Abstract
The human α-globin genes are paralogues, sharing a high degree of DNA sequence similarity and producing an identical α-globin chain. Over half of the α-globin structural variants reported to date are only characterized at the amino acid level. It is likely that a fraction of these variants, with phenotypes differing from one observation to another, may be due to the same mutation but on a different α-globin gene. There have been very few previous examples of hemoglobin variants that can be found at both HBA1 and HBA2 genes. Here, we report the results of a systematic multicenter study in a large multiethnic population to identify such variants and to analyze their differences from a functional and evolutionary perspective. We identified 14 different Hb variants resulting from identical mutations on either one of the two human α-globin paralogue genes. We also showed that the average percentage of hemoglobin variants due to a HBA2 gene mutation (α2) is higher than the percentage of hemoglobin variants due to the same HBA1 gene mutation (α1) and that the α2/α1 ratio varied between variants. These α-globin chain variants have most likely occurred via recurrent mutations, gene conversion events, or both. Based on these data, we propose a nomenclature for hemoglobin variants that fall into this category.
Electronic supplementary material
The online version of this article (doi:10.1007/s00277-008-0624-3) contains supplementary material, which is available to authorized users.
Keywords: α-Globin genes, Paralogues, Gene conversion, Hemoglobin variants, Mutations
Introduction
There are close to 1,000 human hemoglobin (Hb) variants reported to date [1, 2], and new ones are still being discovered. The vast majority of them are rare, while others, e.g., Hb S, Hb C, and Hb E, reach high frequencies in specific population groups [3]. Most Hb variants are clinically and hematologically silent, but some cause hemolytic anemia, e.g., Hb S, some are unstable Hbs, methemoglobinemia (Hbs M), and some cause cyanosis (Hb Kansas) or polycythemia due to their increased oxygen affinity (Hb Ypsilanti, Hb Malmö). There are still other Hb variants that also lead to thalassemic phenotype (Hb E, Hb Knossos).
The human α-like globin gene cluster resides on the telomeric region of chromosome 16. The HBA2 and HBA1 genes are paralogues, i.e., set of homologous genes that have evolved from gene duplication and can be associated with a subsequent divergence of function. Both genes have an almost identical DNA sequence due to sequence homogenization by a gene conversion event [4]. Each gene yields an identical 141-amino acid-long α-globin chain, which participates with a β-like globin chain to form the Hb tetramers. From the 332 α-globin chain variants identified to date, approximately 52% have been only characterized by protein chemistry methods, which creates uncertainty as to which α-globin gene is mutated (Table 1). It is likely that a fraction of these variants may result from the same mutation but on a different α-globin gene. Identification and comparative study of these variants would not only provide useful insights into α-globin gene transcription and translation but can also shed light into the mechanistic properties of sequence diversification of the α-like globin genes. There have been very few examples of Hb variants that have been previously identified at both HBA1 and HBA2 genes, namely Hb Frankfurt [5] and Hb I [6]. Moreover, as the vast majority of Hb variants are rare, identification and comparative analysis of heterozygous cases for these variants is problematic, even for laboratories with large sample volumes.
Table 1.
Number of hemoglobin variants due to a mutant human α-globin gene
| Globin genes | No. of Hb variantsa |
|---|---|
| HBA2 | 95 |
| HBA1 | 54 |
| HBA2 or HBA1 unknown | 173 |
| Found in both HBA2 or HBA1 | 14 |
| Total | 332 |
aData from HbVar database (http://globin.bx.psu.edu/hbvar, assessed in August 2007)
Here, we report our results from a multicenter study to identify and comparatively analyze Hb variants that result from the same mutation but on different α-globin gene paralogues in a large multiethnic population sample. In addition, we performed a systematic study in the HbVar database of Hb variants and thalassemia mutations (http://globin.bx.psu.edu/hbvar; [1, 3]) to reveal other globin chain variants that fall into this category. Based on these results, we propose a nomenclature for related Hb variants.
Materials and methods
Case selection
One hundred and one unrelated individuals, referred to the Unit of Biochemical Genetics, Henri Mondor Hospital (Créteil, France), National Haemoglobinopathy Reference Laboratory, Churchill Hospital, (Oxford, UK), Unit of Prevention of Thalassemia, Trikala General Hospital (Trikala, Greece), and Department of Pathology and Laboratory Medicine, Boston University (Boston, USA), were selected for this study. These subjects were previously identified as carriers of an Hb variant. Hematological indices were measured using a Coulter Cell Counter.
Additionally, 64 unrelated cases were identified in HbVar database of hemoglobin variants and thalassemia mutations (http://globin.bx.psu.edu/hbvar) and comparatively analyzed [7, 8]. Finally, additional data were extracted from Online Mendelian Inheritance in Man, where available (see Tables 2 and 3 for details).
Table 2.
Hematological indices and percentage of Hb variants in either the HBA1 or HBA2 genes and the α2/α1 ratio of the average percentage of the abnormal Hb for heterozygotes with a normal complement of α-globin genes (αα/αα)
| Hb variant | Racial/ethnic background | Samples | Hb (g/dL) | MCV (fL) | Hb X (%) | Hb S (%) | α2/α1 ratio | References | ||
|---|---|---|---|---|---|---|---|---|---|---|
| ΗΒα1 | ΗΒα2 | HBAa | ||||||||
| p.N9S (c.29A>G) | ||||||||||
| Hb Zurich-Hottingen | Portuguese | 1 | 11.7 | 75 | – | N.M. | – | – | N.M. | [24] |
| Hb Zurich-Hottingen | Portuguese | 1 | 13.8 | 78 | – | N.M. | – | – | [24] | |
| Hb Anadour | Moroccan | 1 | Normal | Normal | N.M. | – | – | – | HbVar (ID 2594) b | |
| p.A12D (c.38C>A) | ||||||||||
| Hb J-Paris-I | Asian c | 4 | 12.7 ± 1.8 | 89.2 ± 6.5 | 26.5 ± 1.2 | – | – | – | 0.98 | This report |
| Hb J-Paris-I | N.A. | 1 | Normal | Normal | 20.7 | – | – | – | [14] | |
| Hb J-Paris-I | Asian c | 1 | 14.9 | 91.2 | – | 26 | – | – | This report | |
| Hb J-Paris-I | N.A. | 2 | Normal | Normal | – | 24 | – | – | [14] | |
| p.H20Q (c.63C>A) | ||||||||||
| Hb Le Lamentin | N.A. | 1 | Normal | Normal | – | 28 | – | – | 1.10 | [14] |
| Hb Le Lamentin | British | 7 | 11.4 ± 4.7 | 91.9 ± 6.0 | 25.4 ± 1.1 | – | – | – | This report | |
| Hb Le Lamentin | African | 1 | 11.7 | 96.1 | 14.2 | – | – | 36.9 | This report | |
| p.E27D (c.84G>C) | ||||||||||
| Hb Hekinan | Chinese (Macau) | 1 | 12.8 | 94 | 13.4 | – | – | – | N.A. | [25] |
| Hb Hekinan | Chinese (Macau) | 1 | 10.8 | 92 | 13.7 | – | – | – | [25] | |
| Hb Hekinan | Chinese (Macau) | 1 | Normal | Normal | 12.9 | – | – | – | [25] | |
| Hb Hekinan | Taiwanese | 1 | 9.9 | 72.9 | 14.7 | – | – | – | [26] | |
| Hb Hekinan | French (Guyana) | 1 | Normal | Normal | – | – | 13 | – | [27] | |
| p.D47H (c.142G>C) | ||||||||||
| Hb Hasharon | Jewish (Brazilian) | 1 | 12.6 | 79 | 18 | – | – | – | 1.15 | This report |
| Hb Hasharon | Jewish (British) | 1 | 12.1 | 83 | 18.9 | – | – | – | This report | |
| Hb Hasharon | Jewish (British) | 10 | 14.4 ± 3.8 | 87.5 ± 6.0 | – | 21.2 ± 0.9 | – | – | This report | |
| Hb Hasharon | Jewish | 3 | 14.4 | 95 | – | 17.4 | – | – | [14] | |
| p.H50Q (c.153C>G) | ||||||||||
| Hb Frankfurt | German | 1 | 14.3 | 85 | 24 | – | – | – | 1.13 | [5] |
| Hb Frankfurt | Corsican | 1 | Normal | Normal | – | 27 | – | – | [5] | |
| p.G51R (c.154G>C) | ||||||||||
| Hb Russ | Caucasian/Chinese | 2 | Normal | Normal | 11.5 | – | – | – | 1.53 | [28] |
| Hb Russ | British | 1 | 11.2 | 88 | 14.6 | – | – | – | This report | |
| Hb Russ | British | 1 | 12.9 | 87 | – | 22.3 | – | – | This report | |
| p.V55A (c.167T>C) | ||||||||||
| Hb Gerland | French | 1 | 15 | 81.7 | – | 15.9 | – | – | 1.67d | [29], personal communication |
| Hb Gerland [A1] | Southeast Asian | 1 | 11.5 | 71 | 9.5d | – | – | – | [30] | |
| p.N68H (c.205A>C) | ||||||||||
| Hb St. Truiden | Belgian | 1 | 15.6 | 90 | – | 24 | – | – | 1.28 | HbVar (ID 2591) b |
| Hb Jeddah | Saudi Arabian | 1 | 13.4 | 82.7 | 17.8 | – | – | N.A. | [31] | |
| Hb Jeddah | Yemenite | 1 | 11.6 | 90.3 | 19.6 | – | – | – | [31] | |
| Hb Jeddah | Emirati (Abu Dhabi) | 1 | 12.6 | 72.9 | N.A. | – | – | – | [31] | |
| p.D75Y (c.226G>T) | ||||||||||
| Hb Winnipeg | American | 10 | 12.9 ± 1.3 | 90.7 ± 6.8 | 14.1 ± 0.6 | – | – | – | 1.3 | [32] |
| Hb Winnipeg | French | 38 | 13.0 ± 1.9 | 91.2 ± 7.3 | 13.0 ± 0.7 | – | – | – | This report | |
| Hb Winnipeg | French | 11 | 13.6 ± 0.8 | 91.9 ± 6.7 | – | 16.9 ± 0.9 | – | – | This report | |
| Hb Winnipeg | Greek | 1 | 13.6 | 88.9 | – | 16.1 | – | – | This report | |
| p.P77H (c.233C>A) | ||||||||||
| Hb Toulon | African | 1 | 9.8 | 94.4 | 23 | – | – | – | 1.08 | This report |
| Hb Toulon | British | 1 | 13.3 | 91 | – | 26.3 | – | – | This report | |
| Hb Toulon | French | 1 | Normal | Normal | – | 24 | – | – | [33] | |
| Hb Toulon | Canadian | 1 | Normal | Normal | – | 25.3 | – | – | [34] | |
| Hb Toulon | Italian | 1 | 13.1 | 91 | – | 24 | – | – | [35] | |
| p.N78K (c.237C>A) | ||||||||||
| Hb Stanleyville-II | Central African | 5 | Normal | Normal | – | – | N.A. | – | N.A. | [12] |
| Hb Stanleyville-II | French | 6 | Normal | Normal | – | – | 24 | – | [36] | |
| Hb Stanleyville-II | Caucasian | 1 | 11.0 | 88 | – | 15.3 | – | – | This report | |
| Hb Stanleyville-II | African | 1 | 14.1 | 90 | – | 11.1 | – | 42.7 | This report | |
| p.D94N (c.283G>A) | ||||||||||
| Hb Titusville | Swedish/Finish | 1 | 13.3 | 91 | – | 15.6 | – | – | 1.13 | [37] |
| Hb Titusville | Swedish/Finish | 1 | 13.6 | 88 | – | 16 | – | – | [37] | |
| Hb Titusville | Scottish | 1 | 15.8 | 85.9 | 17 | – | – | – | [38] | |
| Hb Titusville | Scottish | 1 | 14.5 | 91 | 11 | – | – | – | [38] | |
| Hb Titusville | Scottish | 1 | Normal | Normal | 14 | – | – | – | [38] | |
| p.A120E (c.362C>A) | ||||||||||
| Hb J-Meerut | N.A. | 1 | Normal | Normal | 18.4 | – | – | – | 1.22 | [14] |
| Hb J-Meerut | Asian c | 15 | 11.9 ± 2.1 | 85.4 ± 10 | 20.5 ± 2.0 | – | – | – | This report | |
| Hb J-Meerut | N.A. | 1 | Normal | Normal | – | 23 | – | – | [14] | |
N.A. not available, N.M. not measurable, Normal cases where hematological data were normal but hematological indices were not available
aThis percentage was not included in the calculation of the α2/α1 ratio of the average percentage of the abnormal Hb
bDetailed information for these variants can be retrieved from HbVar from the URL: http://globin.bx.psu.edu/cgi-bin/hbvar/query_vars3?mode=output&display_format=page&i=ID, by replacing “ID” with the respective HbVar ID number
cPakistani and Indian
dAverage value from measurements of two independent samples from the same individual (8% and 11%). The variability is most likely due to the slight instability of Hb Gerland.
Table 3.
Hematological indices and percentage of Hb variants in either the HBA1 or HBA2 genes for heterozygotes with an α-thalassemia phenotype (−α/αα or −−/αα)
| Hb variant | Racial/ethnic background | Samples | Hb (g/dL) | MCV (fL) | Hb X (%) | Hb E (%) | Number of α-globin genes | References | |
|---|---|---|---|---|---|---|---|---|---|
| ΗΒα1 | ΗΒα2 | ||||||||
| p.N9S (c.29A>G) | |||||||||
| Hb Zurich-Hottingen | Portuguese | 1 | 16.5 | 91 | – | N.M. | – | −α/αα | [24] |
| p.E27D (c.84G>C) | |||||||||
| Hb Hekinan | N.A. | 1 | 10.9 | 83.9 | – | 23 | −α/αα | [14] | |
| Hb Hekinan | Asian | 1 | 11.3 | 69 | 15.8 | – | 80 | −α/αα | This report |
| Hb Hekinan | Thai | 1 | 10.2 | 83 | 27 | – | 10 | −−/αα | [39] |
| Hb Hekinan | Thai | 1 | 8.2 | 68.6 | 26.5 | – | 9.1 | −−/αα | [39] |
| Hb Hekinan | Burmanese | 1 | 11.2 | 68 | 25 | – | – | −−/αα | [40] |
| p.H50Q (c.153C>G) | |||||||||
| Hb Frankfurt | Portuguese | 1 | 15.2 | 83 | – | 36 | −α/αα | [5] | |
| p.V55A (c.167T>C) | |||||||||
| Hb Gerland | French | 1 | 12.2 | 70.6 | 31 | – | – | −α/αα | [29] |
| Hb Gerland | French | 1 | 13 | 76 | 31 | – | – | −α/αα | [29] |
| p.D75Y (c.226G>T) | |||||||||
| Hb Winnipeg | Greek | 1 | 14.2 | 76.7 | – | 30.1 | – | −α/αα | This report |
| p.N78K (c.237C>G) | |||||||||
| Hb Stanleyville-II | Black | 1 | 11.7 | 87 | 28.6 | – | −α/αα | This report | |
| Hb Stanleyville-II | Black | 1 | 9.3 | 89 | 25.2 | – | – | −α/αα | This report |
| Hb Stanleyville-II | Caucasian | 1 | 15.1 | 80 | – | 27.4 | −α/αα | This report | |
The number of α-globin genes is also provided
N.A. not available, N.M. not measurable
Identification of Hb variants
Each sample was analyzed by cation-exchange high-performance liquid chromatography (HPLC) using the Hb Variant I or II systems with either the 6-min β-Thalassemia short or the Dual Kit program (BioRad Laboratories, Hercules, CA, USA). In addition, samples from the National Haemoglobinopathy Reference Laboratory were analyzed by isoelectric focusing, those from Trikala General Hospital were analyzed by alkaline agarose gel electrophoresis (Hydragel Hemoglobin 7, SEBIA Norcross, GA, USA) and isoelectric focusing, and samples from Henri Mondor Hospital were characterized using a battery of electrophoretic and chromatographic tests (IEF, citrate agar gel electrophoresis, globin chain electrophoresis at acid and alkaline pH and in the presence of Triton X-100, CE-HPLC, RP-HPLC), matching perfectly with results obtained with Hb samples structurally identified as previously described [9].
DNA was isolated from blood leukocytes, and amplification of the HBA2, HBA1, and HBB genes was done using gene-specific primers [10]. DNA sequence analysis was performed with an automated sequencer (ABI 310, Applied Biosystems, Foster City, CA, USA, or CEQ 8000, Beckman Coulter, Fullerton, CA, USA). Detection of α-thalassemia deletional mutants was carried out by a gap-PCR procedure [11].
Results
Fourteen different Hb variants, resulting from identical mutations on either one of the two human α-globin paralogue genes, were identified during this study. From these, nine α-globin chain variants were identified in our study sample, and their hematological and DNA findings were comparatively analyzed with the same Hb variant carriers previously published and documented in HbVar, namely Hb Winnipeg (51 and ten cases, respectively), Hb J-Meerut (15 and two cases, respectively), Hb Hasharon (12 and three cases, respectively), Hb Lamentin (eight and one cases, respectively), Hb J-Paris (five and three cases, respectively), Hb Stanleyville-II (five and 11 cases, respectively), Hb Russ (two and two cases, respectively), Hb Toulon (two and three cases, respectively), and Hb Hekinan (one and nine cases, respectively). Also, five more Hb variants, resulting from identical mutations on either one of the two human α-globin paralogue genes, were selected from HbVar database and/or the literature and included in this study, namely Hb Zurich-Hottingen/Hb Anadour (four cases), Hb Frankfurt (three cases), Hb Gerland (four cases), Hb St. Truiden/Hb Jeddah (four cases), and Hb Titusville (five cases). The hematological indices of the subjects carrying these α-globin chain variants are provided in Tables 2 and 3. With the exception of the mildly unstable Hb Russ and Hb Gerland, the rest of the Hb variants were stable. Furthermore, all Hb variant were found in chromosomes bearing two α-globin genes, except Hb Frankfurt, where the variant was also found in the context of a −α3.7 thalassemia chromosome [5]. All heterozygous or compound heterozygous (with −α3.7 thalassemia) cases were asymptomatic or present with a typical α-thalassemia carrier phenotype, respectively.
Interestingly, however, DNA analysis of the HBA1 and HBA2 genes revealed that in 23 unrelated Hb variant carriers, the mutated α-globin gene was different than the one previously described for each Hb variant. In particular, we identified Hb Hasharon and Hb Toulon resulting from an HBA1, instead of a HBA2, gene mutation; Hb Russ and Hb Winnipeg resulting from a HBA2, instead of a HBA1, gene mutation; while Hb Stanleyville-II mutation was found in both the HBA1 or HBA2 genes (Tables 2 and 3). The mutated gene for Hb Stanleyville-II was previously not known, as this variant was only characterized at the protein level [12].
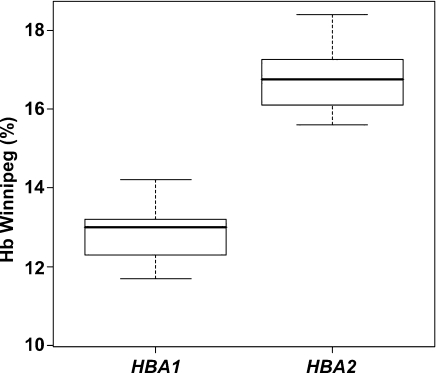
Subsequently, we exploited the large number of unrelated Hb Winnipeg carriers bearing the c.226G>T mutation in either the HBA2 or HBA1 gene to address whether the percentage of the Hb variant can be correlated to the α-globin gene mutation. Statistical analysis, using SPSS 10 software, showed that Hb Winnipeg carriers cluster in two distinct groups, determined by the percentage of the Hb Winnipeg variant. In 38 Hb Winnipeg carriers bearing the HBA1 gene mutation, Hb Winnipeg percentage varied from 10.3 to 14.2 (13.0 ± 0.7), while in 12 Hb Winnipeg carriers bearing the HBA2 gene mutation, the percentage of the variant hemoglobin was between 15.6 and 18.4 (16.9 ± 0.9, Fig. 1). The ration of the average percentage of the abnormal Hb in heterozygotes with HBA2 (α2) and HBA1 mutations (α1) varied from 1.1 to 1.8, with a mean value of 1.3. These results showed that (1) Hb Winnipeg is produced at a higher rate (approx. 23%) when the underlying mutation is in the HBA2 gene, and (2) the percentage of Hb variant could provide initial information regarding the α-globin gene molecular defect.
Fig. 1.
Box plots (p < 0.001) indicating the range and mean values (thick black lines) of the Hb Winnipeg variant percentage between carriers bearing the HBA1 or HBA2 mutation. In our evaluation, the Hb Winnipeg/α-thalassemia compound heterozygous case, as well as the cases described in Ref. [32], is excluded
We then explored the HbVar database to identify other Hb variants with similar properties, e.g., identical mutations in different α-globin genes yielding the same α-globin chain variant. We identified 64 heterozygous and compound heterozygous, with −α3.7 thalassemia cases, originating from various ethnic backgrounds (Tables 2 and 3), creating a total of 14 different Hb variants that fall into this category. Comparative analysis of all 165 carriers, stratified on the basis of the Hb variant, showed that the α2/α1 average Hb variant percentage ranged from 0.98 to 1.67. However, these cases were extremely rare, and therefore, their comparative analysis does not provide statistical confidence compared to the Hb Winnipeg cases. Finally, in three cases, the variant Hb was only characterized by means of protein sequencing; therefore, the mutated α-globin gene could not be identified unambiguously, and these cases were excluded from further analysis.
Discussion
The present study reports the identification and a comparative analysis of the hematological and molecular properties of Hb variants resulting from the same mutation but residing on a different α-globin gene. Our effort was facilitated from the extensive and diverse sample and comprehensive data recording of large diagnostic centers and reference laboratories participating in this study, most of which are members of the ITHANET network (http://www.ithanet.eu).
Structural variants have provided important insights into α-globin gene transcriptional regulation and translation. In particular, using in vitro translation experiments, it has been shown that the HBA2 gene encodes two- to threefold more protein that the HBA1 gene [13]. However, a more detailed study of heterozygous cases for an α-globin gene structural variant showed that the average abnormal Hb percentage in heterozygotes with HBA2 and HBA1 mutations correspond to a α2/α1 ratio of 1.19/1, respectively [14]. This ratio, however, was obtained from the cumulative analysis of all α-globin chain variants, and the relative position of the variant amino acid in the α-globin chain or the relative stability of the variant Hb was not taken into account. The latter may impact on the final amount of α-globin chain, particularly for unstable variants, the translational efficiency or the altered efficiency in hemoglobin tetramer formation. Although the Hb variant carriers reported herein are phenotypically silent, this study provides a much safer approach, since the comparison is more direct and performed between identical mutations in the α-globin genes, yielding identical globin chains. To our knowledge, this is the first attempt to comparatively analyze the mutations in gene paralogues leading to an identical protein product based on data collected from such a large multiethnic sample, not only for the α-globin but also for other gene families.
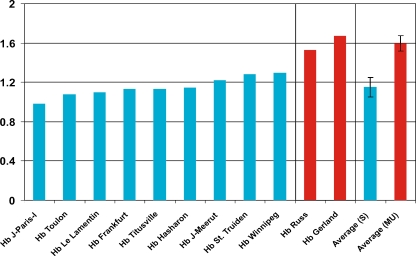
Our data show that the α2/α1 ratio of the average percentage of the abnormal Hb in heterozygotes with HBA2 and HBA1 mutations, respectively, varies from 0.98 (Hb J-Paris-I) to 1.67 (Hb Gerland; Tables 2 and 3). Moreover, comparison of the α2/α1 ratio of the average percentage of the abnormal Hbs showed that stable and mildly unstable abnormal Hbs nicely cluster in two distinct groups with average α2/α1 ratios of 1.15 ± 0.10 and 1.60 ± 0.09, respectively (Fig. 2). Notably, in all cases summarized in Tables 2 and 3, the percentage of variant Hb has been calculated using cation-exchange HPLC. Therefore, although these values come from different centers and published reports, the use of similar analytical method, apparatus, and analysis software makes these values virtually comparable. Also, the large number of Hb Winnipeg cases studied in this context statistically strengthens the above claims (Table 2). These data are comparable with previous results from heterozygous cases for 24 different α-globin chain variants [14] but sharply contradicts with a previous study [13], which indicated that a mutated HBA2 gene yielding a variant α-globin chain is expressed at a two- to threefold higher level than a mutated HBA1 gene. The main supporting data for this claim were the Hb levels of Hb J-Oxford (40%) and Hb Hasharon (35%), which are comparable to levels expected for Hb variant/α-thalassemia compound heterozygotes (see also Table 3). However, these data were obtained from in vitro translation experiments, and hence, this discrepancy is most likely due to the experimental approach rather that directly measuring Hb variant levels in vivo.
Fig. 2.
Comparison of the calculated α2/α1 ratio of the average percentage of the stable (S, shown in blue bars) and mildly unstable abnormal Hbs (MU, shown in red bars) reported in this paper
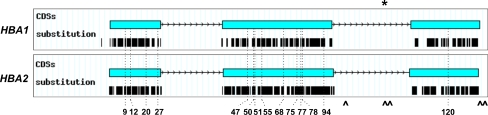
How do these variants occur? Although recurrent mutational events can be a likely cause for some of them, interallelic gene conversion event is the most plausible cause that might have resulted in the same mutation being “transferred” into different genomic contexts. A handful of examples also exist in the human β-like globin genes (reviewed in [15]), as well as in other human multigene families [16]. In favor for this assumption is the fact that 13 out of 14 Hb variants described herein are within exons 1 and 2. These exons, and not exon 3, have been shown to be involved in gene conversion events, as the 3′ end of the human α-globin gene conversion tract is located in intron II (Fig. 3).
Fig. 3.
Schematic drawing of the 14 α-globin chain variants resulting from an identical mutation in either the HBA1 or HBA2 genes. Original graphs have been automatically generated by HbVar graphical display [8]. Thick lines under each coding sequence (CDS) represent the position of each substitution deposited in HbVar. Asterisk 3′ end of the human α-globin gene conversion tract [6], caret PSVs in the promoter and coding sequences of the human α-globin genes (c.300+55G/T, c.301-35_29GGCCCTCdel, c.301-24C/G, c.*+15G/A, c.*+19A/G) deducted from sequence comparison between the HBA2 and HBA1 reference sequences (NG_000006.1; see also Supplementary data)
Importantly, however, the ethnic background of the family or the individual where the mutation is found cannot be always indicative for the likely mechanism that resulted in the mutation in question. For example, the plethora of Hb Winnipeg observed in the French population may indicate recurrent gene conversion events. However, in the cases of Hb Gerland and Hb Stanleyville-II, although the variants have been reported in two families from France and Southeast Asia and in Caucasian and Black families, respectively, recurrent mutations and (recurrent) gene conversion events are equally likely mechanisms to have generated the mutations in the HBA2 and HBA1 genes, and thorough haplotype analysis is required to favor one mechanism over the other.
Apart from gene conversion events, recurrent unequal crossovers cannot be completely ruled out as a likely cause of these Hb variants. Unequal crossovers have been previously shown to be frequent in the human α-globin gene family [17, 18]. In particular, two successive unequal crossover events, i.e., from a normal (αα) to a rearranged [e.g., deletional (α-) or triplicated (ααα)] chromosome and back, will be undistinguishable from a gene conversion event and have the same consequence, e.g., transfer of a DNA segment from one α-globin gene to another. The rearranged (intermediate) chromosome bearing the variant nucleotide can either persist in the population and, therefore, be found, as in the case of Hb Frankfurt [5] or can subsequently be lost.
In general, gene conversion events can be identified from neighboring to the mutation paralogous sequence variants (PSVs) that are also transferred along with the mutation. Therefore, PSVs are valuable, as they provide insights regarding the length of the gene conversion tract. The Cretan type of non-deletional hereditary persistence of fetal hemoglobin stands as a representative example [19, 20]. HBA2 and HBA1 genes have several PSVs outside the 1,436-bp α-globin gene converted region, spanning from positions −868 to +568 relative to the genes’ transcriptional initiation site [4], but very few PSVs within this DNA segment (depicted as “^” in Fig. 3; see also Supplementary data). Therefore, it is very difficult to unambiguously distinguish these recombinational events from recurrent mutations. At present, there is only one well-documented mutagenic gene conversion event in the human α-globin gene locus (Hb I; p.K16E) identified in both HBA1 and HBA2 genes in cis [6]. This resembles Hb F-Charlotte [HBG1:p.I75T and p.A136E; 21], contrary to Hb F-Waynesboro and Hb F-Lesvos [22], which resulted from the same mutation but in the HBG2 gene (HBG2:p.I75T). Also, two variant α-globin gene alleles consisting of a small DNA segment from the other α-globin gene paralogue (α212 and α121) are most likely the result of a single crossover between a normal and a recombinant allele, although a non-reciprocal gene conversion event cannot be completely ruled out [23]. In all cases, extended haplotype analysis around these variants is an absolute requirement, although this is beyond the scope of the present study.
From a formal genetic point of view, despite the fact that these mutations lead to an identical globin chain, they must be considered as different mutational events, since the nucleotide change occurs in another gene. These situations often cause ambiguities when depositing relevant data in locus-specific databases, like HbVar [1]. The same situation is also reported for two mutations leading to α-thalassemia, namely the c.95+5G>A mutation, found on both α-globin genes and the c.1A>G (initiation codon mutation), found on both HBA2 and HBA1 genes and in a hybrid α-globin gene in the context of α-3.7 thalassemia chromosome. In addition, apart from the Hb variants reported herein, there are also Hb variants for which the amino acid change is identical, but the nucleotide change is different. Such Hb variants are clearly the products of independent mutational events, namely Hb G-Philadelphia (p.N68K; HBA2:c.207C>A or HBA1:c.207C>G), Hb J-Broussais (p.K90N; HBA2:c.273G>T or HBA1:c.273G>C), and Hb Manitoba (p.S102R; HBA2:c.307A>C or HBA2:c.309C>A or HBA1:c.309C>A; also documented as Hb Manitoba I, III, and II, respectively). Therefore, we propose the use of an appropriate suffix in the corresponding variant’s name as a solution to this issue. We propose the use of the [A1] suffix for Hb variants due to HBA1 gene and [A2] suffix for HBA2 gene mutations, e.g., Hb Winnipeg [A1] and Hb Winnipeg [A2], referring to the HBA1:c.226G>C and HBA2:c.226G>C mutations, respectively. This proposed nomenclature is different than the one used for δ-globin chain variants, e.g., Hb A2-Yialousa, to avoid confusion.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fast alignment of DNA sequences HBA1 and HBA2 (DOC 33 KB)
Acknowledgments
This work has been the outcome of a collaborative project among FP6 eInfrastructure for Thalassaemia Research Network (ITHANET, RI-2004-026539; http://www.ithanet.eu) and external partners, proposed and coordinated by Erasmus MC, and supported by ITHANET. We are indebted to Dr. Giovanni Ivaldi, Mrs. Sofia Parastratidou, Mr. Jean Riou, Mrs. Eleni Ranou, Miss Emily Lamb, Mrs. Janice McMcarthy, and Mrs. Athina Ritsiou for expert technical assistance.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s00277-008-0624-3) contains supplementary material, which is available to authorized users.
References
- 1.Hardison RC, Chui DHK, Giardine B, Reimer C, Patrinos GP, Anagnou N et al (2002) Hb Var: A relational database of human hemoglobin variants and thalassemia mutations at the globin gene server. Hum Mutat 19:225–233 doi:10.1002/humu.10044 [DOI] [PubMed]
- 2.Patrinos GP, Wajcman H (2004) Recording human globin gene variation. Hemoglobin 28:v–vii [PubMed]
- 3.Patrinos GP, Giardine B, Riemer C, Miller W, Chui DH, Anagnou NP et al (2004) Improvements in the HbVar human hemoglobin variants and thalassemia mutations for population and sequence variation studies. Nucleic Acids Res 32:D537–D541 doi:10.1093/nar/gkh006 [DOI] [PMC free article] [PubMed]
- 4.Michelson AM, Orkin SH (1983) Boundaries of gene conversion within the duplicated human alpha-globin genes. Concerted evolution by segmental recombination. J Biol Chem 258:15245–15254 [PubMed]
- 5.Préhu C, Francina A, Behnken LJ, Prome D, Galacteros F, Wajcman H (2003) An identical mutation carried by different genes: Hb Frankfurt. Haematologica 88:ECR19 alpha50(CE8)His->Gln [PubMed]
- 6.Liebhaber SA, Rappaport EF, Cash FE, Ballas SK, Schwartz E, Surrey S (1984) Hemoglobin I mutation encoded at both alpha-globin loci on the same chromosome: concerted evolution in the human genome. Science 226:1449–1451 doi:10.1126/science.6505702 [DOI] [PubMed]
- 7.Giardine B, Riemer C, Hefferon T, Thomas D, Hsu F, Zielenski J et al (2007) PhenCode: connecting ENCODE data with mutations and phenotype. Hum Mutat 28:554–562 doi:10.1002/humu.20484 [DOI] [PubMed]
- 8.Giardine B, van Baal S, Kaimakis P, Riemer C, Miller W, Samara M et al (2007) HbVar database of human hemoglobin variants and thalassemia mutations: 2007 update. Hum Mutat 28:206 doi:10.1002/humu.9479 [DOI] [PubMed]
- 9.Wajcman H, Préhu C, Bardakdjian-Michau J, Promé D, Riou J, Godart C et al (2001) Abnormal hemoglobins: the laboratory methods. Hemoglobin 25:169–181 doi:10.1081/HEM-100104026 [DOI] [PubMed]
- 10.Lorey F, Charoenkwan P, Witkowska HE, Lafferty J, Patterson M, Eng B et al (2001) Hb H hydrops foetalis syndrome: a case report and review of literature. Br J Haematol 115:72–78 doi:10.1046/j.1365-2141.2001.03080.x [DOI] [PubMed]
- 11.Tan AC, Quah TC, Low PS, Chong SS (2001) A rapid and reliable 7-deletion multiplex polymerase chain reaction assay for alpha-thalassemia. Blood 98:250–251 doi:10.1182/blood.V98.1.250 [DOI] [PubMed]
- 12.Van Ros G, Beale D, Lehmann H (1968) Haemoglobin Stanleyville II (alpha asparagine replaced by lysine). BMJ 4:92–93 [DOI] [PMC free article] [PubMed]
- 13.Liebhaber SA, Cash FE, Ballas SK (1986) Human alpha-globin gene expression. The dominant role of the alpha 2-locus in mRNA and protein synthesis. J Biol Chem 261:15327–15333 [PubMed]
- 14.Molchanova TP, Pobedimskaya DD, Huisman TH (1994) The differences in quantities of alpha 2- and alpha 1-globin gene variants in heterozygotes. Br J Haematol 88:300–306 doi:10.1111/j.1365-2141.1994.tb05022.x [DOI] [PubMed]
- 15.Papadakis MN, Patrinos GP (1999) Contribution of gene conversion in the evolution of the human beta-like globin gene family. Hum Genet 104:117–125 doi:10.1007/s004390050923 [DOI] [PubMed]
- 16.Chen JM, Cooper DN, Chuzhanova N, Férec C, Patrinos GP (2007) Gene conversion: mechanisms, evolution and human disease. Nat Rev Genet 8:762–775 doi:10.1038/nrg2193 [DOI] [PubMed]
- 17.Lam KW, Jeffreys AJ (2006) Processes of copy-number change in human DNA: the dynamics of {alpha}-globin gene deletion. Proc Natl Acad Sci U S A 103:8921–8927 doi:10.1073/pnas.0602690103 [DOI] [PMC free article] [PubMed]
- 18.Lam KW, Jeffreys AJ (2007) Processes of de novo duplication of human alpha-globin genes. Proc Natl Acad Sci U S A 104:10950–10955 doi:10.1073/pnas.0703856104 [DOI] [PMC free article] [PubMed]
- 19.Patrinos GP, Kollia P, Loutradi-Anagnostou A, Loukopoulos D, Papadakis MN (1998) The Cretan type of non-deletional hereditary persistence of fetal hemoglobin [A gamma-158C>T] results from two independent gene conversion events. Hum Genet 102:629–634 doi:10.1007/s004390050753 [DOI] [PubMed]
- 20.Kalamaras A, Chassanidis C, Samara MBB, Chiotoglou I, Vamvakopoulos NK, Papadakis MN et al (2008) The 5′ regulatory region of the human fetal globin genes is a gene conversion hot spot. Hemoglobin (in press) [DOI] [PubMed]
- 21.Gu LH, Oner C, Huisman TH (1995) The G gamma T chain (G gamma 75 Thr; 136 Gly) in Hb F-Charlotte is the product of an A gamma gene with a limited gene conversion and that in Hb F-Waynesboro of a mutated G gamma gene. Hemoglobin 19:413–418 doi:10.3109/03630269509005834 [DOI] [PubMed]
- 22.Papadakis MN, Patrinos GP, Drakoulakou O, Loutradi-Anagnostou A (1996) HbF-Lesvos: an HbF variant due to a novel G gamma mutation (:G gamma 75 ATA->ACA) detected in a Greek family. Hum Genet 97:260–262 doi:10.1007/BF02265278 [DOI] [PubMed]
- 23.Law HY, Luo HY, Wang W, Ho JF, Najmabadi H, Ng IS et al (2006) Determining the cause of patchwork HBA1 and HBA2 genes: recurrent gene conversion or crossing over fixation events. Haematologica 91:297–302 [PubMed]
- 24.Troxler H, Neuheiser F, Kleinert P, Kuster T, Heizmann CW, Sack R et al (2002) Detection of a novel variant human hemoglobin by electrospray ionization mass spectrometry. Biochem Biophys Res Commun 292:1044–1047 doi:10.1006/bbrc.2002.6762 [DOI] [PubMed]
- 25.Zhao W, Wilson JB, Webber BB, Kutlar A, Tamagnini GP, Kuam B et al (1990) Hb Hekinan observed in three Chinese from Macau; identification of the GAG-GAT mutation in the alpha 1-globin gene. Hemoglobin 14:627–635 doi:10.3109/03630269009046971 [DOI] [PubMed]
- 26.Shih HC, Shih MC, Chang YC, Peng CT, Chang TJ, Chang JG (2007) Hb Hekinan in a Taiwanese subject: A G>T substitution at codon 27 of the alpha1-globin gene abolishes an HaeIII site. Hemoglobin 31:495–498 doi:10.1080/03630260701590368 [DOI] [PubMed]
- 27.Merault G, Keclard L, Desfontaines L, Saint-Martin C, Blouquit Y, Rosa J et al (1989) Hemoglobin Hekinan [alpha (2)27(B8)Glu—Asp beta 2] detected in Guyana. Hemoglobin 13:397–402 doi:10.3109/03630268909003402 [DOI] [PubMed]
- 28.Liebhaber SA, Kan YW (1981) Differentiation of the mRNA transcripts originating from the alpha 1- and alpha 2-globin loci in normals and alpha-thalassemics. J Clin Invest 68:439–446 doi:10.1172/JCI110273 [DOI] [PMC free article] [PubMed]
- 29.Lacan P, Aubry M, Couprie N, Francina A (2001) Hb Gerland [alpha55(E4)Val->Ala (alpha2)]: a new neutral alpha chain variant involving the alpha2 gene. Hemoglobin 25:417–420 doi:10.1081/HEM-100107879 [DOI] [PubMed]
- 30.Moradkhani K, Riou J, Francina A, Wajcman H, Prehu C (2008) Hb Gerland [55(E4)Val>Ala] mutation found in the alpha1 gene. Hemoglobin (in press) [DOI] [PubMed]
- 31.Markley KM, Elkhalifa M, Maini A, Hoyer JD (2008) Hemoglobin Jeddah [α68 (E17) Asn>His (alpha1)]: a newly recognized alpha chain variant seen in combination with hemoglobin S and found in three separate families of Middle Eastern origin. Hemoglobin 32:297–302 doi:10.1080/03630260701758908 [DOI] [PubMed]
- 32.Nakatsuji T, Abraham BL, Lam H, Wilson JB, Huisman TH (1983) Hb Winnipeg or alpha 2 75(EF4)Asp leads to Tyr beta 2 in a large Caucasian family living in Georgia, USA. Hemoglobin 7:105–110 doi:10.3109/03630268309038407 [DOI] [PubMed]
- 33.Badens C, Léna-Russo D, Lacan P, Francina A, Promé D, Riou J et al (1999) Hb Toulon [alpha77(EF6)Pro>His]: a new variant due to a mutation in the alpha2 gene found during measurement of glycated hemoglobin. Hemoglobin 23:367–371 [DOI] [PubMed]
- 34.Waye JS, Eng B, Chui DHK, Powers PJ, Lafferty JD (2000) Second report of Hb Toulon [alpha77(EF6)Pro>His] in a Canadian family of Italian descent. Hemoglobin 24:359–360 [DOI] [PubMed]
- 35.Caruso D, Da Riva L, Giavarini F, Galli G, Brambilla S, Luraschi P et al (2002) A hemoglobin variant found during glycohemoglobin measurement, identified as Hb Toulon [alpha77(EF6)Pro->His] by tandem mass spectrometry. Hemoglobin 26:197–199 doi:10.1081/HEM-120005460 [DOI] [PubMed]
- 36.North ML, Darbre PD, Lehmann H, Juif JG (1975) Haemoglobin Stanleyville II (alpha75 [EF 7] Asn yields Lys) found in France. Acta Haematol 53:56–59 [DOI] [PubMed]
- 37.Luo HY, Irving I, Prior J, Lim E, Eung SH, Skelton TP et al (2004) Hemoglobin Titusville, a low oxygen affinity variant hemoglobin, in a family of Northern European background. Am J Hematol 77:384–386 doi:10.1002/ajh.20209 [DOI] [PubMed]
- 38.Deyell R, Jackson S, Spier S, Le D, Poon MC (2006) Low oxygen saturation by pulse oximetry may be associated with a low oxygen affinity hemoglobin variant, hemoglobin Titusville. J Pediatr Hematol Oncol 28:100–102 doi:10.1097/01.mph.0000200685.33291.0a [DOI] [PubMed]
- 39.Fucharoen S, Changtrakun Y, Ratanasiri T, Fucharoen G, Sanchaisuriya K (2003) Complex interaction of Hb Hekinan [alpha27(B8) Glu-Asp] and Hb E [beta26(B8) Glu-Lys] with a deletional alpha-thalassemia 1 in a Thai family. Eur J Haematol 70:304–309 doi:10.1034/j.1600-0609.2003.00049.x [DOI] [PubMed]
- 40.Ngiwsara L, Srisomsap C, Winichagoon P, Fucharoen S, Svasti J (2004) Two cases of compound heterozygosity for Hb Hekinan [alpha27(B8)Glu->Asp (alpha1)] and alpha-thalassemia in Thailand. Hemoglobin 28:145–150 doi:10.1081/HEM-120035913 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
Fast alignment of DNA sequences HBA1 and HBA2 (DOC 33 KB)