Abstract
Objective
To evaluate the ability of third- and fourth-year pharmacy students to identify clinically significant drug-drug interactions (DDIs)
Methods
A questionnaire designed to measure DDI knowledge was disseminated to fourth-year pharmacy students in a school of pharmacy. A second questionnaire was distributed to third-year pharmacy students in 2 schools of pharmacy (schools A and B) and re-administered to students in 1 of the schools 1 year later.
Results
Class of 2005 fourth-year pharmacy students correctly categorized an average of 52% ± 13% DDI pairs on the first questionnaire. Third-year pharmacy students at schools A and B correctly categorized an average of 61% ± 18% and 66% ± 15% of DDI pairs, respectively. The average percentage of correct responses for fourth-year students from the class of 2007 was 65% (± 17%).
Conclusion
Pharmacy students' ability to identify important DDIs is far from optimal, even after completing experiential requirements.
Keywords: drug interaction
INTRODUCTION
The ability to identify potentially harmful drug interactions is a critical facet of the pharmacist's job. Drug interactions have been associated with increased incidence of adverse events, hospitalizations, and death.1-4 As the US population ages and the number of prescriptions dispensed continues to rise, the frequency at which pharmacists will be required to correctly assess the severity of drug-drug interactions and appropriately act upon this information is likely to increase. DDIs occur in 9% to 70% of patients in the outpatient setting.5 Computer programs and drug compendia are commonly used to assist pharmacists in recognizing drug interactions, but the usefulness of these tools is limited. For example, DDI software programs identify both clinically significant and nonsignificant drug interactions, making it challenging for pharmacists to interpret DDI warnings.6,7 Also, software programs and drug compendia do not always recognize all potential DDIs.8-11 Abarca and colleagues investigated the inclusion of major DDIs in 4 commonly used drug compendia (Drug Interaction Facts, Drug Interactions: Analysis and Management, Evaluations of Drug Interactions, and the Micromedex DRUG-REAX program).8 Of the 406 major DDIs investigated, 72% were listed in only 1 compendium.8 Studies have demonstrated similar “holes” in DDI screening software.9-11 Given these system inadequacies, it is critical to train pharmacists to identify potential DDIs and determine their clinical significance without reliance on other resources.
To date, few published studies examine the relationship between pharmacists' level of knowledge of DDIs and how much time pharmacy students spend learning about DDIs. Weidman et al studied the ability of 5 senior pharmacy students and 20 pharmacists at a veterans affairs (VA) medical center to identify potential DDIs using profiles of 2, 4, 8, or 16 drugs in which at least 1 pair of drugs could cause a DDI of moderate or major significance.12 Subjects were able to identify only two-thirds of the potential DDIs in the 2-drug profiles and none were able to detect all of the potential DDIs in the 8- or 16-drug profiles. Except for this report, there has been no published research evaluating the ability of pharmacy students to identify DDIs. Therefore, the purpose of our research was to determine the degree of third- and fourth-year pharmacy knowledge of clinically significant DDIs.
METHODS
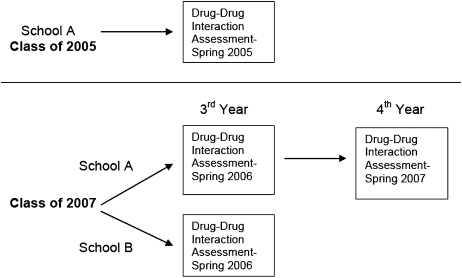
This report describes the assessment of pharmacy students' knowledge of DDIs using 2 separate studies conducted at different times. The overall study design is presented in Figure 1. The selection of programs to evaluate was based on a convenience sample. Two independent questionnaires designed to measure knowledge of DDIs were used in this analysis. The population for the first study consisted of fourth-year PharmD students (n = 68) at an accredited college of pharmacy in the western United States (referred to as school A). A 2-page questionnaire was disseminated to pharmacy students during a convocation immediately prior to graduation to assess knowledge of DDIs. Demographic information on age and sex was also collected.
Figure 1.
Overview of study to determine pharmacy students' ability to identify potential drug-drug interactions.
The items on the first questionnaire consisted of 22 drug pairs, which included 8 pairs that should not be used together, 4 pairs that could be used together safely with monitoring, 7 pairs that could be used safely together without monitoring, and 3 pairs that could have been categorized either as safe to use together with careful monitoring or unsafe to use together, depending on the specific drug interaction compendia cited. The response categories that the students were allowed to select from on the DDI questionnaire were: (1) should not be used together (contraindicated), (2) may be used safely together with monitoring, (3) may be used safely together without monitoring, and (4) not sure. This rating system and approach has been previously used by others.13-15
Due to the lack of consistency in reporting and classification of drug interactions across drug interaction compendia, 2 compendia were chosen to serve as the basis for the correct severity rating assigned to each DDI pair in the questionnaire: Evaluations of Drug Interactions (EDI) and MicroMedex.8,16,17 EDI assigns DDI significance codes based on the potential harm to the patient, frequency and predictability of occurrence, and degree and quality of documentation.17 The significance codes range from 1 to 4, with 1 indicating the greatest potential harm to the patient and 4 indicating the least potential harm (ie, the result of the DDI would not be considered clinically significant).17 MicroMedex also rates DDIs by severity.16 Severity ratings in Micromedex's Drugdex include contraindicated, major, moderate, and minor. Micromedex also provides an indicator regarding the degree of substantiation of evidence pertaining to the particular DDI of interest, and clinical management advice.16 If the 2 compendia were in disagreement over how to categorize or best appropriately manage a DDI, both compendia and corresponding questionnaire responses were considered correct for the purposes of scoring the questionnaire. There were 3 DDI pairs in the first questionnaire that were not cited as DDIs in either EDI or Micromedex (fexofenadine/metoprolol, raloxifene/alendronate and alendronate/conjugated estrogens) at the time of the study; thus, their lack of interaction was implied.
A second study, involving a different set of interactions and using a slightly different format to measure DDI knowledge, was administered to class of 2007 pharmacy students. The subjects who participated in the initial phase of this second separate questionnaire were students in the second semester of their third year of pharmacy school. This was a comparative study of drug interaction knowledge of third-year students in 4-year entry-level PharmD programs in 2 western United States accredited colleges of pharmacy, referred to as school A and school B. The second study also used a survey approach to assess knowledge of DDIs and students' level of confidence when providing responses to identify DDI pairs. The references Evaluations of Drug Interactions and Micromedex were again chosen as the basis of the drug interaction severity ratings. Two of the drug pairs chosen for the questionnaire were considered contraindicated for use together, 5 safe with careful monitoring, and 2 safe without monitoring. The compendia were in disagreement over the most appropriate clinical management strategies for 1 other pair; thus, 2 of the 3 possible responses were acceptable (avoidance of concurrent use and safe use with monitoring). Two of the drug pairs listed were not referenced in EDI or Micromedex (metformin/glyburide and sumatriptan/ibuprofen), and consequently their lack of interaction was implied at the time of the study. The questionnaire response choices were: (1) should not be used together-contraindicated, (2) may be used together with monitoring, and (3) may be used together without monitoring.
The questionnaire also asked respondents to rank their confidence about their answers to the drug combinations using a 6-item scale ranging from 0 (not confident) to 5 (very confident). The dependent variable was the percentage of drug pairs correctly identified by the respondents. The independent variables included demographic information such as age, gender, whether the students worked in a pharmacy setting outside of pharmacy school, and if so, which pharmacy setting and for how many years. In addition, the questionnaire asked students where their knowledge regarding DDIs came from, and whether they would like more class-time dedicated to instruction on DDIs. Another independent variable measured was the students' opinion of how important it is for pharmacists to be aware of DDIs. This questionnaire was reviewed by an expert in DDIs for clarity and minor changes were made prior to administration. In addition to data from students, we queried an experienced clinical faculty member from each school with regard to the total number of hours during the year devoted specifically to addressing DDIs. The purpose of this was to determine if the programs covered DDI in depth, as part of a required coursework.
The questionnaire was distributed without advanced notice to third-year class of 2007 students toward the end of their second semester. Students were asked to complete the voluntary questionnaire without using reference materials, notes, or assistance. Students were given approximately 15 minutes to complete the questionnaire. Correct responses were not shared with the students at that time. This questionnaire was administered again to class of 2007 students from school A during the spring semester of their fourth year of pharmacy school after they had completed all advanced pharmacy practice experiences (APPEs). This second administration of the questionnaire was not planned as part of the original study. However, when an opportunity to resurvey students at school A arose, we submitted a revised protocol to the University's Human Subjects Protection Committee and administered the questionnaire again. Updated demographic information was collected at this time as well. To determine the overall knowledge of drug interactions for each pharmacy class surveyed by year and school, the number of correct responses regarding the classification of DDIs were counted and divided by the total number of combination pairs evaluated. For the survey of the class of 2007's third-year students, Pearson's correlation coefficient was used to evaluate the relationship between actual DDI knowledge and confidence in the correct answer for each combination evaluated. To compare the overall correct knowledge of drug interactions of third-year pharmacy students at school A and school B, we used an independent t test. We also used a t test to compare whether there was a significant difference in the results between third-year and fourth-year students from school A.
The demographic data were analyzed by computing means and standard deviations for continuous variables. Student's t test and Wilcoxon rank sum were used to compare continuous demographic variables. Categorical demographic variables were analyzed using a chi-square test. Linear regression was performed to determine whether DDI knowledge was predicted by any of the following variables: age, gender, school, confidence, and work experience (the sum of work experience across each different work setting). All tests were 2-sided with an alpha level of 0.05.
Based on the hypothesis that students would not perform as well on questionnaire items that included infrequently used medications, another analysis was performed that compared the percentage of correct responses to questionnaire items with whether the drug pairs included at least 1 drug listed in 2006's Top 200 Drugs.
RESULTS
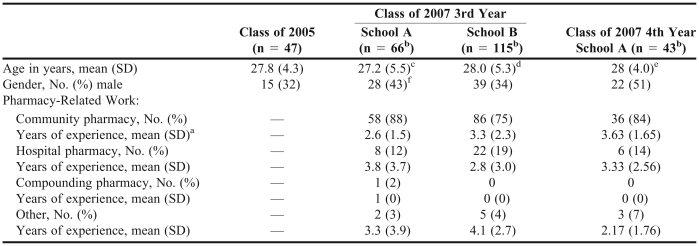
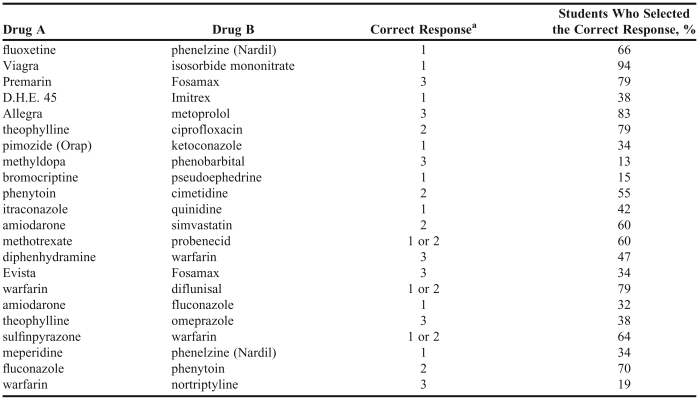
In the first study, 47 (69%) of the 68 fourth-year class of 2005 pharmacy students completed the first questionnaire. Table 1 provides demographic information on these respondents. The average age (SD) of the pharmacy students completing the questionnaire was 27.8 ± 4.3 years, and approximately two-thirds (68%) of the respondents were female. Pharmacy students correctly identified 51.5% ± 12.7% of the drug pairs (Table 2). Of the 8 interactions categorized strictly as those that should be avoided, 44% of the class of 2005 pharmacy students correctly identified them. Of the 7 drug pairs that may be safely used together without monitoring, 45% of the pharmacy students assigned the correct interaction severity level. Of all the drug interaction pairs in the questionnaire, the pair receiving the most correct responses (94%) pertained to sildenafil and isosorbide mononitrate. The pair least likely to generate correct interaction severity responses from the students (13%) was methyldopa and phenobarbital.
Table 1.
Demographics of Students Evaluated for Knowledge of Drug-Drug Interactions
ap < 0.05 between school A and school B
b= unless otherwise noted
cn = 64
dn = 111
en = 42
fn = 65
Table 2.
Class of 2005 Drug-Drug Interaction Knowledge Results, N = 47
aRating scale: 1 = should not be used together (contraindicated), 2 = may be used safely together with monitoring, 3 = may be used safely together without monitoring.
In the second study, 181 third-year class of 2007 students participated; 66 (94.3%) of 70 participated from school A, and 115 (95.8%) of 120 participated from school B. Their demographic data are also shown in Table 1. There were no significant differences between students at the different schools in any of the demographic variables except for the average number of years of work experience in the community practice setting. Students were on average 27.2 ± 5.5 years of age at school A and 28.0 ± 5.3 years of age at school B (p = 0.14). Respondents were predominantly female at both schools (57% female at school A, 66% female at school B, p = 0.22). The majority of students at each school reported working in a community pharmacy; 58 (87.9%) of students at school A and 86 (74.8%) of students at school B. For those students indicating work experience in community pharmacy, the average number of years of work experience for respondents from school A was 2.6 ± 1.5 years and 3.3 ± 2.3 years for respondents from school B (p = 0.02). A smaller number of students reported working in a hospital, a compound pharmacy environment, or other practice setting, including a military institution or for a home infusion company. A faculty representative for school A estimated that the average hours of DDI-related information imparted over the course of the program was about 6 hours, while a representative from school B estimated 5 hours.
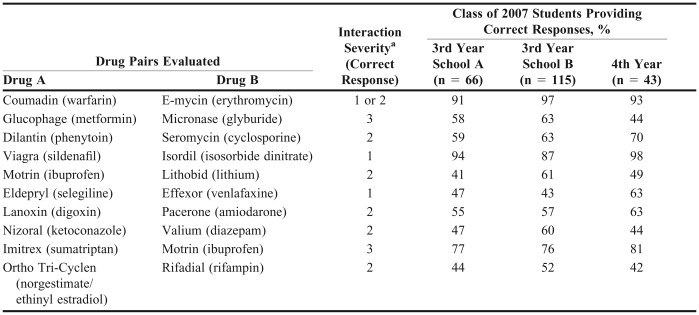
There was no significant difference in the percentage of correct responses by third-year students from each school. Respondents from school A correctly identified an average of 61% ± 18% correct responses, and school B reported an average of 66% ± 15% correct responses (p = 0.06). Students from school A were able to correctly identify the severity level of the sildenafil and isosorbide dinitrate drug pair most often (94% correct responses), whereas students from school B correctly categorized the severity of the warfarin and erythromycin pair most often (97% correct responses; Table 3). The drug interaction pair least likely for school A students to correctly rate was lithium carbonate and ibuprofen (41% correct response rate), and for school B students, venlafaxine and selegiline (43% correct response rate). There was a significant difference between the 2 schools regarding the level of confidence in students' reported responses. School A reported an average confidence score (on a scale of 0 to 5 as described in the methods) of 3.14 ± 0.79 and school B reported a higher confidence level of 3.41 ± 0.79; p = 0.03. Pearson's correlation coefficient test was performed to determine whether there was a high correlation between the number of correct answers and the confidence score for all of the participating third-year class of 2007 students at both schools, and the correlation coefficient was determined to be 0.24.
Table 3.
Pharmacy Students' Responses Regarding Drug-Drug Interactions
aRating scale: 1 = should not be used together (contraindicated), 2 = may be used safely together with monitoring, 3 = may be used safely together without monitoring
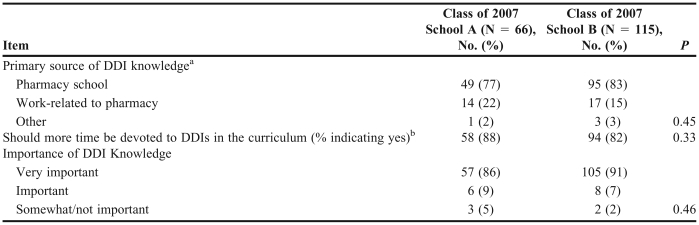
Third-year class of 2007 students were also asked questions regarding their opinions on knowledge of DDIs (Table 4). Students at both schools reported pharmacy school as the primary source of their DDI knowledge: school A = 49 (77%) students; school B = 95 (83%) students (p = 0.45). Fifty-eight students at school A (88%) and 94 students at school B (82%) stated that more time should be spent on DDI in pharmacy school (p = 0.33). In addition, the majority of students at both schools responded that DDI knowledge is very important: school A: 57 (86%) students; school B: 105 (91%) students (p = 0.46).
Table 4.
Student Opinions Regarding Drug-Drug Interaction Knowledge
aThere were 2 missing responses from school A (n = 64).
bThere was 1 missing response from school B (n = 114).
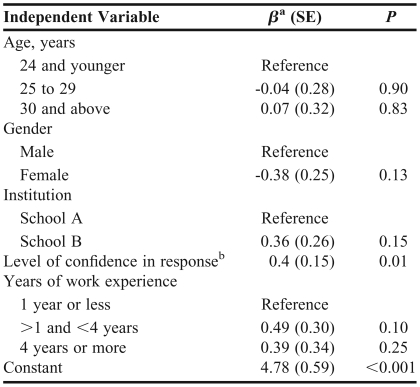
The linear regression analysis to determine whether DDI knowledge was predicted by age, gender, school, confidence, or work experience (Table 5) found that students' confidence was the only significant predictor (p = 0.01, adjusted R-squared = 0.06).
Table 5.
Relationship Between Student Characteristics and Knowledge of Drug-Drug Interactions
aβ, partial regression coefficients adjusted for the other predictors
bConfidence rated using a 6-item scale ranging from 0 (not confident) to 5 (very confident). Adjusted R2 = 0.06.
When the questionnaire was re-administered to class of 2007 students at school A after the completion of their fourth year of pharmacy education, 43 students completed the survey (Table 1). The average percentage of students who provided correct responses was not significantly better than that of the previous year (65% [17%] and 61% [18%], respectively; p = 0.32). These students were most likely to correctly identify the severity of the sildenafil and isosorbide dinitrate drug pair (98% correct responses; Table 3). Norgestimate/ethinyl estradiol and rifampin was the DDI pair with the most incorrect responses (42% correct responses). Students' level of confidence in their responses, as measured by a 6-item scale ranging from 0 (not confident) to 5 (very confident), was significantly better in their fourth year of pharmacy school than in their third year. Class of 2007 students from school A had an average confidence score of 3.1 ± 0.8 during their third year and 3.8 ± 0.6 their fourth year of pharmacy school (p < 0.001).
An analysis was completed for results from the class of 2005 that compared percentage of correct questionnaire responses to whether 1 of the DDI combinations included at least 1 of 2006's most commonly prescribed drugs. Students in the class of 2005 were more likely to correctly classify DDI severity when at least 1 of the drugs from the drug pair was a frequently prescribed medication, defined as 1 of the top 200 most prescribed medications in 2006. In the first questionnaire, there were 6 drug pairs in which neither drug was listed in the Top 200 Drugs of 2006 by prescription count, and on average, 33% of students correctly assigned severity levels to them.18 In contrast, 59% of students correctly assigned severity levels to the drug pairs in which at least 1 of the drugs was listed in the Top 200 Drugs of 2006 by prescription count.18 The questionnaire administered to the class of 2007 had at least 1 Top 200 drug in each pair, so this analysis was not done for this survey's results.18
DISCUSSION
This report found that third- and fourth-year pharmacy students could correctly categorize only 52% to 66% of drug interaction pairs presented to them. To our knowledge, this study is the first known comprehensive evaluation of pharmacy students' ability to identify DDIs. However, results from these questionnaires are consistent with similar studies that have assessed the ability of practicing pharmacists and other clinicians to accurately categorize the severity of drug interactions.12-14 A survey of clinicians, including physicians, nurse practitioners, and physician's assistants, revealed that clinicians were, on average, able to correctly categorize 44% of drug-drug pairs.14 A similar study revealed that senior pharmacy students and practicing pharmacists were able to correctly categorize 66% of drug-interaction pairs in 2-drug prescription profiles presented to them.12
Repetition and familiarity with medications may contribute to students' ability to correctly categorize the severity of DDIs. Supporting this notion is our analysis comparing percentage of correct questionnaire responses to whether 1 of the DDI combinations included at least 1 of 2006's most commonly prescribed drugs. Interestingly, in our analysis of results for the class of 2007, the number of years of pharmacy work experience and the type of pharmacy practice setting were not significant predictors of DDI knowledge.
A majority of students surveyed stated that more time should be spent on drug interaction instruction in pharmacy school, yet when surveyed, the 2 western US colleges of pharmacy reported spending only 5-6 curricular hours on this important facet of the pharmacist's work. Students' desire to learn more about drug interactions is not unwarranted. The clinical consequences of DDIs have been extensively reported in the literature. The prevalence of potential DDIs is far from trivial.1,5,7,19,20 A study of pharmacy records from VA ambulatory care clinics found that the rate of exposure to 25 potential DDIs was 2.2%.20 Another study on the frequency of potential DDIs in a pharmacy benefits manager's drug database revealed that the potential DDI pair with the highest prevalence rate (warfarin/non-steroidal anti-inflammatory drug) occurred at a rate of 279 per 100,000 persons.19
Studies documenting the dangers of DDIs underscore the need to be able to detect and prevent potential drug interactions. For example, a large retrospective database analysis demonstrated a marked increase in the rate of cardiac-related sudden death in individuals who concurrently took erythromycin and a CYP3A inhibitor versus those who had not used a CYP3A inhibitor nor certain antibiotics (incidence-rate ratio 5.35, p = 0.004).4 Even medications regularly prescribed together can cause serious adverse events, warranting intervention on the part of the prescriber, the pharmacist, or both. Knijff-Dutmer et al demonstrated that individuals taking warfarin and a nonsteroidal anti-inflammatory drug (NSAID) are 5.8 times as likely to have a hemorrhage as those on warfarin without an NSAID.2
The introduction of computer software capable of detecting drug interactions at pharmacies has not obviated the need for pharmacists to have a solid understanding of drug interactions. Several studies have demonstrated the inconsistency and overall limited reliability of DDI screening software to warn dispensing pharmacists of potential serious drug interactions.9-11 A study of 9 software programs installed in chain and healthcare management organization (HMO) pharmacies in Washington found that DDI screening software failed to recognize clinically significant DDIs approximately a third of the time.9 A repeat of the study several years later in Tucson, Arizona, investigated the reliability of screening software in 8 community pharmacies and 5 hospital pharmacies, and found that the software in use in the community pharmacies had a median sensitivity of 0.88 (range, 0.81-0.94) and a median specificity of 0.91 (range, 0.67-1.00), while hospital screening software performed slightly worse, with a median sensitivity of 0.38 (range, 0.15-0.94) and median specificity of 0.95 (range, 0.81-0.95).10
Another limitation of DDI screening software is the problem of high signal to noise ratio with respect to DDI warnings. Glassman et al reported that 55% of clinicians perceived excessive nonrelevant alerts as a barrier to using automated drug alerts.14 A survey of VA pharmacists revealed that only 35% of them agreed with the statement “Clinically important DDI alerts are easily differentiated from other warning messages and drug utilization review (DUR) alerts.”21 In another study of DUR programs in community pharmacies, pharmacy staff members overrode approximately 88% of all DUR alerts.6
Yet another limitation of drug screening software is its inability to detect potential DDIs involving nonprescription medications because these products may not be entered into the patient's drug profile. The combination of an NSAID and warfarin is just one example of a DDI with documented clinical significance that may go undetected in pharmacy computer software.2 Pharmacists and pharmacy personnel should always attempt to obtain a complete medication history from the patient, including nonprescription medications and alternative therapies.
This study has several limitations. We assumed that each questionnaire could provide an adequate assessment of student DDI knowledge. We assumed that, in their curriculum, pharmacy students would have been exposed to information about the medications listed in the questionnaire. With regard to the first survey, we assumed that students would be familiar with both brand and generic names of medications since this survey presented either the brand or generic names of drugs, but not both. The students were not permitted to use drug-interaction compendia to help them with their answers, but this is not an accurate reflection of real-world pharmacy practice. We designed both surveys from the perspective of the outpatient community pharmacy setting, which limits the generalizability of the results to other practice settings.
We made the decision to use 2 rather than 1 drug interaction compendium to increase the likelihood that the drug pairs were recognized as DDIs. Often there is conflicting information among the various drug-interaction compendia because classification of the severity of potential drug interactions is not always straightforward. For example, EDI classifies DDI by several factors, including strength of evidence, frequency of occurrence, and potential harm to the patient.17 We might have obtained different results if we had used alternate informational sources or classification schemes. Finally, this study evaluated students' ability to correctly identify a DDI severity rating, but not whether they would take appropriate actions regarding DDIs that would result in the best patient outcomes.
CONCLUSION
Identifying potentially harmful DDIs is a critical component of a pharmacists' job. This study provides a glimpse into how well pharmacy students are being trained to identify potential DDIs. Advanced pharmacy students could identify only 52% to 66% of drug interactions presented to them, and many were unable to recognize drug pairs associated with severe morbidity and mortality. This is concerning given that most students reported that their primary source of DDI knowledge was pharmacy school. Our research findings and those of others underscore the need for more comprehensive, focused education devoted to the area of DDIs in the pharmacy curriculum.
ACKNOWLEDGEMENTS
The authors would like to thank Jennifer Moyers, PharmD, and Martha Mrozowski, PharmD, for their contributions to the design and execution of the class of 2007 DDI questionnaire.
REFERENCES
- 1.Juurlink DN, Mamdani M, Kopp A, et al. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289(13):1652–8. doi: 10.1001/jama.289.13.1652. [DOI] [PubMed] [Google Scholar]
- 2.Knijff-Dutmer EA, Schut GA, van de Laar MA. Concomitant coumarin-NSAID therapy and risk for bleeding. Ann Pharmacother. 2003;37(1):12–6. doi: 10.1345/aph.1C157. [DOI] [PubMed] [Google Scholar]
- 3.Shad MU, Marsh C, Preskorn SH. The economic consequences of a drug-drug interaction. J Clin Psychopharmacol. 2001;21(1):119–20. doi: 10.1097/00004714-200102000-00027. [DOI] [PubMed] [Google Scholar]
- 4.Ray WA, Murray KT, Meredith S, et al. Oral erythromycin and the risk of sudden death from cardiac causes. N Engl J Med. 2004;351(11):1089–96. doi: 10.1056/NEJMoa040582. [DOI] [PubMed] [Google Scholar]
- 5.Jankel CA, Speedie SM. Detecting drug interactions: a review of the literature. Drug Intell Clin Pharm. 1990;24(10):982–9. doi: 10.1177/106002809002401014. [DOI] [PubMed] [Google Scholar]
- 6.Chui MA RM. Evaluation of online prospective DUR programs in community pharmacy practice. J Manage Care Pharm. 2000;6:27–32. [Google Scholar]
- 7.Peng CC, Glassman PA, Marks IR, et al. Retrospective drug utilization review: incidence of clinically relevant potential drug-drug interactions in a large ambulatory population. J Manage Care Pharm. 2003;9(6):513–22. doi: 10.18553/jmcp.2003.9.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abarca J, Malone DC, Armstrong EP, et al. Concordance of severity ratings provided in four drug interaction compendia. J Am Pharm Assoc. 2004;44(2):136–41. doi: 10.1331/154434504773062582. [DOI] [PubMed] [Google Scholar]
- 9.Hazlet TK, Lee TA, Hansten PD, et al. Performance of community pharmacy drug interaction software. J Am Pharm Assoc. 2001;41(2):200–4. doi: 10.1016/s1086-5802(16)31230-x. [DOI] [PubMed] [Google Scholar]
- 10.Abarca J, Colon LR, Wang VS, et al. Evaluation of the performance of drug-drug interaction screening software in community and hospital pharmacies. J Manage Care Pharm. 2006;12(5):383–9. doi: 10.18553/jmcp.2006.12.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavuto NJ, Woosley RL, Sale M. Pharmacies and prevention of potentially fatal drug interactions. JAMA. 1996;275(14):1086–7. [PubMed] [Google Scholar]
- 12.Weideman RA, Bernstein IH, McKinney WP. Pharmacist recognition of potential drug interactions. Am J Health-Syst Pharm. 1999;56(15):1524–9. doi: 10.1093/ajhp/56.15.1524. [DOI] [PubMed] [Google Scholar]
- 13.Glassman PA, Belperio P, Simon B, et al. Exposure to automated drug alerts over time: effects on clinicians' knowledge and perceptions. Med Care. 2006;44(3):250–6. doi: 10.1097/01.mlr.0000199849.08389.91. [DOI] [PubMed] [Google Scholar]
- 14.Glassman PA, Simon B, Belperio P, et al. Improving recognition of drug interactions: benefits and barriers to using automated drug alerts. Med Care. 2002;40(12):1161–71. doi: 10.1097/00005650-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Ko Y, Malone DC, Skrepnek GH, et al. Prescribers' Knowledge of and Sources of Information for Potential Drug-Drug Interactions: A Postal Survey of US Prescribers. Drug Safety. 2008;31(6):525–36. doi: 10.2165/00002018-200831060-00007. [DOI] [PubMed] [Google Scholar]
- 16.Micromedex Healthcare Series. Thomson Healthcare. http://www.thomsonhc.com Accessed January 21, 2009.
- 17.Evaluations of Drug Interactions. San Bruno, CA: First DataBank, Inc, The Hearst Corporation 2006.
- 18.Top 200 Prescription Drugs of 2006. Pharmacy Timeshttp://pharmacytimes.com/Article.cfm?Menu=1&ID=4629. Accessed January 21, 2008.
- 19.Malone DC, Hutchins DS, Haupert H, et al. Assessment of potential drug-drug interactions with a prescription claims database. Am J Health-Syst Pharm. 2005;62(19):1983–91. doi: 10.2146/ajhp040567. [DOI] [PubMed] [Google Scholar]
- 20.Mahmood M, Malone DC, Skrepnek GH, et al. Potential drug-drug interactions within Veterans Affairs medical centers. Am J Health-Syst Pharm. 2007;64(14):1500–5. doi: 10.2146/ajhp060548. [DOI] [PubMed] [Google Scholar]
- 21.Ko Y, Abarca J, Malone DC, et al. Practitioners' views on computerized drug-drug interaction alerts in the VA system. J Am Med Inform Assoc. 2007;14(1):56–64. doi: 10.1197/jamia.M2224. [DOI] [PMC free article] [PubMed] [Google Scholar]