 | 94 | 115 | 196 | 120 | 160 | 210 |
| Ex–in vivo film formation timec (d ± 35 sec) | 130 | 160 | 210 | 110 | 152 | 230 |
| Feeling of warmth and subsequent cooling sensationc (e ± 1 min) | 12 | 14 | 15 | 12 | 14 | 16 |
| Appearance of the filmc | + | + | ++ | + | + | ++ |
| Dermal adhesion and flexibility of the filmc | ++ | ++ | ++ | ++ | +++ | +++ |
| Water washabilityc | ++ | ++ | ++ | +++ | +++ | ++ |
a Y 1 is the amount of drug transported (μg) per ml at the end of first hour
b Y 2 is the time (min) required to transport 50% of the drug (t 50%)
cMeasured for placebo batches replacing fluconazole with blend of alcohol and acetone
Fig. 1.
In vitro drug transport from batches B1–B6 through nylon membrane; B1 (-◆-), B2 (-□-), B3 (-△-), B4 (- -), B5 (-
-), B5 (- -), B6 (-○-)
-), B6 (-○-)
Factorial Design
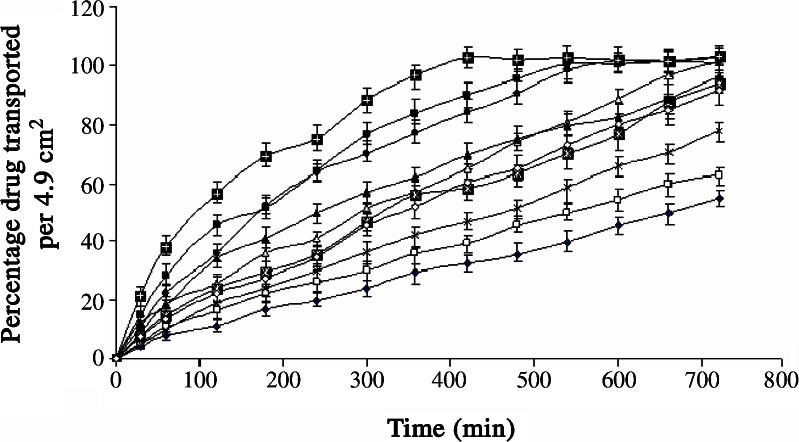
A 32 full factorial design was used for optimization of formulated product. The concentration of Eudragit® RS100 (X1) and ethyl cellulose (X2) were selected as independent variables, whereas the amount of drug transported in 1 h/ml (Y1) and the time required to transport 50% of the drug (t50%, Y2) were selected as dependent variables. Tables II and III show the composition, design layout for the optimization study and the responses. The method for preparation and evaluation of the formulated batches (B7–B16) was similar to that described under preliminary studies. The results are expressed in Fig. 2 and Tables II and III.
Table II.
Composition and Evaluation of Fluconazole Sprays Based on 32 Full Factorial Design
| Ingredient (% w/w) | Batch Code | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B7 | B8 | B9 | B10 | B11 | B12 | B13 | B14 | B15 | B16 | |
| Fluconazole | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Ethyl cellulose | 7.5 | 5 | 3 | 7.5 | 5 | 3 | 7.5 | 5 | 3 | 5.25 |
| Eudragit® RS100 | 15 | 15 | 15 | 10 | 10 | 10 | 5 | 5 | 5 | 10.6 |
| PEG 400 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Eutectic blend | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Alcohol and acetone blend (q.s.) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Tests | Average results (n = 3) | |||||||||
| Viscosity (a ± 2.7 cPs) | 128.3 | 92.8 | 70.8 | 69.7 | 48.9 | 38.1 | 52.5 | 34.0 | 23.7 | 52.9 |
| Volume of solution delivered upon each actuation (b ± 0.03 ml) | 0.14 | 0.18 | 0.2 | 0.21 | 0.26 | 0.31 | 0.26 | 0.3 | 0.33 | 0.24 |
| Spray angle (c ± 0.4o) | 91.5 | 88.2 | 83.8 | 84.2 | 81.8 | 80.6 | 82.6 | 79.8 | 78.7 | 82.6 |
| Ex–in vivo film formation timea (d ± 35 sec) | 532 | 435 | 390 | 372 | 328 | 287 | 340 | 282 | 249 | 335 |
| Feeling of warmth and subsequent cooling sensationa (e ± 1 min) | 19 | 18 | 17 | 18 | 16 | 15 | 17 | 13 | 13 | 17 |
| Appearance of the filma | +++ | ++ | ++ | ++ | ++ | + | ++ | + | + | ++ |
| Dermal adhesion and flexibility of the filma | +++ | +++ | +++ | +++ | +++ | +++ | +++ | ++ | ++ | +++ |
| Water washabilitya | + | + | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
aMeasured for placebo batches replacing fluconazole with blend of alcohol and acetone
Table III.
Design Layout for 32 Factorial Design
| Batch Code | Real Values | Transformed Values | Dependent Variables | |||
|---|---|---|---|---|---|---|
| X 1 | X 2 | X 1 | X 2 | Y 1 | Y 2 | |
| B7 | 15 | 7.5 | 1 | 1 | 4.53 | 660 |
| B8 | 15 | 5 | 1 | 0 | 6.15 | 540 |
| B9 | 15 | 3 | 1 | –1 | 10.7 | 292 |
| B10 | 10 | 7.5 | 0 | 1 | 5.73 | 466 |
| B11 | 10 | 5 | 0 | 0 | 8.49 | 320 |
| B12 | 10 | 3 | 0 | −1 | 12.85 | 165 |
| B13 | 5 | 7.5 | −1 | 1 | 10.56 | 240 |
| B14 | 5 | 5 | −1 | 0 | 16.15 | 165 |
| B15 | 5 | 3 | −1 | −1 | 21.7 | 107 |
| B16* | 10.6 | 5.25 | 0.12 | 0.1 | 7.91 | 347 |
B16* is the point batch, X 1 is the concentration of Eudragit® RS100 (% w/w), X 2 is the concentration of ethyl cellulose (% w/w), Y 1 is the amount of drug transported (μg) per milliliter at the end of first hour, Y 2 is the time (min) required to transport 50% of the drug (t 50%)
Fig. 2.
In vitro drug transport from batches B7–B16-A; B7 (-♦-), B8 (-□-), B9 (-△-), B10 (- -), B11 (-
-), B11 (- -), B12 (-●-), B13 (-▲-), B14 (-■-), B15 (-
-), B12 (-●-), B13 (-▲-), B14 (-■-), B15 (- -), B16 (-◇-), B16-A (-○-)
-), B16 (-◇-), B16-A (-○-)
Evaluations
Viscosity
The viscosity of the solutions was measured at 25 ± 1°C using Brookfield viscometer (digital viscometer model DV-II+, Stoughton, MA, USA). The ULA spindle was rotated at 1 rpm.
-
2.
Volume of solution delivered upon each actuation
The volume of solution delivered upon each actuation was calculated using eq. 1.
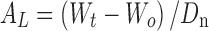 |
1 |
where AL is the volume of solution delivered upon each actuation, Wt is weight of formulation after actuation, Wo is the initial weight of the formulation before actuation, and Dn is the density of the formulation.
-
3.
Spray angle
The method of impingement of spray on a piece of paper was used for the study. Sudan red (10 mg) was dissolved in formulation to facilitate visualization. The sprays were actuated in horizontal direction onto a white paper mounted at a distance of 15 cm from the nozzle. The radius of the circle, formed on the paper, was recorded in triplicate from different directions. Spray angle (θ) was calculated by eq. 2.
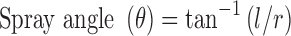 |
2 |
where l is the distance of paper from the nozzle, and r is the average radius of the circle.
-
4.
Ex–in vivo physical evaluation
Placebo batches (B1P–B16P) were actuated on the left-hand palms of three healthy human volunteers of 21–27 years in age, four times every 10 s from a distance of 15 cm. The purpose of the study was fully explained and the volunteers gave written consent. The departmental review board approved the study. The time required for film formation, appearance of the film, dermal adhesion and flexibility of the film, feeling of warmth and subsequent cooling sensation, irritation potential and water washability were recorded. The appearance of the film was graded as shiny and transparent (+) or shiny and translucent (++) or dull and opaque (+++). Dermal adhesion, flexibility and water washability of the film were graded as poor (+), moderate (++) or good (+++). To check dermal adhesion of the film, the palms of each volunteer were rotated for 10 min in anti-clock wise direction with occasional opening and closing of palm, after 8 min of actuation of the spray. During the study (720 min), the nature of the film was carefully evaluated for any fracture, separation or removal. After 720 min, water washability of the film was checked.
-
5.
In vitro drug transport
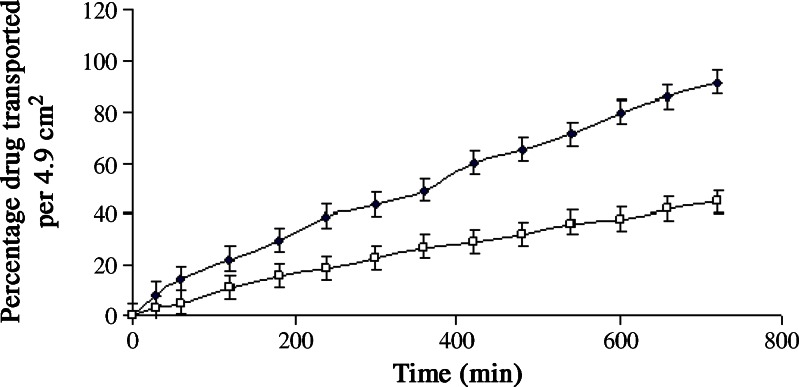
A nylon membrane with pore rating equal to 0.22 μm was mounted in a Franz cell. The surface area of membrane available for drug transport, was 4.9 cm2. One milliliter of the drug formulation and 88 ml of solution (pH 7.4, 100 rpm) containing 0.9% w/v sodium chloride and 1% w/v sodium lauryl sulfate were filled in donor and receptor compartments, respectively. Throughout the experiment, the temperature of media present in the receptor compartment was maintained at 32 ± 2°C using a hot-water jacket (37 ± 2°C). Sodium lauryl sulfate was used to provide sink condition. Aliquots of 10 ml samples were withdrawn at different time intervals from the receptor compartment, and fluconazole was estimated spectrophotometrically at 264 nm (5). Equal amount of fresh dissolution medium was replaced after each withdrawal. Figures 1 and 2 show the drug transport profiles. The amount of drug transported in 1 h/ml (Y1) and the time required to transport 50% of the drug (Y2) were found. The UV spectrum of fluconazole was observed for fluconazole–formulation excipient interaction. The in vitro drug transport from the optimized batch B16 and batch B17 was also determined using shed snake skin as a membrane in Franz diffusion cell (Fig. 4). The shed snake skin was procured from the local snake habitat (Sundarvan, Ahmedabad, India). The composition of batches B16 and B17 was similar except that batch B17 was formulated without using the eutectic mixture.
Fig. 4.
In vitro drug transport from batches B16 and B17 through shed snake skin; B16 (closed diamonds), B17 (open squares-)
Kinetics of Drug Transport
The method of Bamba et al. was adopted to ascertain kinetics of drug transport from the formulated batches B1–B16 (17). In vitro drug transport data of batches B1–B16 were analyzed by zero-order, first-order, Higuchi, Hixson–Crowell, Korsmeyer–Peppas and Weibull models (18–23). A FORTRAN software, developed in-house, was used. The least value of sum of square of residuals (SSR) and Fisher’s ratio (F) were used to select the most appropriate kinetic model.
Similarity Factor
The similarity factor (f2) was calculated by comparing test and reference in vitro drug transport profile using eq. 3 (24).
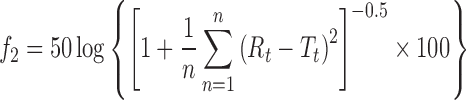 |
3 |
where n is the number of pull points, Rt and Tt are percentage drug transported from reference and test products, respectively, at time “t.”
Criteria for Optimized Batch
The film formation time was arbitrarily fixed as less than 8 min. The film should be mucoadhesive and flexible in nature to allow day-to-day activity yet should be water washable. Viscosity of the formulations should be less than 80 cPs. The selected limits for drug transport were: (1) Y1: amount of drug transported in 1 h/ml should be equal to the minimum inhibitory concentration of fluconazole (minimum inhibitory concentration (MIC) = 8 μg/ml) ± 5%, and (2) Y2: time required to transport 50% of the drug (t50%) should be equal to 360 min ± 5%.
Statistical Analysis
Unpaired t test with equal variance was used to find any statistically significant difference in the in vitro drug transport profile between batches B16 and B17 through shed snake skin at 5% level of significance.
Stability Study
Optimized formulation (batch B16) was stored for 2 months at 25 ± 2°C away from light. At the end of second month, the formulation was subjected to various tests like volume of solution delivered upon each actuation, pH, viscosity, solution delivered upon each actuation, spray angle, ex–in vivo physical characteristics and in vitro drug transport. The procedure employed for the study was identical to that described above. The results are shown in Table IV.
Table IV.
Results of 2 Months Stability Study of Optimized Batch B16
| Tests | Average results (n = 3) |
|---|---|
| Viscosity (a ± 2.9 cPs) | 54.7 |
| Volume of solution delivered upon each actuation (b ± 0.06 ml) | 0.24 |
| Spray angle (c ± 0.4°) | 83.4 |
| Ex–in vivo film formation timea (d ± 30 s) | 341 |
| Feeling of warmth and subsequent cooling sensationa (e ± 0.5 min) | 16 |
| Appearance of the filma | ++ |
| Dermal adhesion and flexibility of the filma | +++ |
| Water washabilitya | ++ |
aMeasured for placebo batches replacing fluconazole with blend of alcohol and acetone
RESULT AND DISCUSSION
A eutectic mixture is a mixture of two or more solids which has lower melting temperature than any of its constituents. Various substances such as ibuprofen, menthol, chloral hydrate, beta naphthol, lidocaine, and prilocaine form eutectic mixtures. The primary criterion for eutectic formation is the mutual solubility of the components in the liquid. Camphor and menthol also forms a hydrophobic eutectic mixture. Camphor and menthol are powerful penetration enhancers (8–14). Camphor and menthol cause leaching of the lipids present in the skin and thus cause subsequent pore formation (25). In the present study, attempt was made to use eutectic mixture of camphor and menthol as multifunctional excipient (solvent for the film formers, powerful permeation enhancer and antifungal agent).
Preliminary Batches Containing Ethyl Cellulose
The solubility of fluconazole in a mixture consisting of 80 parts of alcohol (80% v/v) and 20 parts of acetone and the 1:1 eutectic mixture of camphor and menthol was 150 and less than 2 mg/ml, respectively. Acetone and primary alcohols are preferred solvents in concentration of 40–65% in formulation of topical solutions (26). As per US Department of Health and Human Services-Food and Drug Administration (US FDA) inactive ingredient guide, limit of alcohol and acetone in topical solutions is more than 83% and 12%, respectively (27). Acetone was used along with alcohol in the vehicle blend to facilitate faster film formation (<8 min) on skin. Ethyl cellulose, a film former, was soluble (>100 mg/ml) in the eutectic mixture. Hence, fluconazole was dissolved in vehicle blend of alcohol and acetone, whereas ethyl cellulose was dissolved in eutectic mixture of camphor and menthol.
The pH of formulated batches (B1–B3) ranged from 5 to 7. The pH of human skin is in between pH 5.5 and 6.5 (28). Hence, the pH adjustment was unnecessary. Table I shows that the viscosity of the formulated batches containing ethyl cellulose as film former (B1–B3) ranged from 15 to 43.5 cPs. The viscosity of the formulations increased with ethyl cellulose concentration. Formulated batches (B1–B3) showed good sprayability. The volume of solution delivered upon each actuation and spray angle ranged from 0.26 to 0.32 ml and 78° to 81°, respectively. The stated parameters were correlated with polymer concentration and viscosity of the formulation. Ex–in vivo film formation of placebo batches (B1P–B3P) passed the desired criteria of film formation, which varied from 130 to 210 s after actuation. The film turned from transparent to translucent on increase in viscosity. Dermal adhesion and flexibility of films were moderate. Feeling of warmth and subsequent cooling sensation were perceived after application of spray (around 12 min) because of camphor and menthol in the formulation. None of the placebo formulations resulted in irritation, rashes and itching in any of the volunteers. Water washability of the placebo batches (B1P–B3P) was moderate.
The formulation excipients did not show absorbance at 264 nm. The UV spectrum remained unchanged during in vitro drug transport study, indicating stability of fluconazole during study. The excipients or membrane constituents did not show absorbance at 264 nm. The MIC of fluconazole against Candida species is 8 μg/ml (29). The problem of dose dumping and lack of sustained drug transport was seen with formulated batches (B1–B3) with values of Y1 greater than 12 μg/ml and Y2 less than 200 min (Table I and Fig. 1). Considering the results of viscosity, the concentration of ethyl cellulose was not increased beyond 7.5% w/w.
Preliminary Batches Containing Eudragit® RS100
Eudragit® RS100 was soluble (>120 mg/ml) in the eutectic mixture. Hence, Eudragit® RS100 was dissolved in eutectic mixture of camphor and menthol. The pH of formulated batches (B4–B6) ranged from 5 to 7. Table I shows that the viscosity of batches B4–B6 increased with polymer concentration and ranged from 7.6 to 51.6 cPs. The formulated batches containing Eudragit® RS100 as a film former (B4–B6) showed good sprayability. The volume of solution delivered upon each actuation and spray angle ranged from 0.24 to 0.39 ml and 76° to 82°, respectively. The polymer concentration and viscosity were found to affect the stated two parameters. Ex–in vivo film formation of placebo batches (B4P–B6P) passed the desired criteria of film formation. The appearance of film changed from shiny transparent to translucent on increase in viscosity. Dermal adhesion and flexibility of films were good except batch B4P containing lower concentration of Eudragit® RS100. Eudragit® RS100 is a widely used polymer in formulation of mucoadhesive dosage forms (30). Feeling of warmth and subsequent cooling sensation were perceived after application of spray (around 12 min). None of the placebo formulations resulted in irritation, rashes and itching in any of the volunteers. Water washability of the placebo batches (B4P–B6P) was good except batch B6P containing higher concentration of Eudragit® RS100. Concentration of Eudragit® RS100 was not increased beyond 15% w/w, considering the results of water washability. The formulated batches containing Eudragit® RS100 as a film former (B4–B6) failed to meet the desired in vitro drug transport criteria with values of Y1 greater than 11.75 μg/ml and Y2 less than 215 min (Fig. 1). Considering the results of preliminary study, a combination of ethyl cellulose and Eudragit® RS100 was tried to achieve desired in vitro drug transport.
Factorial Design
A two-factor, three-level full factorial design was used for formula optimization. The levels of independent variables were determined from the results of preliminary batches. For Eudragit® RS100, the low, medium and high levels were 5%, 10% and 15%, respectively. Ethyl cellulose was used at low (3%), medium (5%) and high (7.5%) levels. The pH of formulated batches (B7–B15) ranged from 5 to 7. Table II shows that viscosity, volume of solution delivered upon each actuation and spray angle of formulated batches (B7–B15) ranged from 23.7 to 128.3 cPs, 0.14 to 0.33 ml and 78.7° to 91.5°, respectively. Viscosity of the liquids increased with total increase in polymeric concentration. All placebo batches (B7P–B15P) passed ex–in vivo film formation criteria (<8 min) except batch B7P containing high level of Eudragit® RS100 and ethyl cellulose. The appearance of film changed from shiny transparent to dull and opaque on increase in viscosity. Dermal adhesion and flexibility of films were good except batches B14P and B15P. Feeling of warmth and subsequent cooling sensation were perceived after application of spray (around 13 min). None of the placebo formulations resulted in irritation, rashes and itching in any of the volunteers. Water washability of the placebo batches (B7P–B15P) was moderate except batches B7P and B8P, which showed poor water washability because of presence of higher level of Eudragit® RS100 along with moderate to low level of ethyl cellulose.
The values of Y1 (amount of drug transported at the end of first hour) and Y2 (time required to transport 50% of the drug) for formulated batches (B7–B15) varied from 4.53 to 21.7 μg/ml and 107 to 660 min, respectively (Table III and Fig. 2). Hence, it can be concluded that selected independent variables exhibit significant influence on the values of Y1 and Y2. Further optimization was carried out by evolving mathematical models using linear regression analysis. Equation 4 shows the relationship between the amount of drug transported at the end of first hour (Y1) and the independent variables.
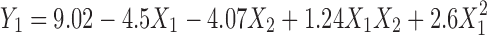 |
4 |
(Multiple R = 0.994, p < 0.05)
Equation 5 shows the relationship between the time required to transport 50% of the drug (Y2) and the independent variables.
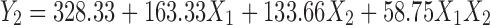 |
5 |
(Multiple R = 0.992, p < 0.05)
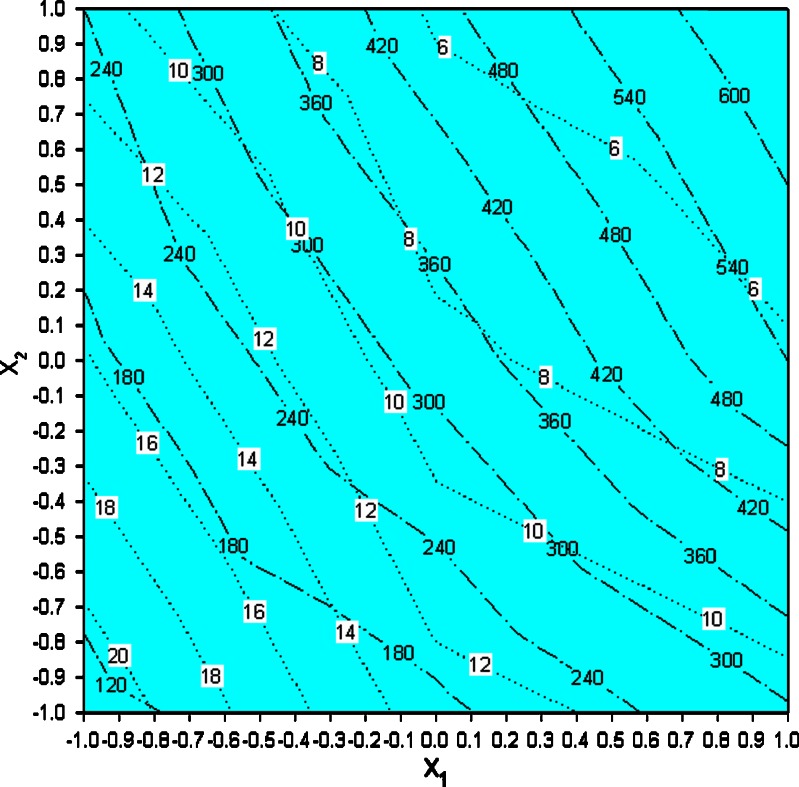
Contour plots of Y1 and Y2 were overlapped to locate the region of acceptability. The critical observation of the contour plot (Fig. 3) reveals that if X1 and X2 is varied from −0.5 to 0.12 and 0.1 to 1, respectively, the responses Y1 and Y2 are close to the target values of 8 μg/ml and 360 min, respectively. This limit may be considered for fine tuning of the formulation. Three formulations (A: X1 = −0.1; X2 = 0.4, B: X1 = −0.45; X2 = 1; and C = batch B16: X1 = 0.12; X2 = 0.1) theoretically satisfy the values of Y1 and Y2. A check-point batch (B16) was prepared. The experimental values of Y1 and Y2 for batch B16 were 7.91 μg/ml and 347 min, respectively (Table III). Batch B16 showed good sprayability. Ex–in vivo film formation of batch B16 passed the desired criteria of film formation (<8 min). The appearance of the film was shiny translucent. Dermal adhesion and flexibility of the film was good, whereas water washability was moderate. Thus, looking to overall results of sprayability, water washability and in vitro drug transport, batch B16 was ranked as one of the optimized batches and taken for further study.
Fig. 3.
Overlapped contour plots; dotted line Y 1, dashes and dots Y 2
A spray formulation (batch B16-A, Fig. 2) was prepared to evaluate the effect of acetone on in vitro drug transport by replacing acetone with alcohol (80% v/v ethanol) in batch B16. The Y1 and Y2 of batch B16-A was 16 μg/ml and 190 min, respectively. The probable reason for faster drug transport is delayed ex–in vivo film formation (720 s). The similarity factor (f2 = 38.5) calculated using in vitro drug transport data of batches B16 and B16-A as reference and test profile, respectively, indicated significant difference in drug transport between both the batches (24). It is therefore concluded that acetone is indispensable in getting desired drug transport profile.
The in vitro drug transport data were analyzed for determining kinetics of drug transport. Model fitting was done using an in-house computer program, developed by the authors. Zero-order, first-order, Higuchi, Hixson–Crowell, Korsmeyer–Peppas and Weibull models were tested. Korsmeyer–Peppas model showed the least of SSR and therefore, it was used for further data analysis. The slope and intercept values were 0.706, 0.799, 0.737 and −2.031, −2.413, −2.161, respectively for the formulations B9, B10, and B11. Equations 6 and 7 for slope and intercept, respectively, were evolved using linear regression analysis taking slope or intercept as dependent variable and ratio of X1 and X2 in the formulation as independent variable. The values of multiple R were greater than 0.85 in both the equations indicating good correlation. The unified equation (eq. 8) was evolved by combining the equations of slope and intercept.
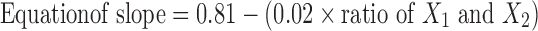 |
6 |
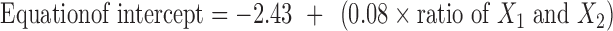 |
7 |
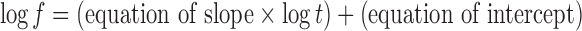 |
8 |
Where f and t are fraction of drug transported and time, respectively. Model validation was done by comparing the values of observed and predicted drug transport for three formulations (batches B9–B11) as well as for a check-point batch B16 containing 10.6% Eudragit® RS100 and 5.25% ethyl cellulose. Similarity factor (f2) was calculated considering calculated and observed drug transport data as reference and test profile, respectively (24). Batches B9–B11 and B16 showed insignificant difference in observed and predicted drug transport with similarity factor (f2) greater than 64 in each case.
The effect of eutectic mixture on drug permeation was checked using shed snake skin as a biological membrane (31,32) in Franz diffusion cell. The UV spectrum remained unchanged during in vitro drug transport study, indicating stability of fluconazole during the analysis. Figure 4 shows that the amount of drug transported and present in the receptor compartment per milliliter at the end of first hour, Y1 was 8.18 and 2.78 μg/ml for batches B16 and B17, respectively. The Y2 values were 367 and >720 min, respectively. Incomplete drug transport (less than 45%) was seen from the batch B17 formulated without using eutectic mixture. The probable reason for this difference could be presence of camphor and menthol in batch B16. It is reported that camphor and menthol works as penetration enhancer (8). Camphor and menthol causes leaching of the lipids present in the skin and thus causes pore formation (24). The difference in drug transport between batches B16 and B17 through shed snake skin was found to be significant at 5% level with 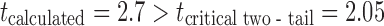 . Hence, it can be concluded that eutectic mixture of camphor and menthol significantly improved the drug permeation. Batch B16 showed similar in vitro drug transport through nylon (reference profile) and biological membrane (test profile) with similarity factor (f2) of 86. Probable reason for this behavior could be high concentration (10%) of eutectic mixture in the formulation.
. Hence, it can be concluded that eutectic mixture of camphor and menthol significantly improved the drug permeation. Batch B16 showed similar in vitro drug transport through nylon (reference profile) and biological membrane (test profile) with similarity factor (f2) of 86. Probable reason for this behavior could be high concentration (10%) of eutectic mixture in the formulation.
Stability Study
Short-term stability study of the optimized batch B16 was carried out for 2 months at 25 ± 2°C. Table IV shows that pH, viscosity, volume of solution delivered upon actuation, spray angle and ex–in vivo physical characteristics of optimized batch B16 remained unchanged during the study. Unpaired t test with equal variance indicated insignificant difference in the in vitro drug transport from the optimized batch B16 at p = 5% with 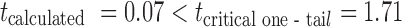 . The amount of drug transported at the end of 1 h (Y1) and time required to transport 50% of the drug (Y2, t50%) of optimized batch (B16) at the end of stability study were 7.78 μg/ml and 364 min, respectively.
. The amount of drug transported at the end of 1 h (Y1) and time required to transport 50% of the drug (Y2, t50%) of optimized batch (B16) at the end of stability study were 7.78 μg/ml and 364 min, respectively.
CONCLUSION
Modified transport transdermal spray of fluconazole was developed using 32 full factorial design. The optimized batch B16 containing 10.6% Eudragit® RS100 and 5.25% ethyl cellulose yielded mucoadhesive and flexible film on the human skin. The in vitro drug transport at the end of first hour was equal to the minimum inhibitory concentration (8 ± 0.4 μg/ml) with t50% of (360 ± 18 min). The eutectic mixture showed increased penetration of the drug through shed snake skin. Batch B16 passed short-term stability study, carried out at 25 ± 2°C, with no change in performance characteristics of the product.
Acknowledgments
We are grateful to Indian Council of Medical Research (ICMR) for providing senior research fellowship (SRF) to one of the authors for this project. We are thankful to Zydus Cadila (India) and Degussa Pvt. Ltd. (India) for kindly providing the gift samples.
Abbreviations
- f2
similarity factor
- MIC
minimum inhibitory concentration (μg/ml)
- PEG 400
polyethylene glycol
- X1
concentration of Eudragit® RS100 (% w/w)
- X2
concentration of ethyl cellulose (% w/w)
- Y1
the amount of drug transported (μg) per milliliter at the end of first hour
- Y2
the time (min) required to transport 50% of the drug (t50%)
- US FDA
US Department of Health and Human Services-Food and Drug Administration
Contributor Information
Mukesh C. Gohel, Phone: +91-79-26302746, FAX: +91-79-26304865, Email: mukeshgohel@hotmail.com
Stavan A. Nagori, Email: stavanpharma2000@gmail.com
References
- 1.Susan B. Merck index. 12. NJ: Merck & Co. Inc.; 1996. [Google Scholar]
- 2.United States Pharmacopoeia, XXIX, NF XIV, The United States Pharmacopoeial Convention Inc.; 2006. pp. 911–3.
- 3.Laura K, Stephanie DT, Christopher JP, Charles LC, David MS. Assessing pregnancy risks of azole antifungals using a high throughput aromatase inhibition assay. AAPS PharmSciTech. 2002;28:129–40. doi: 10.1081/erc-120015045. [DOI] [PubMed] [Google Scholar]
- 4.Ayub AC, Gomes AD, Lima M, Vianna-Soares CD, Ferreira LA. Topical delivery of fluconazole: In vitro skin penetration and permeation using emulsions as dosage forms. Drug Dev Ind Pharm. 2007;33:273–80. doi: 10.1080/03639040600829989. [DOI] [PubMed] [Google Scholar]
- 5.El-laithy HM, El-Shaboury KMF. The development of cutina lipogels and gel microemulsion for topical administration of fluconazole. AAPSPharmSciTech. 2002;3:77–85. doi: 10.1208/pt030435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivera PA, Martinez-Oharriz MC, Rubio M, Irache JM, Espuelas S. Fluconazole encapsulation in PLGA microspheres by spray-drying. J Microencapsulation. 2004;21:203–11. doi: 10.1080/02652040310001637811. [DOI] [PubMed] [Google Scholar]
- 7.Kavitha K, Sivaramkrishnan M, Nalini CN, Nappinnai M. Formulation and evaluation of topical drug delivery system of fluconazole. Indian Drugs. 2003;40:720–3. [Google Scholar]
- 8.Khan M. Bioavailability of vitamin B12 using a small volume nebulizer opthalmic drug delivery system. Clin Exp Ophthalmol. 2005;33:402–7. doi: 10.1111/j.1442-9071.2005.01030.x. [DOI] [PubMed] [Google Scholar]
- 9.Ho H, Chen L, Lin H, Sheu M. Penetration enhancement by menthol combined with a solubilization effect in a mixed solvent system. J Control Release. 1998;51:301–11. doi: 10.1016/S0168-3659(97)00184-3. [DOI] [PubMed] [Google Scholar]
- 10.Flashner-Barak M, Lerner I, Rosenberger V, Moldavski N, inventors. Menthol solutions of drugs. Patent WO/2004/073686. September 2, 2004.
- 11.Aqil M, Ahad A, Sultana Y, Ali A. Status of terpenes as skin penetration enhancers. Drug Discov Today. 2007;12:1061–7. doi: 10.1016/j.drudis.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Rhee Y, Choi J, Park E, Chi S. Transdermal delivery of ketoprofen using microemulsions. Int J Pharm. 2001;228:161–70. doi: 10.1016/S0378-5173(01)00827-4. [DOI] [PubMed] [Google Scholar]
- 13.Jain R, Aqil M, Ahad A, Aliand A, Khar RK. Basil oil is a promising skin penetration enhancer for transdermal delivery of labetolol hydrochloride. Drug Dev Ind Pharm. 2008;34:384–9. doi: 10.1080/03639040701657958. [DOI] [PubMed] [Google Scholar]
- 14.Williams AC, Barry BW. Terpenes and the lipid–protein–partitioning theory of skin penetration enhancement. Pharm Res. 1991;8:17–24. doi: 10.1023/A:1015813803205. [DOI] [PubMed] [Google Scholar]
- 15.Pattnaik S, Subramanyam VR, Bapaji M, Kole CR. Antibacterial and antifungal activity of aromatic constituents of essential oils. Microbios. 1997;89:39–46. [PubMed] [Google Scholar]
- 16.Nürnberg E. Welche galenischen Grundlagen werden heute für die. Hautbehandlung eingesetzt? Hautarzt. 1978;29:61–7. [PubMed] [Google Scholar]
- 17.Bamba M, Puisievx F, Marty JP, Carstensen JT. Release mechanism in gel forming sustained release formulation. Int J Pharm. 1979;2:307–15. doi: 10.1016/0378-5173(79)90037-1. [DOI] [Google Scholar]
- 18.Gibaldi M, Feldman S. Establishment of sink conditions in dissolution rate determinations: theoretical considerations and application to nondisintegrating dosage forms. J Pharm Sci. 1967;56:1238–42. doi: 10.1002/jps.2600561005. [DOI] [PubMed] [Google Scholar]
- 19.Korsmeyer R, Gurny R, Doelker E, Buri P, Peppas N. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35. doi: 10.1016/0378-5173(83)90064-9. [DOI] [PubMed] [Google Scholar]
- 20.Higuchi T. Rate of release of medicaments from ointment bases containing drugs in suspension. J Pharm Sci. 1961;50:874–5. doi: 10.1002/jps.2600501018. [DOI] [PubMed] [Google Scholar]
- 21.Higuchi T. Mechanism of sustained-action medication. J Pharm Sci. 1963;52:1145–9. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- 22.Higuchi T. Analysis of data on the medicament release from ointments. J Pharm Sci. 1962;51:802–4. doi: 10.1002/jps.2600510825. [DOI] [PubMed] [Google Scholar]
- 23.Langenbucher F. Linearization of dissolution rate curve by Weibull distribution. J Pharm Pharmacol. 1972;24:979–89. doi: 10.1111/j.2042-7158.1972.tb08930.x. [DOI] [PubMed] [Google Scholar]
- 24.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for Industry: Dissolution Testing of Immediate Release Solid Dosage Forms, August 1997.
- 25.Chew N, Reed B, Morgan T, Finnin B, inventors. Topical delivery of antifungal agents. US Patent US20040081684. April 29, 2004.
- 26.Warren R, Reed J, inventors. Topical tanning compositions. European patent EP19870306848. February 8, 1989.
- 27.http://www.accessdata.fda.gov/scripts/cder/iig/getiigWEB.cfm. Accessed on February 1, 2009.
- 28.Nadakatti S, Naik V. Detergent bar and process of manufacture, inventors. US patent US2006287206. December 21, 2006.
- 29.Pfaller MA, Diekema DJ, Sheehan DJ. Interpretive breakpoints for fluconazole and Candida revisited: A blueprint for the future of antifungal susceptibility testing. Clin Microbiol Rev. 2006;19:435–47. doi: 10.1128/CMR.19.2.435-447.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adriana T, Valentino L, Nunzio D, Angela L, Annalisa C, Massimo F, et al. Eudragit RS 100 microparticles containing 2-hydroxypropyl-β-cyclodextrin and glutathione: Physicochemical characterization, drug release and transport studies. E J Pharm Sci. 2007;30:64–74. doi: 10.1016/j.ejps.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Li GL, Geest RVD, Chanet L, Zanten EV, Danhof M, Bouwstra JA. In vitro iontophoresis of R-apomorphine across human stratum corneum: structure–transport relationship of penetration enhancement. J Control Release. 2002;84:49–57. doi: 10.1016/S0168-3659(02)00259-6. [DOI] [PubMed] [Google Scholar]
- 32.Higuchi T, inventor. Method for in vitro determination of transdermal absorption US Patent US4771004. August 29, 1986.