Abstract
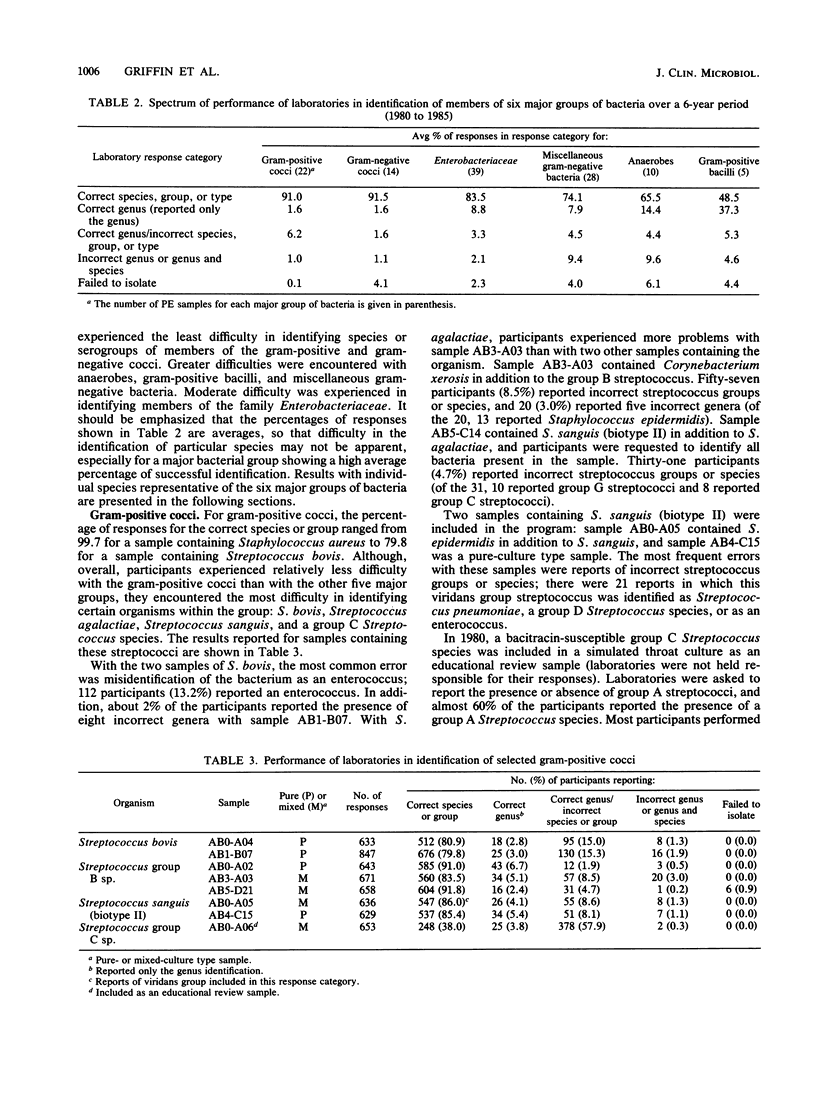
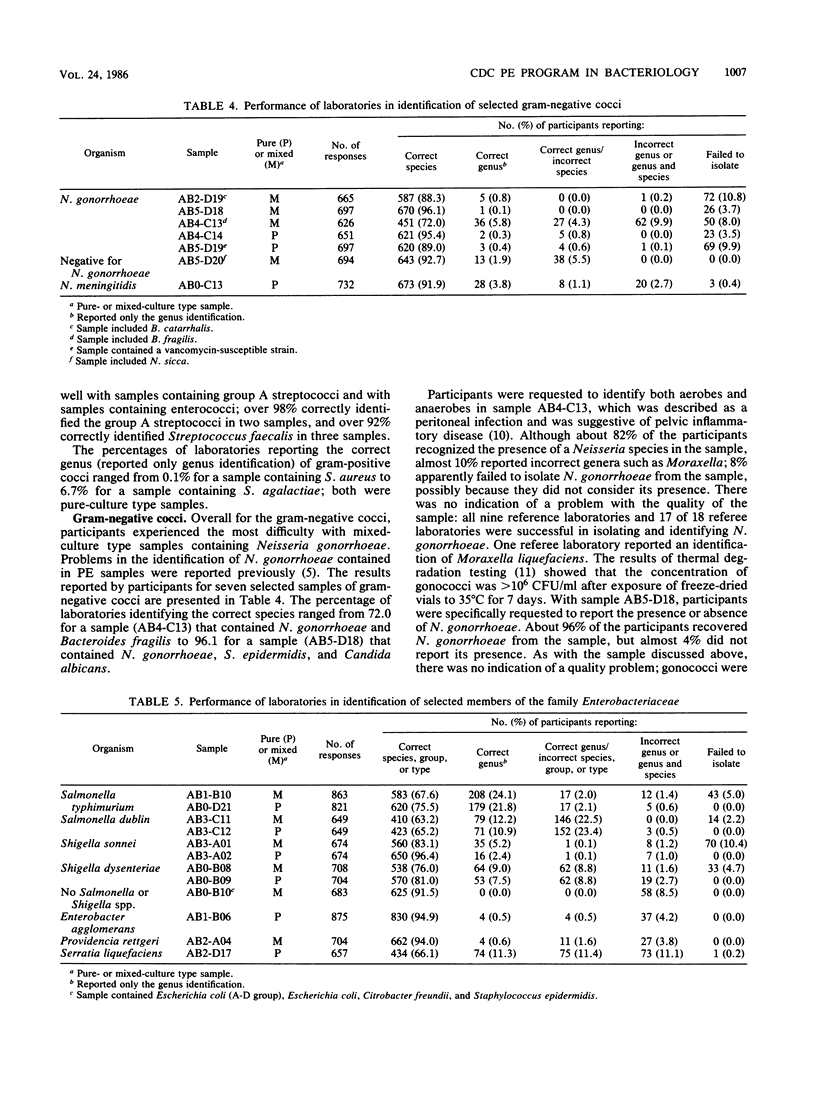
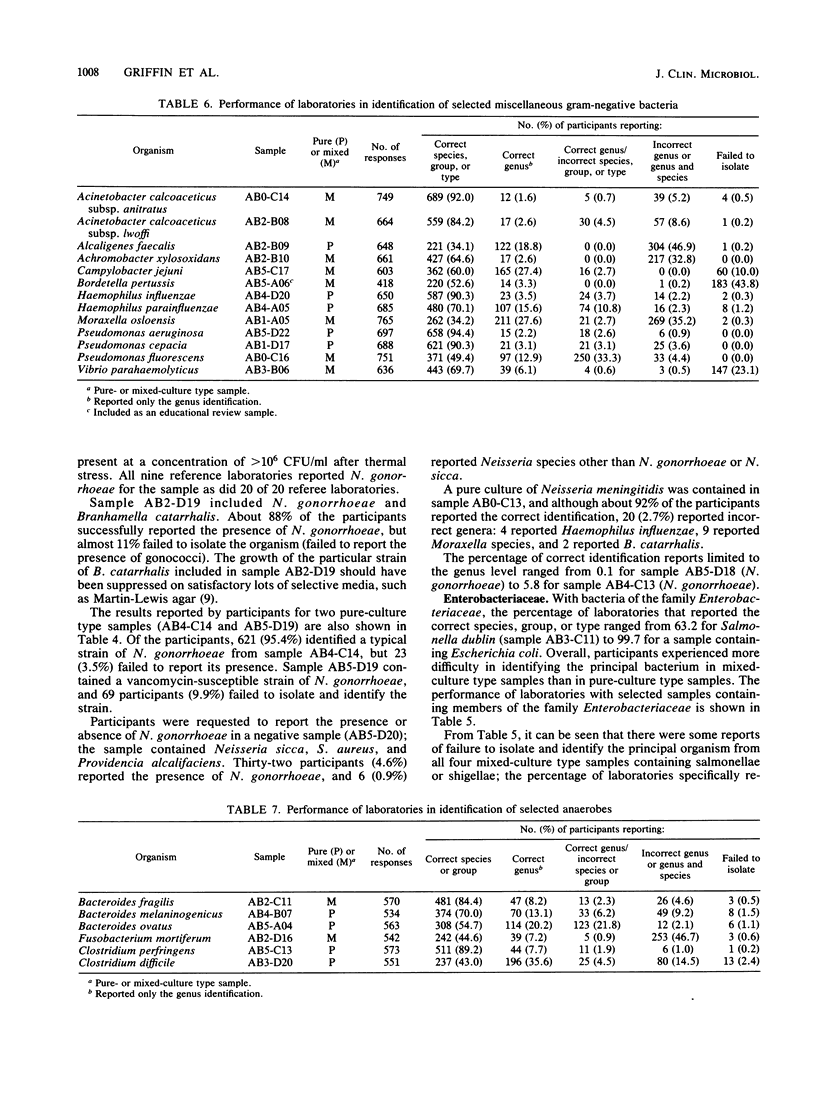
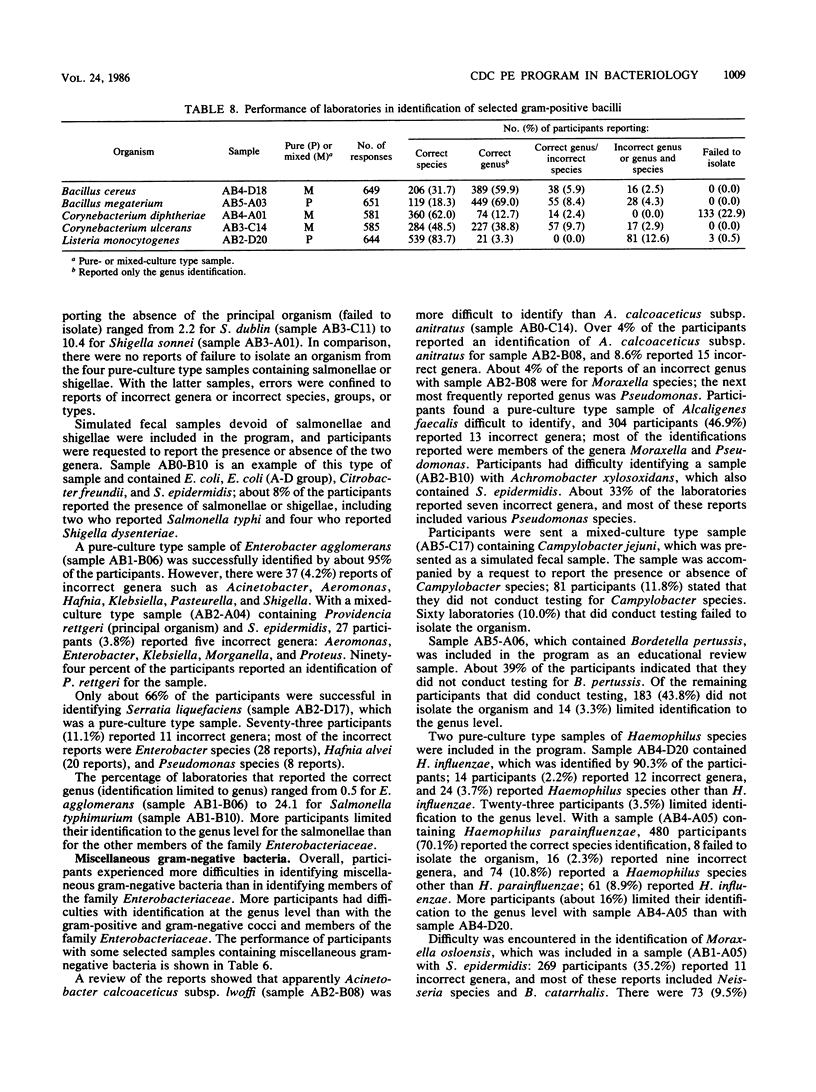
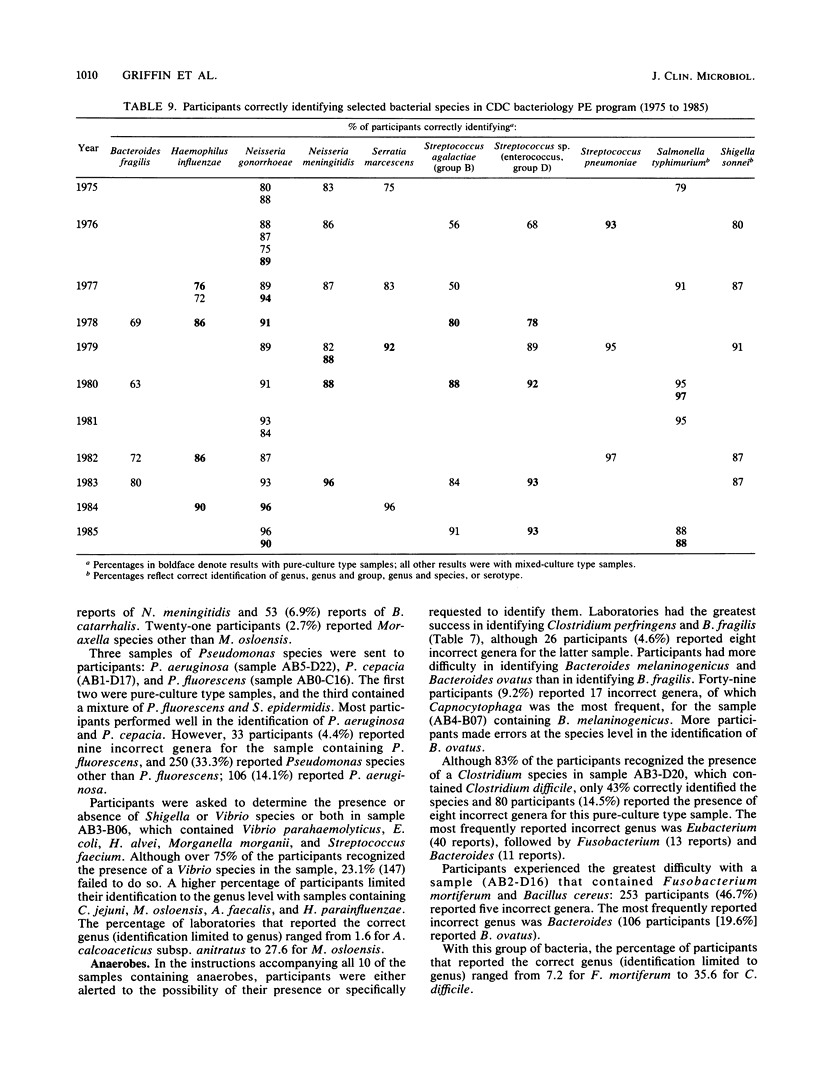
This report presents a summary and analysis of data from the Centers for Disease Control performance evaluation program in bacteriology for the 6-year period 1980 to 1985. During this period, the number of laboratories enrolled in the program ranged from about 750 to 1,000. Identification results reported by participating laboratories for representative species of six major groups of bacteria were placed into five response categories that were based on the level and accuracy of the identifications. Data on the performance of participants with bacterial groups and performance with selected species within each major group were analyzed. Overall, participants experienced the least difficulty in identifying species or serogroups of members of the gram-positive and gram-negative cocci. Participants encountered greater difficulties with anaerobes, gram-positive bacilli, and miscellaneous gram-negative bacteria. Identification of members of the family Enterobacteriaceae was of moderate difficulty. Problems in identifying certain bacterial species were probably related to a number of factors such as the characteristics of the species, its frequency of occurrence, the state of technology available for identification, and the state of proficiency and quality control in individual laboratories at the time of testing. Examples are given of improvements over time in the identification of certain bacterial species. Laboratories participating in an external proficiency testing program should take full advantage of the benefits of participation by instituting measures to correct testing deficiencies identified by the program.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Griffin C. W., 3rd, Mehaffey M. A., Cook E. C., Blumer S. O., Podeszwik P. A. Relationship between performance in three of the Centers for Disease Control microbiology proficiency testing programs and the number of actual patient specimens tested by participating laboratories. J Clin Microbiol. 1986 Feb;23(2):246–250. doi: 10.1128/jcm.23.2.246-250.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin C. W., 3rd, Mehaffey M. A., Cook E. C. Five years of experience with a national external quality control program for the culture and identification of Neisseria gonorrhoeae. J Clin Microbiol. 1983 Nov;18(5):1150–1159. doi: 10.1128/jcm.18.5.1150-1159.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehaffey M. A., Cook E. C., Griffin C. W., 3rd Preparation and stability of freeze-dried Neisseria gonorrhoeae cultures used for external quality control. J Clin Microbiol. 1984 Dec;20(6):1126–1129. doi: 10.1128/jcm.20.6.1126-1129.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


