Abstract
Using data for 555,038 pregnancies conceived in the Czech Republic in 1987-1990, we show that pronounced differences in fetal survival in the middle trimester of pregnancy by marital status, educational level, and labor force attachment become much smaller at full term; survival differences by age at conception and number of previous deliveries show relatively constant proportional hazards throughout gestation. Social inequalities in postpartum life chances have been documented previously, but we show that similar inequalities exist before birth.
Patterns of social inequality in infant survival after live birth in Western populations are well known. Small differences in relative risks of death in the neonatal period increase as infancy progresses (Cramer 1987; Durant 1994; Eberstein and Parker 1984; Gardos and Rychtarikova 1996; Hogue and Hargraves 1993; Rogers 1989). Such widening survival disparities usually are seen as effects of differences in living conditions that accumulate with age. By contrast, we know little about prenatal loss patterns.
Existing research on fetal loss chiefly examines the absolute magnitude and shape of the risk function during gestation (Abramson 1973; Bakketeig, Seigel, and Sternthal 1978; Hilden et al. 1991; James 1970; Modvig, Schmidt, and Damsgaard 1990; Rychtarikova 1981a, 1981b; Shapiro, Jones, and Denson 1962; Susser 1983; Susser and Kline 1986; Wilcox et al. 1988). Clinical research suggests that the true risk of spontaneous loss accelerates exponentially from birth back through pregnancy to conception (Wilcox et al. 1985). In contrast to the literature on infant mortality, research on the risks of fetal loss has devoted little attention to differences across social categories (Figa-Talamanca and Repetto 1988; Freedman, Coombs, and Friedman 1966; Kiely 1991; Mittendorf et al. 1993). We map such differences using unique data from the Czech Republic.
ADVANTAGES OF CZECH REGISTER DATA
The Institute of Health Information and Statistics of the Czech Ministry of Health furnished individual machinereadable records (Syrovatka and Rychtarikova 1990) for all live births, stillbirths, and induced and spontaneous abortions registered in the Czech Republic during calendar years 1986 through 1992. We exclude 1986 because abortion records from that year used a format not consistent with later years, making them unusable on important items. Problems with coding labor force attachment on the 1992 records also resulted in the deletion of that year from study. To combine abortion and birth records in one analysis, we include cases only if conception can be inferred (based on the date of the event and weeks of gestation) to have occurred in calendar years 1987 through 1990 inclusive. Because some conceptions in 1990 resulted in births occurring in 1991 and in a smaller number of spontaneous losses in 1991, the study is based on data for the period 1987-1991 concerning conceptions inferred to the period 1987-1990. To avoid statistical effects peculiar to extreme categories, we eliminate records for women who were aged 40 or older at conception or who had more than three previous deliveries. (Four percent of births and 3% of abortions were to women with more than three previous deliveries.) A few cases with ineligible reported combinations of values for mother's age, marital status, and education also are excluded.
Coverage and Completeness of Reporting
Many early spontaneous abortions occur before women are in contact with doctors and hospitals, so the event often goes unreported. Many more are not even recognized by the woman. Reported spontaneous abortions thus increasingly give a biased sample of all such events, the earlier the gestational duration (Wilcox et al. 1985, 1988). This produces the well-known “backward-bending” curve of apparent risk generated by registration data for the first trimester (Abramson 1973; Modvig et al. 1990; Shapiro, Jones, and Denson 1962). Therefore we analyze fetal loss starting with the 10th week after the onset of the last normal menses.
Even coverage back to the 10th week is rarely achieved. Kristensen and Mac (1992) used late fetal deaths (28 completed weeks of gestation) from the Danish Medical Birth Register, but restricted their study to cases of 31 or more completed weeks of gestation. The U.S. National Fetal Mortality Survey of 1980 contains similar information, but extends back only to the 20th week of pregnancy. Bakketeig et al. (1978) constructed a life table from Norway's register of births, extending from Week 16 of gestation to the end of the first year of life. Their data contained 5,845 fetal losses and 5,144 infant deaths following a live birth.
By comparison, records considered here contain 7,027 fetal losses and 4,655 infant deaths if we consider only events after 16 weeks of gestation. Weeks 10 to 16 in the Czech data include an additional 19,480 fetal deaths. Clearly the Czech data present a more comprehensive picture of fetal loss, even though omitting the first nine weeks eliminates nearly half of all available records (mostly early induced abortions), including all records with unknown gestational age. We assume that all cases with unknown gestational age occurred before the beginning of the 10th week.
Induced Versus Spontaneous Abortions
In some settings (e.g., in the Norwegian birth register and in the U.S. Fetal Mortality Survey), induced and spontaneous abortions may be recorded in separate and incompatible data bases. Absence of induced abortions makes it impossible to calculate exposures correctly because exposure contributed before an induced abortion is not included (Hilden et al. 1991; Susser 1983; Susser and Kline 1986).
In the Czech abortion data, gestational age at termination was recorded for both spontaneous and induced abortions, dated from the onset of the last normal menses. The same certificate was used for both types of events. Type of abortion was recorded on the certificate at the same time and in the same medical site as for other information. Virtually all pregnancy terminations took place in hospitals in the Czech Republic. Because all physicians were state employees who worked in hospitals, they could not maintain private practices in other locations.
Misclassification of induced abortions as spontaneous has proved a serious defect in abortion records in other settings, but Czech society at the end of the 1980s was essentially free of conditions that would lead aborting women or medical staff to misreport induced abortions as spontaneous. There was no legal stigma attached to induced abortion. There were no financial costs to women who had an induced abortion for medical reasons. Induced abortions other than for medical indications cost about the equivalent of a day's wages for a typical worker. The reporting system was embedded in an established universal health coverage, which included free access to prenatal care. Such care was eagerly and widely sought by virtually all expectant women (CDC/WHO 1995). In fact, a special benefit paid to mothers upon delivery was calculated on the basis of the number of prenatal visits, so pregnant women had a strong incentive to enter the care/registration system as early as possible. Misrepresentation by physicians or birth attendants of an induced abortion as spontaneous, if discovered, would have entailed serious risk for the attendant; it also would entail a financial penalty because no fee could be charged for a spontaneous abortion. For all these reasons, we dismiss possible misreporting of induced abortions as spontaneous.
Spontaneous Abortions, Stillbirths, and Live Births
Evidence from California (Goldhaber 1989) documents a boundary problem for defining spontaneous abortions versus stillbirths. In that study, late fetal deaths at this boundary of gestational age count more often as stillbirths when they are heavier or larger and as spontaneous abortions when they are severely growth-retarded (small for gestational age), which may introduce biases when stillbirth and spontaneous abortion are studied as separate events. We combine stillbirths with spontaneous abortions in our definition of fetal loss, so this distinction does not affect our analysis.
The definition of a live birth in the Czech data conforms to accepted international definitions based on presence of any one of the four recognized signs of life (heartbeat, respiration, muscle movement, or pulsation of the umbilical cord). After 1988 this definition was modified to exclude births weighing less than 500 grams, but no such case was recorded before 1988.
Multiple-fetus pregnancies have loss patterns that differ sharply from those of singletons (Cooperstock et al. 1998). Therefore we exclude multiple births from our study rather than count each pregnancy or birth as a separate event.
Coding of Covariates
Because we combine birth and abortion records into a unified data set, we can use only covariates that appear in both types of records. This limits attention to demographic characteristics of women only, such as the pregnant woman's age at conception and number of previous deliveries. In addition, we include her marital status at parturition, her educational level, and an indicator of her labor force attachment. Only the birth record gives the date of marriage, so we cannot infer marital status at conception for abortions. We distinguish between single, married, and divorced women and omit records for widows (who are very few). Women are grouped by educational level into those with basic education, vocational secondary-level training, or gymnasium and higher. In our tables and figures, we call the third of these levels higher education. For more information about our variable for educational levels, see Carlson and Hoem (1999).
Women's occupations appear in both birth and abortion records, though in some cases a woman was recorded as being without occupation. We do not find important differences or clear patterns in risks of fetal loss by occupation, net of the effect of other variables, except that women without any occupation consistently have higher risks than all others. Therefore we take the presence or absence of an occupation as a binary indicator of labor force attachment. Such information is not available in U.S. records.
PATTERNS IN CRUDE RATES OF FETAL LOSS
Starting with the 10th week of gestation, all cases of pregnancy termination in a given week contribute half a week, and continuing pregnancies a full week, of exposure in that week. Both spontaneous abortions and stillbirths count as fetal losses (26,507 cases). Pregnancies ending in induced abortion in Week 10 or later (27,839 cases) and pregnancies ending in a live birth (500,692) are right-censored from the point of the event. Results shown for multiweek intervals are aggregated from single-week counts of occurrences and calculations of exposure to risk. Fetal losses per 1,000 person-weeks in utero measure risk for each time interval and each category of each variable.
Baseline Hazard by Gestational Age
The unadjusted or crude (occurrence/exposure) rate of fetal loss by gestational age (Table 1, panel 1, column 4) shows strong agreement with the pattern observed in previous studies from Norway (Bakketeig et al. 1978) and Kaiser-Permanente cohorts (Goldhaber and Fireman 1991). This provides further evidence of the reliability of our data.
TABLE 1.
CRUDE, RELATIVE, AND ADJUSTED RATES OF FETAL LOSS
| Pregnancies at Risk (1) | Person-Weeks of Exposurea (2) | Fetal Lossesb (3) | Crude Ratec (4) | Relative Rated (5) | Adjusted Ratee (6) | |
|---|---|---|---|---|---|---|
| Weeks Since Last Normal Menstrual Periodf | ||||||
| 10-12 | 555,038 | 1,595,374.5 | 13,731 | 8.61 | 10.81 | 9.56 |
| 13-16 | 515,055 | 2,046,031.0 | 5,749 | 2.81 | 3.53 | 3.30 |
| 17-20 | 508,617 | 2,029,808.0 | 2,191 | 1.08 | 1.36 | 1.28 |
| 21-24 | 506,033 | 2,019,511.5 | 1,935 | 0.96 | 1.20 | 1.14 |
| 25-28 | 503,690 | 2,010,271.0 | 1,139 | 0.57 | 0.71 | 0.68 |
| 29-32 | 501,380 | 1,998,560.5 | 329 | 0.16 | 0.21 | 0.20 |
| 33-36 | 497,275 | 1,965,057.5 | 556 | 0.28 | 0.36 | 0.34 |
| 37-38 | 479,500 | 914,034.0 | 339 | 0.37 | 0.47 | 0.46 |
| 39-41 | 422,912 | 675,804.0 | 538 | 0.80 | 1.00 | 1.00 |
| Age at Conception | ||||||
| 15-17 | 23,825 | 637,500.0 | 963 | 1.51 | 1.01 | 0.27 |
| 18-19 | 87,552 | 2,464,764.0 | 3,451 | 1.40 | 0.94 | 0.74 |
| 20-24 | 246,980 | 6,939,329.5 | 10,346 | 1.49 | 1.00 | 1.00 |
| 25-29 | 128,169 | 3,518,432.5 | 6,564 | 1.87 | 1.25 | 1.40 |
| 30-34 | 52,252 | 1,336,637.5 | 3,624 | 2.71 | 1.82 | 1.94 |
| 35-39 | 16,260 | 357,788.5 | 1,559 | 4.36 | 2.92 | 2.76 |
| Previous Births | ||||||
| None | 263,933 | 7,459,409.5 | 11,671 | 1.56 | 1.00 | 1.00 |
| One | 205,356 | 5,747,349.0 | 9,304 | 1.62 | 1.03 | 0.94 |
| Two | 67,863 | 1,658,435.5 | 4,188 | 2.53 | 1.61 | 0.94 |
| Three | 17,886 | 389,258.0 | 1,344 | 3.45 | 2.21 | 0.82 |
| Marital Status | ||||||
| Single | 42,426 | 899,725.5 | 5,281 | 5.87 | 4.11 | 3.16 |
| Married | 499,150 | 14,055,148.5 | 200,88 | 1.43 | 1.00 | 1.00 |
| Divorced | 13,462 | 299,578.0 | 1,138 | 3.80 | 2.66 | 1.51 |
| Educational Level | ||||||
| Basic | 86,480 | 2,086,025.0 | 7,421 | 3.56 | 2.46 | 1.32 |
| Vocational | 211,271 | 5,906,232.0 | 8,541 | 1.45 | 1.00 | 1.00 |
| Higher education | 257,287 | 7,262,195.0 | 10,545 | 1.45 | 1.00 | 0.89 |
| Labor Force Attachment | ||||||
| No occupation | 52,996 | 1,083,081.5 | 7,314 | 6.75 | 4.99 | 4.08 |
| With occupation | 502,042 | 14,171,370.5 | 19,193 | 1.35 | 1.00 | 1.00 |
A pregnancy contributes half a week of risk in a week when it ends, or a full week if it continues intact.
Includes spontaneous abortions and stillbirths combined.
Fetal losses per thousand person-weeks in utero for each category, unadjusted for other factors.
Cmde rate for each category divided by crude rate for reference category of each predictor.
Relative rates adjusted by multiple indirect standardization with no interactions (proportional hazards).
Pregnancies surviving to the beginning of each gestational interval reflect attrition by induced abortion and live birth as well as by spontaneous fetal losses shown here.
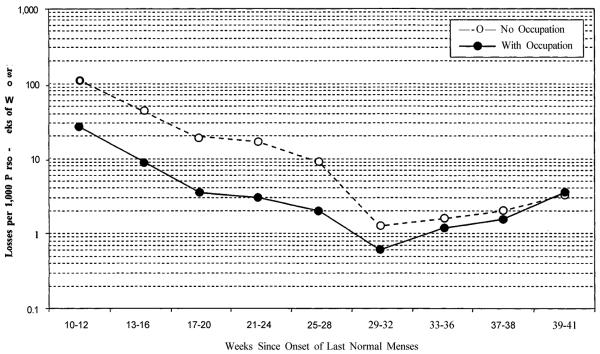
After the 10th week following the last normal menstrual period, spontaneous loss rates drop rapidly during the second trimester (Figure 1). An added sharp drop late in the second trimester occurs because high-risk fetuses reach a developmental stage that results in extremely premature live birth followed by almost certain infant death (Davies et al. 1992; Fejgin et al. 1992; Romero 1987) instead of in fetal death as at earlier gestational ages (Ekwo et al. 1992; Witkin and Ledger 1992). Premature live birth removes high-risk cases from the fetal population before they die (Feldman 1992; Gravett et al. 1986; Morgan et al. 1994). As a result, we observe lower risks for the remaining population in utero. We are now investigating the trend in risk of involuntary reproductive losses before and after parturition combined, with time since conception as a unifying dimension.
FIGURE 1.
ABSOLUTE RISKS OF FETAL LOSS BY GESTATIONAL AGE AND WOMAN'S LABOR FORCE ATTACHMENTa
aStandardized for woman's age, birth order, and marital status.
As full term approaches, the typical live-born infant gradually shifts from a high-risk pregnancy (Lieberman et al. 1987; Savitz, Blackmore, and Thorp 1991) to a more normal profile (Mittendorf et al. 1993), the risk-selective effect of live birth fades, and fetal mortality rises again, producing a reversed J-shaped pattern. At 39 to 41 weeks after the last normal menstrual period (the mode for live-birth events), the unadjusted rate is similar to the loss rate observed at the end of the second trimester.
After full term, the fetal population remaining in utero shrinks to a small group of postterm pregnancies with atypically high risk factors (Divon et al. 1998). This postterm period is not considered here because too few pregnancies went beyond the 41st week of gestation to support our detailed statistical analysis.
Biodemographic and Social Factors
Table 1 (column 4) also shows crude or unadjusted rates of fetal loss for the covariates we consider. Crude rates divided by the corresponding rate for the reference category of each variable yield relative rates (column 5). We see a familiar J-shaped pattern of unadjusted rates of fetal loss for increasing ages of women at conception in panel 2 of Table 1 (Santow and Bracher 1989). The unadjusted rates increase with increasing parity (panel 3). Married women have much lower unadjusted rates than do unmarried women (panel 4). There is little difference in rates of fetal loss between Czech women with a vocational education and those with a higher education, but ending school early constitutes a clear risk factor (panel 5).
In the Czech Republic, with great stress on full employment and with payments for maternity leave based on one's regular wages or salary, only the most unhealthy or unlucky women got pregnant before establishing an occupational history. This special group had much higher rates of fetal loss than women with a recorded occupation (last panel in Table 1).
MAIN EFFECTS FOR STANDARDIZED RATES
In addition to computing unadjusted occurrence/exposure rates for covariates and gestational intervals, we use hazard (or intensity) regression to produce estimates of the risk of fetal loss for each category of a covariate relative to the corresponding risk for a selected reference category, adjusted for all other covariates. Gestational age constitutes the time variable of the analysis. This well-known type of event-history analysis is an improved and multivariate version of the indirect standardization technique often used by demographers and epidemiologists (Hoem 1987, 1993).
Changes Revealed by Standardization
The main effects of this proportional-hazards model appear in the last column of Table 1. Marital status, educational level, and labor force attachment each show smaller contrasts after adjustment. Each variable considered separately also picks up effects of the others. The increase in rates of fetal loss with increasing parity actually reverses to a small decrease. Even more important, the J-shape of the age profile becomes a nearly linear increase with increasing age. Women with higher-order deliveries and those at the youngest ages seem to have high rates of fetal loss only because they include more than their share of unmarried, unemployed, and poorly educated women. Geronimus (1987) argued that high risks of infant death (i.e., after birth) for teenage women in the United States represent a concentration of disadvantaged women among those bearing children as teenagers, and not an intrinsic age effect. The same argument seems to apply to fetal mortality in the Czech Republic.
Confirmation of Low Risk at Young Ages
The nearly linear increase in adjusted rates of fetal loss with increasing age at conception includes extremely low rates of loss for women aged 15 to 17. These rates are so low, in fact, that one may suspect them of being an artifact produced by the multivariate indirect standardization instead of reflecting actual conditions of risk among the youngest women. If the underlying model projected patterns of relative risk among older age groups into these youngest ages, where distributions of the social variables are different, where cases are few, and where the influence on model coefficients is consequently weak, very different real patterns of risk at the youngest ages might be distorted and concealed.
To eliminate this possibility, we return in Table 2 to un-adjusted crude rates for a detailed cross-classification of young women with no previous deliveries. No women in the youngest age group (15-17) were divorced or had completed higher education, so we do not include those categories in the analysis.
TABLE 2.
CRUDE RATESa OF FETAL LOSS FOR YOUNG NULLIPAROUS WOMEN, BY SOCIAL CATEGORIES
| Ages at Conception |
|||
|---|---|---|---|
| 15-17 | 18-19 | 20-24 | |
| Single Women | |||
| With occupation | |||
| Basic education | 1.819 | 3.226 | 3.302 |
| Vocational education | 1.738 | 2.916 | 2.583 |
| No occupation | |||
| Basic education | 1.764 | 3.630 | 4.385 |
| Vocational education | 4.091 | 9.219 | 8.423 |
| Married Women | |||
| With occupation | |||
| Basic education | 0.241 | 0.919 | 1.472 |
| Vocational education | 0.148 | 0.262 | 0.430 |
| No occupation | |||
| Basic education | 0.261 | 1.990 | 4.459 |
| Vocational education | 0.484 | 3.270 | 5.927 |
Fetal losses per 1,000 person-weeks in utero for women with no previous deliveries, youngest three age groups only. Divorce, widowhood, and higher academic degrees generally do not apply to the youngest age group (15-17) and so are not included in these comparisons.
Differences between the rows of this table indicate sharp contrasts in the risk of fetal loss for different social groups at each age, in line with the more comprehensive patterns reported in the final column in Table 1. Still, within each row it is clear that the lowest risk actually occurs for the youngest women. For each group of single women, the risk increases sharply between the first and second age group and then remains at the latter level in the age group 20-24. For each group of married women, the (consistently lower) risk increases almost linearly across all three age groups. Any suspicion of gross model distortion is put to rest. Adjustment (indirect standardization) removes the confounding effects of social composition within age groups as intended. The statistical procedures used have worked properly, and they reveal a dramatic truth about underlying patterns of risk.
In addition, we notice particularly high relative risks of fetal loss for single as well as married women with vocational training but no recorded occupation. The lack of firm labor force attachment for a woman at this educational level may signal health problems or some other problematic situation.
RISK TRAJECTORIES DURING GESTATION
Do demographic and social factors shown in Table 1 operate in the same manner throughout gestation? Can the proportional-hazards model intrinsic in computations for the last column in Table 1 withstand scrutiny? Table 3 considers the interaction of gestational age with each of the other covariates in turn. Each panel shows relative risks by gestational age interacted with a chosen covariate. In each case, remaining covariates in complete interaction are entered as a control term, with separate models fitted to produce each panel.
TABLE 3.
TRAJECTORIES FOR PREDICTORS BY GESTATIONAL DURATION: RISK OF FETAL LOSS RELATIVE TO BASELINE CATEGORYa
| Weeks Since Onset of Last Normal Menses |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 10-12 | 13-16 | 17-20 | 21-24 | 25-28 | 29-32 | 33-36 | 37-38 | 39-41 | |
| Age at Conception | |||||||||
| 15-17 | 0.323 | 0.363 | 0.397 | 0.526 | 0.519 | 0.395 | 0.579 | 0.344 | 0.403 |
| 18-19 | 0.766 | 0.736 | 0.770 | 0.840 | 0.915 | 0.664 | 0.705 | 0.749 | 1.052 |
| 20-24 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| 25-29 | 1.325 | 1.290 | 1.495 | 1.401 | 1.088 | 1.416 | 1.214 | 1.199 | 1.324 |
| 30-34 | 1.669 | 1.726 | 2.208 | 1.701 | 1.466 | 1.811 | 1.383 | 1.284 | 1.649 |
| 35-39 | 2.189 | 2.436 | 3.381 | 2.683 | 2.067 | 1.849 | 2.203 | 1.734 | 2.698 |
| Previous Births | |||||||||
| 0 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| 1 | 0.962 | 0.978 | 0.920 | 0.820 | 0.574 | 0.919 | 0.748 | 0.765 | 0.739 |
| 2 | 0.811 | 0.924 | 1.177 | 0.813 | 0.715 | 1.134 | 0.562 | 0.540 | 0.648 |
| 3 | 0.633 | 0.950 | 1.136 | 0.750 | 0.778 | 0.879 | 0.562 | 0.610 | 0.807 |
| Marital Status | |||||||||
| Single | 3.905 | 3.419 | 2.280 | 2.079 | 1.756 | 1.800 | 1.860 | 1.175 | 1.258 |
| Married | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Divorced | 1.394 | 1.766 | 1.769 | 1.698 | 1.058 | 0.720 | 0.988 | 0.600 | 1.004 |
| Educational Level | |||||||||
| Basic | 1.335 | 1.632 | 2.088 | 2.161 | 2.728 | 1.250 | 1.242 | 1.025 | 1.336 |
| Vocational | 1.029 | 1.016 | 1.023 | 1.283 | 1.406 | 1.177 | 1.295 | 1.307 | 1.547 |
| Higher education | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Labor Force Attachment | |||||||||
| No occupation | 4.144 | 4.959 | 5.151 | 5.477 | 4.387 | 1.754 | 1.310 | 1.281 | 0.950 |
| With occupation | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
For each factor, the relative risks have been calculated by multivariate indirect standardization, with the selected covariate in interaction with gestational duration and with the remaining covariates in complete interaction as a control term. (Using only main effects of the remaining covariates produces essentially the same effects.) Each panel represents a separate model.
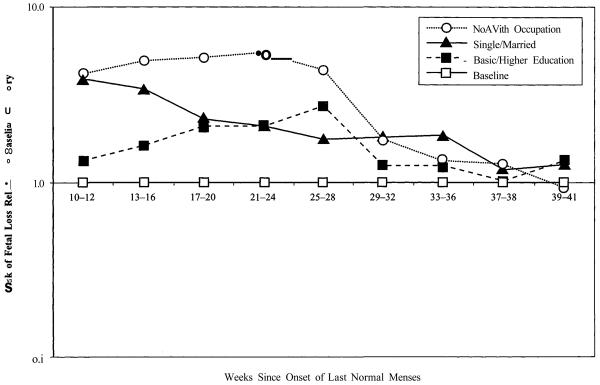
If the proportional-hazards model is appropriate, figures in each row should remain about the same across the table. Instead, two different patterns appear in Table 3. For the demographic factors (age at conception and number of previous births), proportionality exists and the interaction does not reveal important new features. For the social factors (marital status, educational attainment, and labor force attachment), however, high excess risk for disadvantaged groups during the second trimester declines as gestational age increases and is much smaller at full term.
Figure 2 highlights this pattern for the social variables in Table 3. It presents standardized loss-risk trajectories for single women relative to the baseline category of married women, for women with a basic education relative to the baseline category of higher education, and for women with no occupation relative to the baseline category of those with a known occupation. The trajectory of the relative risk for women without a recorded occupation is particularly dramatic: It peaks late in the second trimester at more than five times the corresponding rate for women with a recorded occupation, and then drops to a level only one-third or so above the more advantaged group in the middle of the third trimester.
FIGURE 2.
RISKS OF FETAL LOSS RELATIVE TO BASELINE CATEGORIES FOR LABOR FORCE ATTACHMENT, MARITAL STATUS, AND EDUCATIONa
aRelative risks for each factor are standardized for other factors.
The results in Table 3 and Figure 2 confirm our assessment that misreporting of induced abortions as spontaneous does not confound the results. If such an effect were present, it should affect only the first gestational interval that we study. About 95% of all recorded induced abortions occurred before the 10th week of gestation and thus were never even considered in our analysis. Over 95% of the remaining 27,839 recorded induced abortions occurred in weeks 10 through 12 and actually outnumbered spontaneous losses from the same period of pregnancy; thus, there cannot have been much hesitancy in reporting induced abortion at any gestational age. The wide social differences in spontaneous fetal loss persist throughout the second trimester and are strongly reduced only in the third trimester, at which time induced abortion is no part of the picture.
Other possible reporting problems also seem unlikely to have influenced our results. For incomplete reporting of early spontaneous abortions to produce our findings, there would have to be more complete reporting by the higher-risk women (women who are unmarried, who have only basic education, and who have no recorded occupation), particularly at early gestational ages. There is no plausible reason to expect such a reporting bias, as coverage by the prenatal care system begins early and is virtually universal in the population. If anything, undercoverage is normally suspected for the less advantaged groups. Similarly, if observed differences early in the second trimester were to be attributed to misreporting of gestational age, time elapsed since the last normal menstrual period would have to be systematically overstated by high-risk women relative to low-risk women. Again, there is no reason to expect such a pattern, and even if it existed, it is not apparent why the effect should disappear during the third trimester. That the narrowing of survival differences from the second to the third trimester does not appear for either women's age at conception or number of previous births further suggests that results for marital status, education, and labor force attachment are real effects.
LIVE BIRTH AS A COMPETING RISK
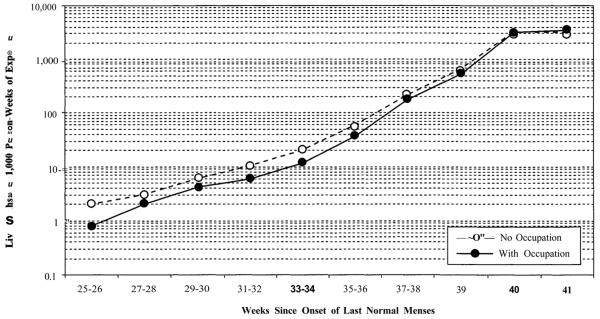
The strong reduction of social inequality in risk of fetal loss during the third trimester has a complex explanation. First, frail fetuses are selected out more strongly for disadvantaged women than for other women, progressively reducing their risk disadvantage. This “intensity” effect is partly a result of fetal mortality, but also may be related to differences in rates of premature live births, which remove cases from the fetal population in significant numbers from gestational Week 26 onward. Risks of fetal loss in the third trimester are complicated by the appearance of this competing risk. Rates of live birth increase during the third trimester, as shown in Figure 3 for categories of labor force attachment.
FIGURE 3.
ABSOLUTE RISKS OF LIVE BIRTH BY GESTATIONAL AGE AND WOMAN'S LABOR FORCE ATTACHMENTa
aStandardized for woman's age, birth order, and marital status.
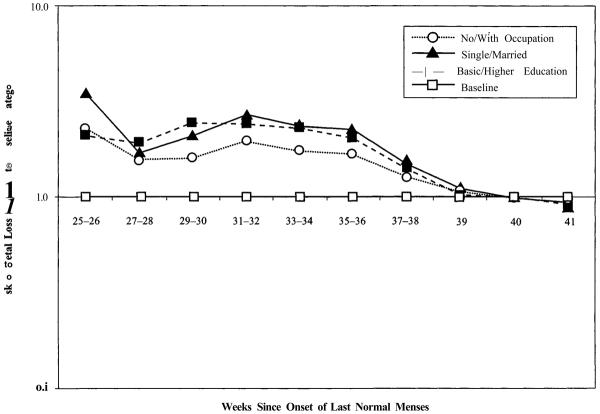
As already noted, the earliest premature live births reduce fetal mortality around the start of the third trimester, as the threshold of viability is reached, because high-risk pregnancies terminate in live birth followed by almost immediate and certain infant death rather than in fetal death. Figure 4 shows that there are clear social gradients in the risk of premature live birth at the beginning of the third trimester. The same categories of social disadvantage associated with higher fetal mortality (unmarried, poorly educated, without occupation) are also associated with higher rates of premature live birth. These live-birth risk differences disappear at full term, however, just as risks of fetal loss converged in Figure 2.
FIGURE 4.
RISKS OF LIVE BIRTH RELATIVE TO BASELINE CATEGORIES FOR LABOR FORCE ATTACHMENT, MARITAL STATUS, AND EDUCATIONa
aRelative risks for each factor are standardized for other factors.
Second, at each stage in the third trimester, high-risk pregnancies of women who are less advantaged result in a live birth more readily, whereas women who are more advantaged retain such pregnancies longer and have a live birth at a later stage of fetal development. This “timing” effect could disproportionately increase the apparent later risk of fetal loss among women who are more advantaged and thus reduce loss-risk differences at higher gestational ages. The effect would also fit with the observed mortality advantage of premature births to disadvantaged women: A premature live birth would be a stronger distress signal for a woman in an advantaged group (see Carlson and Hoem 1999).
CONTINUITIES IN PRENATAL AND POSTNATAL LOSS
The usual and obvious explanation of differential trajectories of infant mortality is that the effects of social inequalities in living conditions accumulate as children grow older. One might expect a similar cumulative insult mechanism to operate before birth, producing increasing social differences in the risks of fetal loss as pregnancies mature. We have found just the opposite pattern. Czech women exhibit considerable differences in risks of fetal loss by marital status, educational level, and labor force attachment, but the contrasts are greatest early in the pregnancy (as far back as we can observe it reliably). Differences become much smaller at full term.
This trajectory of fetal loss is the mirror image rather than a parallel of differences known to appear in infancy. Systematic social inequalities in the risk of involuntary reproductive loss increase in both directions with distance from full-term births, obscured only by a local minimum centered on that critical point.
Acknowledgments
We acknowledge support for this research from the National Institute of Child Health and Development, Grant R01-HD31146, and express our appreciation to the Czech Ministry of Public Health for providing the data. During some of this work, the second author enjoyed the hospitality of the Center for Advanced Study in the Behavioral Sciences as a Fellow with economic support from Stockholm University and the Bank of Sweden Tercentennial Foundation. The third author spent three semesters in residence at the Department of Sociology, University of South Carolina. We thank Michael Bracher, Tonji Durant, Isaac Eberstein, Joan Herold, Ernest Hook, Carlton Hornung, Robert Hummer, Vicki Lamb, Ronald Lee, Michelle Marcus, Gigi Santow, Shripad Tuljapurkar, and Kenneth Wachter for useful comments on our study.
REFERENCES
- Abramson F. Spontaneous Fetal Death in Man. Social Biology. 1973;20:375–403. doi: 10.1080/19485565.1973.9988069. [DOI] [PubMed] [Google Scholar]
- Bakketeig L, Seigel D, Sternthal P. A Fetal-Infant Life Table Based on Single Births in Norway, 1967-1973. American Journal of Epidemiology. 1978;107:216–25. doi: 10.1093/oxfordjournals.aje.a112528. [DOI] [PubMed] [Google Scholar]
- Carlson E, Hoem J. Low-Weight Neonatal Survival Paradox in the Czech Republic. American Journal of Epidemiology. 1999;149:447–53. doi: 10.1093/oxfordjournals.aje.a009832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC/WHO . 1993 Czech Republic Reproductive Health Survey: Final Report. Centers for Disease Control and Prevention; Atlanta: 1995. [Google Scholar]
- Cooperstock MS, Bakewell J, Herman A, Schramm W. Effects of Fetal Sex on Risk of Very Preterm Birth in Twins. American Journal of Obstetrics and Gynecology. 1998;179:762–65. doi: 10.1016/s0002-9378(98)70079-1. [DOI] [PubMed] [Google Scholar]
- Cramer J. Social Factors and Infant Mortality: Identifying High-Risk Groups and Proximate Causes. Demography. 1987;24:299–322. [PubMed] [Google Scholar]
- Davies R, Goldenberg R, Boots L, Hoffman H, Copper R, Cutler G, DuBard M, Cliver S, Smith R. Elevated Levels of Midtrimester Maternal Serum Oc-Fetoprotein Are Associated With Preterm Delivery But Not With Fetal Growth Retardation. American Journal of Obstetrics and Gynecology. 1992;167:596–600. doi: 10.1016/s0002-9378(11)91556-7. [DOI] [PubMed] [Google Scholar]
- Divon M, Haglund B, Nisell H, Otterblad P, Westgren M. Fetal and Neonatal Mortality in the Postterm Pregnancy: The Impact of Gestational Age and Fetal Growth Restriction. American Journal of Obstetrics and Gynecology. 1998;178:726–31. doi: 10.1016/s0002-9378(98)70482-x. [DOI] [PubMed] [Google Scholar]
- Durant T. South Carolina Infant Mortality: Cause-Specific Analysis of Racial Mortality Differences, 1980-1987. Paper presented at the annual meeting of the Southern Demographic Association; Atlanta: 1994. [Google Scholar]
- Eberstein I, Parker J. Racial Differences in Infant Mortality by Cause of Death. Demography. 1984;21:301–21. [PubMed] [Google Scholar]
- Ekwo E, Gosselink C, Woolson R, Moawad A. Risks for Premature Rupture of Amniotic Membranes. International Journal of Epidemiology. 1993;22:495–503. doi: 10.1093/ije/22.3.495. [DOI] [PubMed] [Google Scholar]
- Fejgin M, Amiel A, Goldberger S, Barnes I, Zer T, Kohn G. Placental Insufficiency as a Possible Cause of Low Maternal Serum Human Gonadatropin and Low Maternal Serum Unconjugated Estrio Levels in Triploidy. American Journal of Obstetrics and Gynecology. 1992;167:766–67. doi: 10.1016/s0002-9378(11)91586-5. [DOI] [PubMed] [Google Scholar]
- Feldman G. Prospective Risk of Stillbirth. Obstetrics and Gynecology. 1992;79:547–53. [PubMed] [Google Scholar]
- Figa-Talamanca I, Repetto F. Correcting Spontaneous Abortion Rates for the Presence of Induced Abortion. American Journal of Public Health. 1988;78:40–42. doi: 10.2105/ajph.78.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Coombs L, Friedman J. Social Correlates of Fetal Mortality. Milbank Memorial Fund Quarterly. 1966;44:327–44. [PubMed] [Google Scholar]
- Gardos E, Rychtarikova J. Sante et Mortalite en Europe: Chaire Quetelet 1994. Academia-Bruylant/L/Harmattan; Louvainla-Neuve: 1996. Recent Trends in Health and Mortality in Central and Eastern Europe; pp. 437–67. [Google Scholar]
- Geronimus A. On Teenage Childbearing and Neonatal Mortality in the United States. Population and Development Review. 1987;13:245–79. [Google Scholar]
- Goldhaber M. Fetal Death Ratios in a Prospective Study Compared to State Fetal Death Certificate Reporting. American Journal of Public Health. 1989;79:1268–70. doi: 10.2105/ajph.79.9.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhaber M, Fireman B. The Fetal Life Table Revisited: Spontaneous Abortion in Three Kaiser-Permanente Cohorts. Epidemiology. 1991;2:33–39. [PubMed] [Google Scholar]
- Gravett M, Hummel D, Eschenbach D, Holmes K. Preterm Labor Associated With Subclinical Amniotic Infection and With Bacterial Vaginosis. Obstetrics and Gynecology. 1986;67:229–37. doi: 10.1097/00006250-198602000-00013. [DOI] [PubMed] [Google Scholar]
- Hilden J, Modvig J, Damsgaard M, Schmidt L. Estimation of the Spontaneous Abortion Risk in the Presence of Induced Abortions. Statistics in Medicine. 1991;10(2):285–97. doi: 10.1002/sim.4780100211. [DOI] [PubMed] [Google Scholar]
- Hoem J. Statistical Analysis of a Multiplicative Model and Its Application to the Standardization of Vital Rates: A Review. International Statistical Review. 1987;55:119–52. [Google Scholar]
- Hoem J. Classical Demographic Methods of Analysis and Modern Event-History Techniques. IUSSP: 22nd International Population Conference, Montreal; Canada. 1993.1993. pp. 281–91. [Google Scholar]
- Hogue C, Hargraves M. Class, Race and Infant Mortality in the United States. American Journal of Public Health. 1993;83:9–12. doi: 10.2105/ajph.83.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. The Incidence of Spontaneous Abortion. Population Studies. 1970;24:241–45. doi: 10.1080/00324728.1970.10406127. [DOI] [PubMed] [Google Scholar]
- Kiely J. Some Conceptual Problems in Multivariable Analyses of Perinatal Mortality. Paediatrics and Perinatal Epidemiology. 1991;5:243–57. doi: 10.1111/j.1365-3016.1991.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Kristensen F, Mac F. Life Table Analysis of Infant Mortality and Feto-Infant Mortality Distributed on Causes of Death in Denmark 1983-1987. International Journal of Epidemiology. 1992;21:320–23. doi: 10.1093/ije/21.2.320. [DOI] [PubMed] [Google Scholar]
- Lieberman E, Ryan K, Monson R, Schoenbaum S. Risk Factors Accounting for Racial Differences in the Rate of Premature Birth. New England Journal of Medicine. 1987;317:743–48. doi: 10.1056/NEJM198709173171206. [DOI] [PubMed] [Google Scholar]
- Mittendorf R, Williams M, Berkey C, Lieberman E, Monson R. Predictors of Human Gestational Length. American Journal of Obstetrics and Gynecology. 1993;168:480–84. doi: 10.1016/0002-9378(93)90476-y. [DOI] [PubMed] [Google Scholar]
- Modvig J, Schmidt L, Damsgaard M. Measurement of Total Risk of Spontaneous Abortion: The Virtue of Conditional Risk Estimation. American Journal of Epidemiology. 1990;132:1021–38. doi: 10.1093/oxfordjournals.aje.a115744. [DOI] [PubMed] [Google Scholar]
- Morgan M, Berkowitz K, Thomas S, Reimbold P, Quilligan E. Abruptio Placentae: Perinatal Outcome in Normotensive and Hypertensive Patients. American Journal of Obstetrics and Gynecology. 1994;170:1595–99. [PubMed] [Google Scholar]
- Rogers R. Ethnic and Birth Weight Differences in Cause-Specific Infant Mortality. Demography. 1989;26:335–43. [PubMed] [Google Scholar]
- Romero R, Emanmian M, Wan M, Quintero R, Hobbins J, Mitchell M. Protaglandin Concentrations in Amniotic Fluid of Women With Intra-Amniotic Infection and Preterm Labor. American Journal of Obstetrics and Gynecology. 1987;157:1461–67. doi: 10.1016/s0002-9378(87)80245-4. [DOI] [PubMed] [Google Scholar]
- Rychtarikova J. Unpublished doctoral thesis. Department of Geography and Geodemography; University Karlova, Prague: 1981a. “Reprodkcni ztraty v tehodenstvi a behem prvniho roku zivota” (Prenatal and Infant Reproductive Loss) [Google Scholar]
- Rychtarikova J. “Prenatalni pomer pohlavi u cloveka” (Prenatal Sex Ratio in Man) Demografie. 1981b;23:295–300. [PubMed] [Google Scholar]
- Santow G, Bracher M. Do Gravidity and Age Affect Pregnancy Outcome? Social Biology. 1989;3 6:9–22. doi: 10.1080/19485565.1989.9988716. [DOI] [PubMed] [Google Scholar]
- Savitz D, Blackmore C, Thorp J. Epidemiologic Characteristics of Preterm Delivery: Etiologic Heterogeneity. American Journal of Obstetrics and Gynecology. 1991;164:467–71. doi: 10.1016/s0002-9378(11)80001-3. [DOI] [PubMed] [Google Scholar]
- Shapiro S, Jones E, Densen P. A Life Table of Pregnancy Terminations and Correlates of Fetal Loss. MilbankMemorial Fund Quarterly. 1962;40:7–45. [PubMed] [Google Scholar]
- Susser E. Spontaneous Abortion and Induced Abortion: An Adjustment for the Presence of Induced Abortion When Estimating the Rate of Spontaneous Abortion From Cross-Sectional Studies. American Journal of Epidemiology. 1983;118:305–308. doi: 10.1093/oxfordjournals.aje.a113542. [DOI] [PubMed] [Google Scholar]
- Susser E, Kline J. Effects of Induced Abortion on Spontaneous Abortion Rates. In: Hemminki K, Sorsa M, Vainio H, editors. Occupational Hazards and Reproduction. Hemisphere Publishing Corp; Washington, DC: 1985. pp. 183–91. [Google Scholar]
- Syrovatka A, Rychtarikova J. “Demograficke aktuality pro pediatry” (Current Demographic Facts for Pediatrics) Pokroky v pediatrii. 1990;11:183–207. [Google Scholar]
- Wilcox A, Weinberg C, Wehmann R, Armstrong E, Canfield R, Nisula B. Measuring Early Pregnancy Loss: Laboratory and Field Methods. Fertility and Sterility. 1985;44:366–74. [PubMed] [Google Scholar]
- Wilcox A, Weinberg C, O'Connor J, Baird D, Schlatterer J, Canfield R, Armstrong E, Nisula B. Incidence of Early Loss of Pregnancy. New England Journal of Medicine. 1988;319:189–94. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- Witkin S, Ledger W. Antibodies to Chlamydia tracomatis in Sera of Women With Recurrent Spontaneous Abortions. American Journal of Obstetrics and Gynecology. 1992;167:135–38. doi: 10.1016/s0002-9378(11)91647-0. [DOI] [PubMed] [Google Scholar]