Abstract
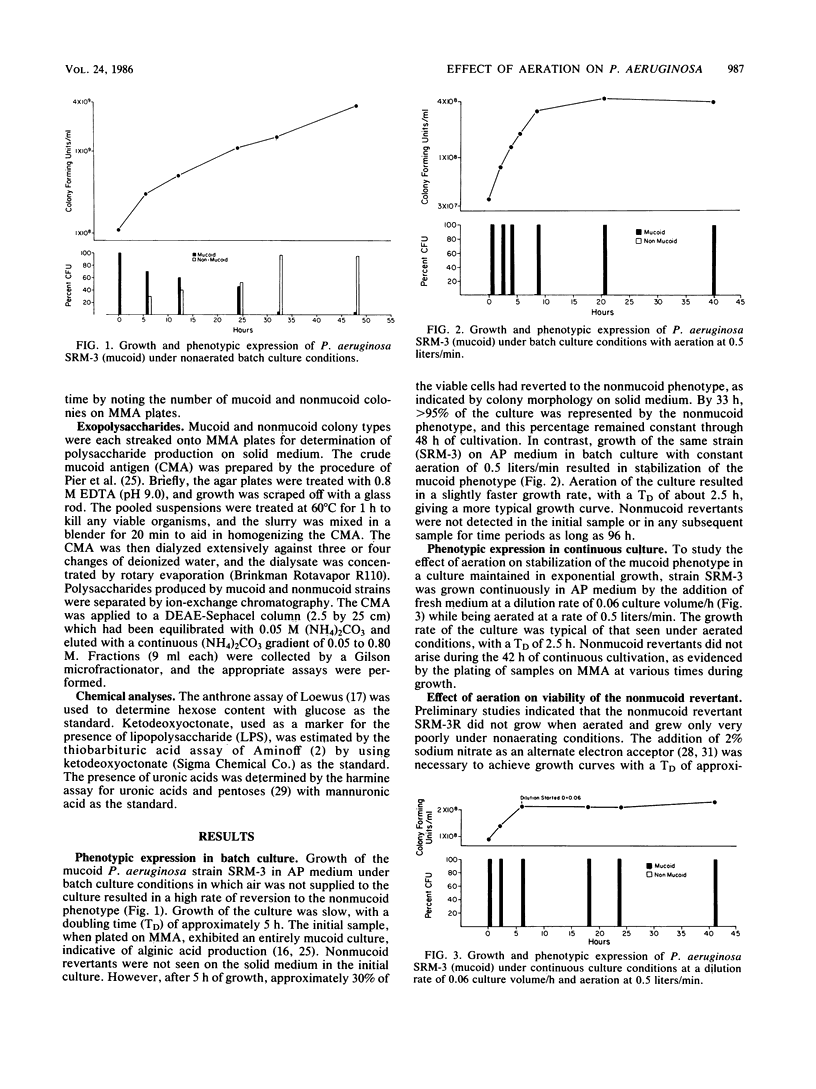
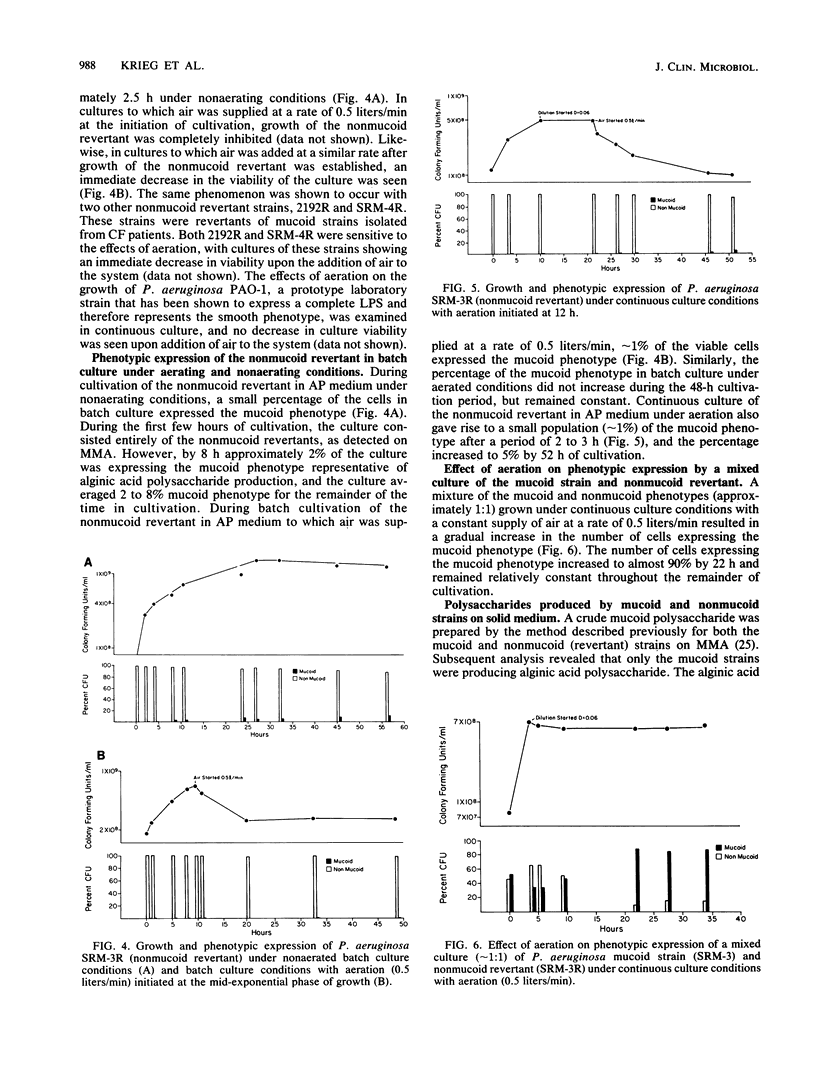
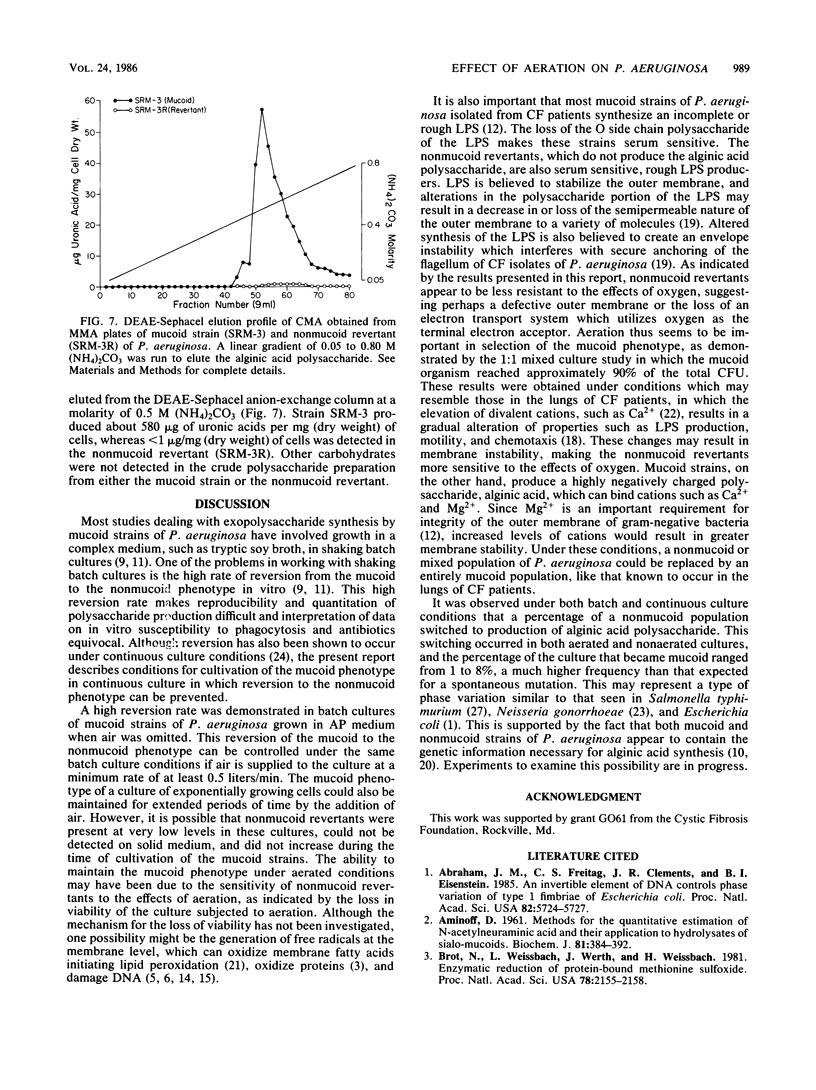
A mucoid strain of Pseudomonas aeruginosa isolated from a patient with cystic fibrosis and its nonmucoid revertant were grown in a chemically defined alginate-promoting medium under batch and continuous culture conditions. Selection for the mucoid and nonmucoid phenotype was accomplished by varying the levels of air available to the culture. The addition of air at a rate of 0.5 liters/min to the nonmucoid revertant growing under batch or continuous culture conditions resulted in a greater than 50% decrease in viability over a 10-h incubation period. In contrast, aeration of the mucoid culture maintained a totally mucoid population and there was no decrease in viability over a 55-h incubation. Aeration of a mixed population of the mucoid and nonmucoid phenotype (1:1) resulted in selection for the mucoid phenotype within the first 20 h of cocultivation. The correlation between the mucoid phenotype and alginic acid was demonstrated by the production of 580 micrograms of uronic acid per mg (dry weight) of cells by the mucoid phenotype and less than 1 microgram of uronic acid per mg (dry weight) of cells by the nonmucoid revertant. These results suggest that nonmucoid revertants may have an unusual sensitivity to aeration, which may indicate a mechanism for natural selection of the mucoid phenotype in vivo.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham J. M., Freitag C. S., Clements J. R., Eisenstein B. I. An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5724–5727. doi: 10.1073/pnas.82.17.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brot N., Weissbach L., Werth J., Weissbach H. Enzymatic reduction of protein-bound methionine sulfoxide. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2155–2158. doi: 10.1073/pnas.78.4.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson D. M., Matthews L. W. Polyuronic acids produced by Pseudomonas aeruginosa. Biochemistry. 1966 Sep;5(9):2817–2822. doi: 10.1021/bi00873a006. [DOI] [PubMed] [Google Scholar]
- Cathcart R., Schwiers E., Saul R. L., Ames B. N. Thymine glycol and thymidine glycol in human and rat urine: a possible assay for oxidative DNA damage. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5633–5637. doi: 10.1073/pnas.81.18.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Linn S. 5,6-Saturated thymine lesions in DNA: production by ultraviolet light or hydrogen peroxide. Nucleic Acids Res. 1982 Jun 25;10(12):3781–3789. doi: 10.1093/nar/10.12.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. R., Linker A. Production and characterization of the slime polysaccharide of Pseudomonas aeruginosa. J Bacteriol. 1973 Nov;116(2):915–924. doi: 10.1128/jb.116.2.915-924.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govan J. R., Fyfe J. A., McMillan C. The instability of mucoid Pseudomonas aeruginosa: fluctuation test and improved stability of the mucoid form in shaken culture. J Gen Microbiol. 1979 Jan;110(1):229–232. doi: 10.1099/00221287-110-1-229. [DOI] [PubMed] [Google Scholar]
- Govan J. R., Fyfe J. A. Mucoid Pseudomonas aeruginosa and cystic fibrosis: resistance of the mucoid from to carbenicillin, flucloxacillin and tobramycin and the isolation of mucoid variants in vitro. J Antimicrob Chemother. 1978 May;4(3):233–240. doi: 10.1093/jac/4.3.233. [DOI] [PubMed] [Google Scholar]
- Govan J. R. Mucoid strains of Pseudomonas aeruginosa: the influence of culture medium on the stability of mucus production. J Med Microbiol. 1975 Nov;8(4):513–522. doi: 10.1099/00222615-8-4-513. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Mutharia L. M., Chan L., Darveau R. P., Speert D. P., Pier G. B. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: a class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect Immun. 1983 Oct;42(1):170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiby N. Epidemiological investigations of the respiratory tract bacteriology in patients with cystic fibrosis. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Aug;82(4):541–550. [PubMed] [Google Scholar]
- Hollstein M. C., Brooks P., Linn S., Ames B. N. Hydroxymethyluracil DNA glycosylase in mammalian cells. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4003–4007. doi: 10.1073/pnas.81.13.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINKER A., JONES R. S. A POLYSACCHARIDE RESEMBLING ALGINIC ACID FROM A PSEUDOMONAS MICRO-ORGANISM. Nature. 1964 Oct 10;204:187–188. doi: 10.1038/204187a0. [DOI] [PubMed] [Google Scholar]
- Levin D. E., Hollstein M., Christman M. F., Schwiers E. A., Ames B. N. A new Salmonella tester strain (TA102) with A X T base pairs at the site of mutation detects oxidative mutagens. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7445–7449. doi: 10.1073/pnas.79.23.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzar M. A., Montie T. C. Avirulence and altered physiological properties of cystic fibrosis strains of Pseudomonas aeruginosa. Infect Immun. 1985 Nov;50(2):572–576. doi: 10.1128/iai.50.2.572-576.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzar M. A., Thomassen M. J., Montie T. C. Flagella and motility alterations in Pseudomonas aeruginosa strains from patients with cystic fibrosis: relationship to patient clinical condition. Infect Immun. 1985 Nov;50(2):577–582. doi: 10.1128/iai.50.2.577-582.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. R. Mucoid variation in Pseudomonas aeruginosa induced by the action of phage. J Med Microbiol. 1973 Feb;6(1):111–118. doi: 10.1099/00222615-6-1-111. [DOI] [PubMed] [Google Scholar]
- Mearns M. B., Hunt G. H., Rushworth R. Bacterial flora of respiratory tract in patients with cystic fibrosis, 1950-71. Arch Dis Child. 1972 Dec;47(256):902–907. doi: 10.1136/adc.47.256.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. F., Mlawer N., So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982 Aug;30(1):45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- Mian F. A., Jarman T. R., Righelato R. C. Biosynthesis of exopolysaccharide by Pseudomonas aeruginosa. J Bacteriol. 1978 May;134(2):418–422. doi: 10.1128/jb.134.2.418-422.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Matthews W. J., Jr, Eardley D. D. Immunochemical characterization of the mucoid exopolysaccharide of Pseudomonas aeruginosa. J Infect Dis. 1983 Mar;147(3):494–503. doi: 10.1093/infdis/147.3.494. [DOI] [PubMed] [Google Scholar]
- Pseudomonas aeruginosa infections: persisting problems and current research to find new therapies. Ann Intern Med. 1975 Jun;82(6):819–831. doi: 10.7326/0003-4819-82-6-819. [DOI] [PubMed] [Google Scholar]
- Simon M., Zieg J., Silverman M., Mandel G., Doolittle R. Phase variation: evolution of a controlling element. Science. 1980 Sep 19;209(4463):1370–1374. doi: 10.1126/science.6251543. [DOI] [PubMed] [Google Scholar]
- Van Hartingsveldt J., Marinus M. G., Stouthamer A. H. Mutants of Pseudomonas aeruginosa bblocked in nitrate or nitrite dissimilation. Genetics. 1971 Apr;67(4):469–482. doi: 10.1093/genetics/67.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardi A. H., Allen W. S., Varma R. A simple method for the detection and quantitative determination of hexuronic acids and pentoses. Anal Biochem. 1974 Jan;57(1):268–273. doi: 10.1016/0003-2697(74)90072-4. [DOI] [PubMed] [Google Scholar]
- Wood R. E., Boat T. F., Doershuk C. F. Cystic fibrosis. Am Rev Respir Dis. 1976 Jun;113(6):833–878. doi: 10.1164/arrd.1976.113.6.833. [DOI] [PubMed] [Google Scholar]
- YAMANAKA T., OTA A., OKUNUKI K. A nitrite reducing system reconstructed with purified cytochrome components of Pseudomonas aeruginosa. Biochim Biophys Acta. 1961 Oct 28;53:294–308. doi: 10.1016/0006-3002(61)90442-5. [DOI] [PubMed] [Google Scholar]
- di Sant'agnese P. A., Davis P. B. Cystic fibrosis in adults. 75 cases and a review of 232 cases in the literature. Am J Med. 1979 Jan;66(1):121–132. doi: 10.1016/0002-9343(79)90491-1. [DOI] [PubMed] [Google Scholar]



