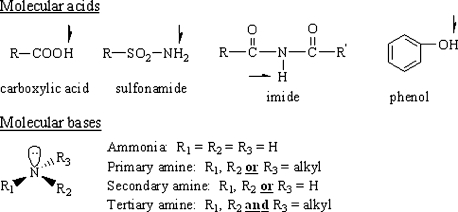Abstract
Despite repeated exposure to the principles underlying the behavior of organic acids and bases in aqueous solution, some pharmacy students remain confused about the topic of acid-base chemistry. Since a majority of organic drug molecules have acid-base character, the ability to predict their reactivity and the extent to which they will ionize in a given medium is paramount to students' understanding of essentially all aspects of drug action in vivo and in vitro. This manuscript presents a medicinal chemistry lesson in the fundamentals of acid-base chemistry that many pharmacy students have found enlightening and clarifying
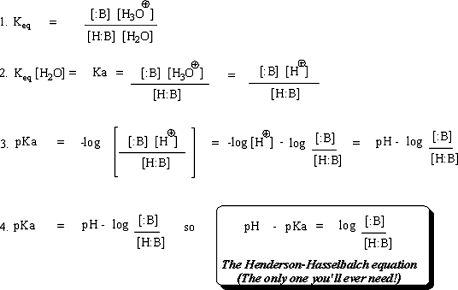
Keywords: medicinal chemistry, acid-base chemistry, acid-base equilibria, Henderson-Hasselbalch equation
INTRODUCTION
By the time pharmacy students encounter required coursework in medicinal chemistry, they have received repeated and often in-depth exposure to acid-base chemistry. Yet, despite having covered this essential content in inorganic, organic, biochemistry, and pharmaceutics courses, students often demonstrate an inability to work competently and confidently with acid-base chemistry principles. The ability to predict the behavior of organic acids and bases in solution is paramount to the comprehensive understanding of drug action that all pharmacists are expected to have. It is therefore essential that pharmacy educators identify teaching and learning strategies that foster a true and lasting comprehension of this critical and omnipresent aspect of drug behavior in vivo and in vitro.
The 2-semester, 5-credit-hour medicinal chemistry course sequence required of second-professional year (P2) pharmacy students at Creighton University has been recently described in detail.1,2 The 2 Chemical Basis of Drug Action courses take a practice-oriented approach that emphasizes the relevance of chemistry to the contemporary practice of pharmacy. They were purposefully designed to give students the skills necessary to predict biological properties and therapeutic activities of drug molecules.3,4 Comprehensive and conversational lesson handouts, learning objectives, and lesson summaries (entitled Med. Chem. To Go) are provided via the course web site, and students take a collaborative open book quiz over material provided in the lesson handout prior to class. This allows the use of class time to delve into topics in greater depth, apply concepts, and solve problems. To assist students in achieving higher-order learning goals, several optional worksheets, exercises, case studies, and past examinations are made available on the course web site.
The first 4 lessons of the Chemical Basis course sequence are focused on acid-base chemistry. As previously noted, despite repeated and prolonged exposure to these important principles, many students lack confidence in their ability to: (1) recognize acids and their conjugate bases from chemical structure, (2) predict the behavior of acids and bases in solution, and (3) solve problems using the Henderson-Hasselbalch equation. Each year before the first acid-base chemistry lesson, students who have studied acid-base chemistry in the past are asked to raise their hand. As expected, every hand goes up. Students are then asked to keep their hands raised if they understand acid-base chemistry. It is rare if more than 10% of the class admits to an understanding of these essential principles.
The first lesson handout on acid-base chemistry provided to Chemical Basis students (Appendix 1) has routinely been viewed by pharmacy students as an effective presentation of a traditionally problematic topic area. Students have verbally shared that this approach to the instruction of acid-base chemistry allowed them to envision the equilibrium process and apply it accurately to predict drug behavior. Assessment of student performance has validated anecdotal commentary by documenting that students are capable of working competently with these principles and using them to solve practice-based problems, both shortly after content presentation and after a 6.5 month period.
DESIGN
The equilibrium process described by the Henderson-Hasselbalch equation is really a simple and straightforward concept, but is confounded by the fact that acids and their conjugate bases can be charged (eg, protonated amines) or uncharged (eg, carboxylic acids). Medicinal chemistry students who had previously memorized a faculty-derived Henderson-Hasselbalch equation were often unable to explain what it meant and/or did not know how to apply it to a specific acid or base under consideration. Others who had memorized 2 equations (one for ionized acids and another for unionized acids) could not explain the circumstances under which each should be applied. After reflecting upon the barriers to an enduring understanding of acid-base chemistry, the 2 most important issues to be confronted were identified: the rote memorization of equations, which was essentially meaningless to students; and students' narrow focus on how individual instructors expected them to be utilized.
A major goal in the design of the acid-base chemistry lessons presented in the Chemical Basis courses was to help students visualize equilibrium processes so that the critical equations and concepts could be truly understood and consistently applied. A concerted effort was made to demystify concepts through the use of conversational, student-friendly language, analogy, in-class demonstrations (including acting out the equilibrium processes), and the illustrative incorporation of drug structure. An acid-base understanding roadmap was conceptualized and applied in the sequencing of ideas within the lesson in order to build logically from basic principles to more advanced concepts and, ultimately, to application. Thus, the derivation of a Henderson-Hasselbalch equation that could be universally applied to all molecules with acid-base character was preceded by a general discussion of acid-base equilibria and the need to balance charge. This, in turn, was followed by guidelines for recognizing the charged or uncharged nature of specific acids and bases, and for appropriately applying the Henderson-Hasselbalch equation to predict which conjugate would predominate in solution at a given pH. With a clear understanding of acid-base equilibria and the impact of pH on conjugate predominance, students could make sense of ionization (titration) curves, and were shown a facile method for constructing curves for drugs that were either ionized or unionized in acidic form (unionized or ionized in conjugate base form, respectively), as well as for neutral quaternary ammonium salts and drugs containing only unionized, neutral functional groups. These scientific skills were then brought to bear on the clinically relevant issues of absorption, distribution, metabolism, and excretion (ADME); therapeutic intervention in the case of drug toxicity; and drug-drug incompatibility in solution.
The acid-base chemistry lessons were also purposefully designed to assist students in making critical connections between the concepts presented. Rather than assume that these essential connections would be apparent to the learners, concept threads woven throughout the lesson were made explicit, and key principles were reinforced throughout the lesson. Students were given “sneak previews” of concepts to come, as well as prompted to think back on concepts previously covered that were closely tied to the concept currently being addressed. Taking this approach allowed the “big picture” of acid-base chemistry to emerge, a picture deemed essential by the instructor to a lasting understanding of this important chemistry.
Although the acid-base chemistry concepts covered in the first fall semester Chemical Basis examination are reinforced throughout the 2 semester Chemical Basis course sequence, an abbreviated assessment was conducted with the same cohort in spring 2007 to specifically measure retention of acid-base chemistry understanding. Ten points of content addressing major concepts in acid-base chemistry were added to a regularly scheduled examination on antineoplastic agents, and students were awarded bonus points for correct responses. The time gap between these 2 formal acid-base chemistry assessments was 6.5 months.
Students were not guided in any formal way in preparation for the spring semester acid-base chemistry retention assessment. Via e-mail, they were told only that acid-base chemistry bonus questions would be included on the antineoplastics examination and the general types of information the examination might cover (eg, working with the Henderson-Hasselbalch equation, recognizing acidic and basic salts by name, interpreting ionization curves). All of these concepts were emphasized in the examination administered during the fall term and, since the instructor returns graded examinations back to students, they would have known what kinds of questions to expect even without this courtesy announcement.
ASSESSMENT
Performance on Chemical Basis examination questions evaluating students' ability to: (1) recognize the impact of acid-base equilibria on the behavior of drug molecules in solution, and (2) use acid-base concepts to solve patient-centered therapeutic problems has routinely documented the success of this approach in fostering in-depth understanding of these important principles. Student performance on the acid-base chemistry examination administered in fall 2006 is provided in Table 1. The data from the fall 2006 examination show that both campus-based students and distance (web-based) students mastered acid-base chemistry concepts at a very high level. The complete examination, which also included questions on acid-base strength, acid-base functional groups, receptor chemistry, and drug metabolism, is available from the author upon request.
Table 1.
Student Performance on Chemical Basis Examination Questions Addressing Acid-Base Chemistry Principles (2006-2007 Academic Year)
*33 points out of a 100-point examination on acid-base chemistry, acid-base strength, acid-base functional groups, receptor chemistry, and drug metabolism
†10 points added as bonus questions to a 100 point antineoplastics examination
The performance of both campus and distance students on the retention assessment questions asked in spring 2007 clearly demonstrated that the understanding they documented in fall 2006 had endured (Table 1). The case around which the retention assessment examination was constructed described a patient who had inadvertently overdosed on transdermally administered fentanyl. Students were informed that, in addition to the unionized conjugate base found in the patch formulation, fentanyl is also marketed as the citrate salt. This information should have alerted them to the fact that fentanyl is ionized in conjugate acid form and unionized in conjugate base form. Answers to Questions 2 and 3 on the retention assessment were directly linked to the student's response to Question 1, and credit was only awarded for correct thinking. For example, if a student selected the wrong Henderson-Hasselbalch equation to describe fentanyl in Question 1, credit was only issued for Questions 2 (identifying the ratio of ionized:unionized fentanyl conjugates at a given urinary pH) and 3 (selecting an appropriate course of action to treat the fentanyl overdose) if those answers were consistent with the misassigned equation. Thus, if a student selected the keyed answer(s) to Question 2 and/or 3 based on flawed chemical reasoning, no credit was awarded.
When announcing the inclusion of the acid-base chemistry retention assessment on the antineoplastics examination, the instructor assured students that they were not expected to actively study for this bonus section of the examination. It was assumed that few would, since the antineoplastic agents represent the most mechanistic and difficult chemistry the students encounter in the 2 semester Chemical Basis course sequence and student study is traditionally intense prior to this examination. To test this assumption, students were asked after the examination to inform the instructor if they had actively prepared for the acid-base chemistry retention assessment questions and, out of 157 enrolled students, 28 (18%) responded that they had studied to some extent. Of these 28 students, 18 (12% of the class) said they had skimmed through course notes or other learning resources for less than 10-15 minutes, while the remainder (10 students or 6% of the class) did not specify their study time. The fact that 94% of the Chemical Basis class either did not study for these acid-base chemistry retention assessment questions or only breezed through their notes or old examination, and still performed close to a “B” level on the assessment, documents an enduring understanding of acid-base chemistry principles.
Anecdotal evidence suggests that students are able to apply the knowledge of acid-base chemistry gained from the Chemical Basis courses to other pharmacy coursework. For example, before the acid-base chemistry retention assessment was conducted, the instructor received 2 unsolicited e-mail messages from students who shared how their understanding of acid-base chemistry allowed them to correctly reason through a question on a recent pharmacology examination relating to the anticipated acid-base equilibrium behavior of drugs. These testimonials were particularly gratifying in light of the instructor's previously stated goal of fostering an understanding of acid base chemistry that is applicable to any and all courses.
DISCUSSION
Armed with a firm and enduring awareness of acid-base chemistry principles, students are able to approach the study of drug action with a clearer understanding of how organic molecules behave in solution. They can better predict drug distribution patterns, the likelihood of absorption vs. excretion at various urinary pH values, the ability (or lack thereof) of reaching sites of metabolic biotransformation, and the relative risk of CNS side effects. They can envision drug binding to receptors via electrostatic forces and better predict amino acid residues that will serve as electrostatic anchors to charged drugs (eg, protonated amines would only bind to anionic amino acids, limiting the viable candidates to 2 out of 20).
Over the 25 years this instructor has been teaching, pharmacy students have shared that after the completion of our acid-base lesson series (which also includes lessons on acid-base strength and acid-base functional groups) they finally “get it.” It is my sincere belief that, once students truly understand how to look at a molecule with acid-base character and learn to predict its behavior, it is a skill that will never leave them.
CONCLUSION
Helping pharmacy students understand the behavior of drug molecules with acid-base character paves their way to being the drug expects they are being educated to be. Their health professions colleagues will depend on this level of understanding and their patients deserve nothing less.
Appendix 1. Acid-Base Chemistry Lesson
ACID-BASE CHEMISTRY LESSON
Guiding Principle: If drugs are chemicals, and pharmacists are drug experts, then pharmacists are the chemical experts of the health care team.
General Concepts
The vast majority of the drug molecules you will encounter as pharmacists have acidic and basic functional groups and, therefore, acidic and basic properties. This can serve as an advantage both in vivo and in vitro. For example, in vivo, ionizable compounds are well transported in the aqueous-based general circulation, and are often the form of the drug that binds to biological receptors and serum proteins. The unionized component of an acid-base conjugate pair, however, is the form that will penetrate membranes to reach sites of action, biotransformation (metabolism) and excretion. In vitro, a compound with acid-base character can be formulated into aqueous-based injectable dosage forms.
The extent of molecular ionization will have a dramatic influence on all aspects of drug bioavailability, including absorption, distribution, the opportunity for metabolism, and excretion. The extent of ionization is totally dependent on two chemical parameters.
The chemical structure of the drug, and therefore the pKa value(s) of any functional groups with acid-base character. You can't change this unless you get in the lab and synthesize a new drug.
The pH of the aqueous medium in which the drug is found. You can change this by adding acid or base to the medium (although you have to be careful in vivo, as changing the pH of the blood can life-threatening).
In our analysis of the acid-base character of drug functional groups, we will most commonly use the Lowry-Bronsted definition of an acid as a proton donor, and combine the Lowry-Bronsted and Lewis definitions of a base as a functional group that accepts a proton by sharing an electron pair with that totally electron-deficient species.
Charges on Acids and Bases
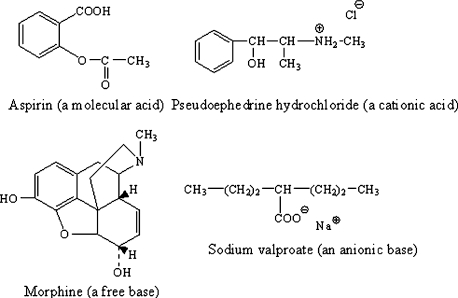
Both acids and bases can be either positively charged (cationic), negatively charged (anionic), or uncharged (unionized). Important Terminology Note: In addition to “unionized”, acceptable synonyms for the word “uncharged” include “molecular” and “free”. While it is commonly used, the term “neutral” should not be used to mean uncharged. In the chemist's language, the term “neutral” means the absence of acid-base character. Therefore, the phrase “neutral acid” or “neutral base” is chemical nonsense.
In drug chemistry, we will most commonly encounter acids that are either uncharged or cationic. Our most commonly encountered bases will be either molecular or anionic (see Figure 1. for examples).
Figure 1.
Examples of charged and molecular acids and bases.
Acid-Base Equilibrium
Acids and bases differ from one another by the presence or absence of an ionizable hydrogen atom. Acids have it….bases want it. When an acid donates its ionizable hydrogen atom as proton (H+), a conjugate base species is generated that lacks the proton. The extent to which the conjugate base is willing to take the proton back is determined by its structure and the pH of the medium in which it is found.
In other words, there is an equilibrium established between an acid and its conjugate base. The prevailing direction of the equilibrium is determined by those two all-important properties, the pKa of the functional group in question (unchangeable) and the pH of the medium (changeable).
As in any equilibrium process, the charges on each side of the equilibrium expression must balance. So let's look at the acid-base equilibrium in terms of the charge (or lack thereof) on our dissociating acid.
Cationic Acids
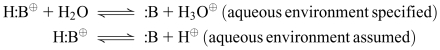 |
Note that, in the acidic conjugate, a shared pair of electrons holds the ionizable hydrogen atom (H) to the rest of the molecule (B). When the acid acts as acid and relinquishes the hydrogen atom as proton (H3O+ or H+), both electrons of the pair stay behind with the rest of the molecule (:B). The electrons are no longer shared…they are “alone”, so they are referred to as a “lone pair” of electrons. Since these electrons could conceivably take on proton again (to form the original acid), :B meets our definition of a base (both Lowry-Bronsted and Lewis), and is called the conjugate base of the original acid.
Since the original acid carried a +1 charge, and proton also is +1, the need for a charged-balanced equation dictates that the conjugate base of cationic (+1) acids must be unionized. General Rule: Cationic (+1) acids have unionized conjugate bases.
Remember, how readily the acid conjugate wants to donate the proton, and how readily the conjugate base will pick it up again, depends entirely on the pKa of the acidic form and the pH of the aqueous medium the drug is in. More on that in a minute!
Molecular Acids (aqueous environment assumed)
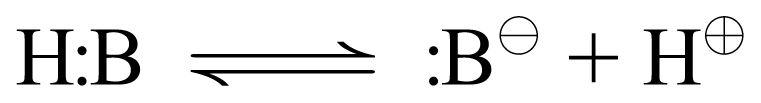 |
Everything we just said about cationic acids holds true for molecular acids except the charge on the conjugate base. Since a molecular acid is uncharged to begin with, when it gives up proton (+1 charge) you are left with a conjugate base that carries a −1 charge. General Rule: Molecular acids have anionic conjugate bases. Again, the direction of the equilibrium will depend on the strength of the acid form (pKa) and the concentration of proton already in the medium (pH).
Acid Strength: Qualitative
Here are some important “rules” to keep in mind as we discuss acid-base equilibria:
-
1. The stronger an acid, the weaker its conjugate base will be. Doesn't this make sense? Remember that an acid is defined as a proton donor. Therefore, the stronger the acid, the more willing it is to act as an acid and donate its proton. If the conjugate base that forms was also strong, it would pick that discarded proton right back up to re-form the acid which (since we claimed it was strong) would throw it right back again. This would get us nowhere!
Chemically speaking, the reason strong acids are strong is because they dissociate to form something (a conjugate base) that is much weaker and less likely to react. Chemists refer to weak species as stable species. Think of a strong acid as an agitated, unstable or discontented structure, one that needs to do something (give up H+) in order to be chemically content. This concept leads us to our second and third “rules.”
2. Strong species are reactive, weak species are stable, and chemical reactions always proceed to give the most stable (weakest) products possible under the circumstances.
3. The stronger an acid, the less time it spends in acid form, and the more time it spends as its conjugate base. For example hydrochloric acid, a very strong inorganic acid, readily donates its hydrogen when dissolved in water, dissociating almost completely into proton (H+) and its conjugate base, chloride ion (Cl−).
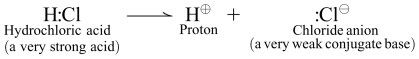 |
Note that I did not draw a “reverse arrow” showing the conjugate base, chloride ion, taking up the proton to reform HCl. The reaction is effectively “one way”, and essentially none of the HCl is left in acid form. It dissociates so completely because chloride ion is so stable (e.g., weak). This is because halogen is both electron withdrawing (meaning it is willing and able to accept the negative charge) and large enough to disperse the negative charge over its surface, which stabilizes it. Speaking anthropomorphically, it is quite happy in conjugate base form, and has no desire to form the highly reactive and chemically “unhappy” acid form.
4. The weaker the acid, the more time it spends in acid form and the less time it spends in conjugate base form. Hopefully you can understand why the reverse of rule #3 is true. If an acid is weak, it is relatively stable and comfortable with its chemical structure in conjugate acid form. It can donate proton, but it does so more reluctantly than a strong acid.
5. The conjugate acid form of a drug is promoted in acidic media. The conjugate base form of a drug is promoted in basic media. Think about it! An acidic medium already has plenty of proton floating around and doesn't need any more from the acidic drug conjugate. The proton on the drug is more likely to “stay put” in an acidic medium because the medium has enough of what it is offering (proton). The more acidic the medium, the less likely the acidic drug is to dissociate and the more it will exist in conjugate acid form.
Conversely, when the medium is basic, there is a lack of proton. In fact, there is hydroxide ion (OH−) in basic media, and that hydroxide reacts readily with the drug's acidic proton to form water (H+ + OH− = H2O). The acid will more willingly give up its proton to a basic medium. In fact, you can think of the hydroxide ion in the basic medium forcing the acid to relinquish its proton, thereby generating the conjugate base of the original acid. The more basic the medium, the faster the acidic drug will donate its proton, and the more it will exist in conjugate base form. We will qualify this idea just a bit later on. For now, it's important to understand the general concept of the influence that the pH of the medium has on an acid's willingness to dissociate to yield proton and its conjugate base.
Acid Strength: Quantitative
Well, here it is…what you've all been waiting for…the Henderson Hasselbalch equation! This important equation ties together the two critical concepts governing the extent of acid dissociation in solution: the pH of the medium and the pKa of the acidic functional group in question.
Remember that the pKa is a measure of the strength of each acidic functional group found on a given drug and is totally dependent on the drug's structure. The pKa tells you how inherently stable or unstable the acid is…how willing or unwilling the acid is to donate proton when dissolved in water. The pH of an aqueous solution tells you the concentration of proton already in the medium. Since pH is the negative log of the H+ concentration, the lower the number, the higher the concentration of protons (e.g., an aqueous solution with a pH of 1 is more acidic than one with a pH of 5).
To work out the Henderson-Hasselbalch equation, we need to first restate our acid dissociation equation. Please note that I'm going to do this without assigning any charges to the acidic form or the conjugate base form. You already know that if the acid form is uncharged, the conjugate base form will be anionic (−1) and that if the acid form is cationic (+1), the conjugate base form will be unionized. So bear with me for a bit, and I'll show you where the charges fit in very shortly.
Are you ready??? Here we go! (See Figure 2 for the derivation of the Henderson-Hasselbalch equation used in the lesson handout).
Figure 2.
Derivation of the Henderson-Hasselbalch equation.
Henderson-Hasselbalch and ionization
Now it's time to consider the charge on acids and their conjugate bases and relate it all back to the Henderson-Hasselbalch equation.
Cationic Acids
Remember that the Henderson-Hasselbalch equation is always:
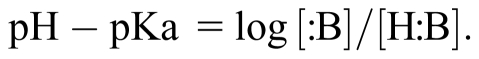 |
Also remember that the equation describing the dissociation of a cationic acid to proton and the unionized conjugate base is:
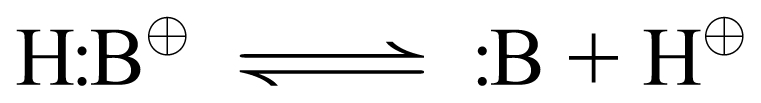 |
Given these two truths, it is clear that, for all compounds that are cationic (+1) in conjugate acid form and unionized in conjugate base form, we can express the Henderson-Hasselbalch equation as:
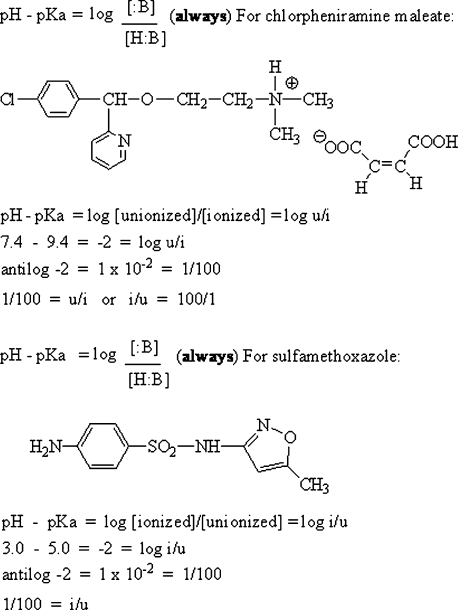
For example, the popular OTC antihistamine chlorpheniramine maleate is a cationic (+1) acid with an unionized conjugate base (soon you will be able to tell this from the name of the salt form of the drug). The pKa of chlorpheniramine conjugate acid is approximately 9.4. Calculate the ratio of ionized to unionized drug forms that would exist in the plasma at physiological pH (7.4) [see Figure 3 for the calculation provided in the lesson handout].
Figure 3.
Calculation of ionized/unionized ratios using the Henderson-Hasselbalch equation.
The percentage of chlorpheniramine that exists in ionized form at pH 7.4 can be easily calculated from the i/u ratio:
Unionized Acids
Again, the Henderson-Hasselbalch equation is always:
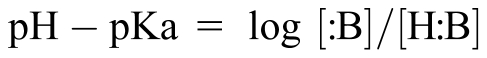 |
The acid dissociation expression for a molecular acid is:
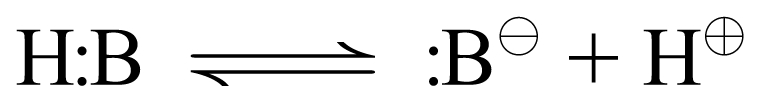 |
Therefore, the Henderson-Hasselbalch expression for molecular acids, which have anionic (-1) conjugate bases, is:
For example, the antibacterial sulfamethoxazole is an unionized acid with a pKa of approximately 5.0. What would be the i/u ratio of this drug in buffered gastric fluid of pH 3.0? [See Figure 3 for the calculation provided in the lesson handout]. The percentage of sulfamethoxazole that would exist in ionized form at pH 3.0 is readily calculated as before:
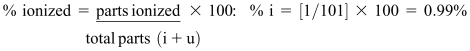 |
Important Note: When the pH of the medium is equal in value to the pKa of the acid, the drug exists 50% in the ionized form and 50% in the unionized form. Prove it yourself with the Henderson-Hasselbalch equation.
Ionization Curves
The two examples provided above illustrate a point about how pH influences ionization of acids (both cationic and molecular) in predictable ways. Remember how we said that the acidic conjugate of a drug will be favored in acidic media and the basic conjugate will be favored in basic media? (See Acid Strength: Qualitative, rule #5). As promised, we're now going to qualify this statement just a bit by saying that the acidic conjugate predominates in media with a pH value that's lower (“more acidic”) than the pKa of the drug, and that the basic conjugate predominates in media with a pH value that's higher (“more basic”) than the pKa.
1. The 50:50 point is when the pH of the media is equal in value to the pKa of the acid.
2. When the pH of the medium is two pH units below (“more acidic than”) the pKa of the acid, you are 99.01% in acidic form. Remember the rule: The acidic conjugate is favored in media “more acidic than” the pKa. If the acidic form of the drug is ionized, that means your drug is almost totally ionized. If the acidic form of the drug is molecular, it means your drug is almost totally unionized.
Of course, if your medium is two pH units below the pKa of the acid, your drug would be only 0.99% in conjugate base form. Whether that's ionized or unionized depends on the chemical structure of the starting acid and its conjugate base. Remember that molecular acids have anionic (−1) conjugate bases, and cationic (+1) acids have unionized conjugated bases.
3. When the pH of the medium is two pH units above (“more basic than”) the pKa of the acid, you are 99.01% in the conjugate base form. Again, the rule states that: The basic conjugate is favored in media “more basic than” the pKa. If the basic conjugate is anionic, your drug would be almost totally ionized. If the basic conjugate is molecular, your drug would be almost totally unionized.
An ionization (or titration) curve is simply a plot which shows how the percent of a given drug existing in ionized form varies with the pH of the medium. To draw an ionization curve for any compound with acid-base character, you must first know whether the compound is cationic or molecular in acid form, which then also tells you what the conjugate base is. You can get this information either by looking at the structure or from the name of the drug salt. Once you have this information, simply do the following
Graph your first data point by finding the pH value that is equal to the pKa of the acid, and put your data point at 50% ionized.
Next find the pH value that is 2 pH units below (“more acidic than”) the pKa value. If your acid is unionized in acidic form, put a data point close to 0% ionized. If your acid is cationic in acid form, put this data point close to 100% ionized.
Finally, find the pH value that is 2 pH units above (“more basic than”) the pKa value. If your acid is unionized in acidic form the conjugate base will be anionic, so put your third data point close to 100% ionized. If your acid is cationic in acid form the conjugate base will be free, and your third data point would be close to 0% ionized.
Connect these three data points and complete the curve by extending the “ends” of this graph out towards both 0% and 100% ionized, as appropriate.
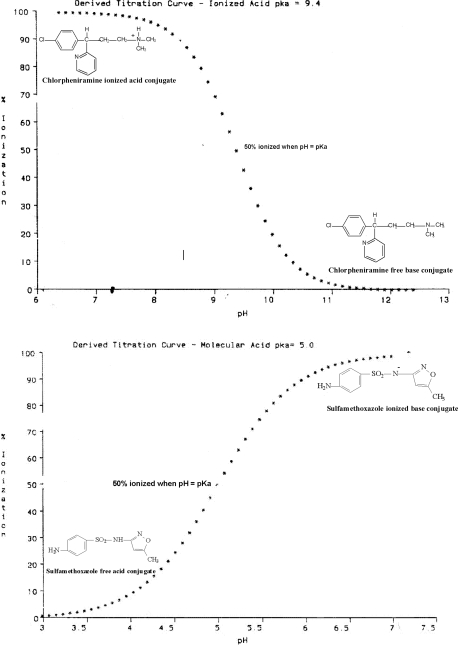
Check out the examples of the titration curves shown in Figure 4 for cationic acids like chlorpheniramine maleate and molecular acids like sulfamethoxazole. Specifically note how, on both curves, when the pH of the medium is equal in value to the pKa of the acid, the compound is 50% ionized. Also note that when you are 2 pH units above or below the pKa, the compound is almost entirely (99.01%) in the basic or acidic conjugate form, respectively. Whether these forms are ionized or unionized depends, of course, on the type of acid you have (molecular or cationic).
Figure 4.
Titration curves of chlorpheniramine and sulfamethoxazole.
All cationic acid ionization curves will have the same shape as chlorpheniramine. What will change is where the curve lies on the graph. This will be dictated by the pKa of the acid because that always tells you where the “50% ionized” point is. Likewise, all molecular acid ionization curves will have the same shape as sulfamethoxazole but, again, where the curve lies on the graph will be dictated by the pKa.
And your point is…?? No doubt, the burning question in your mind right now is…So what?! What is the real value of knowing how much of a drug is ionized or unionized at any given moment? The answer is, because each conjugate has a different role to play in promoting drug action. Let's start with a discussion of the role of each conjugate in vivo.
The unionized conjugate is usually highly lipophilic and this promotes drug action by: 1) increasing transport across biological membranes (including the blood brain barrier), 2) promoting absorption into the general circulation from sites of administration, and 3) inhibiting excretion, as unionized forms can be reabsorbed across renal tubular membranes. For these reasons, the unionized form is considered the absorbable form.
The ionized conjugate is usually hydrophilic, and this promotes drug action by: 1) promoting a wide systemic distribution through the bloodstream and polar extracellular fluids, 2) establishing molecular contact with membranes, which is a requirement for efficient penetration, and 3) promoting drug excretion, as these compounds are soluble in urinary water. For these reasons, the ionized form is considered the excretable form.
Remember that any drug with acid-base character in aqueous media will always exist simultaneously in both ionized and unionized forms, and that the amount of each conjugate present at any moment will depend on the pKa of the acid and the pH of the medium. We can't change the pKa of a given drug because it is totally dependent on chemical structure, but we can change the pH of some body fluids (within narrow limits) to influence the relative amounts of ionized and unionized drug in order to accomplish therapeutic purposes. This is most commonly done with urine to promote excretion in the case of overdose. Sometimes it can be done with gastric pH to influence the rate of absorption of drug from the stomach.
Example 1: Let's say that you have a patient who has overdosed on phenobarbital. Phenobarbital is an unionized acid with a pKa of 7.4, and, therefore, the conjugate base is anionic. The patient's urinary pH is 5.4. In order to speed recovery, would you want to increase urinary pH (e.g., to 6.4) or decrease pH (e.g., to 4.4)? In other words, would you want to promote a more basic urine or a more acidic urine?
To solve this problem, first conceptualize what you want to happen. Then, work through the Henderson-Hasselbalch equation to ensure that what you conceptualize matches what you calculate. If the two don't jive, check your math! If your math is correct, recheck your thinking about the acid-base concepts.
Conceptually
The patient needs rapid excretion of the phenobarital
The excretable form is ionized
The ionized conjugate of phenobarbital is basic
The basic conjugate will predominate in the more basic media
Therefore, you want to basify the urine to treat the phenobarbital overdose
Mathematically
pH – pKa = log [:B]/[H:B] (always)
pH − pKa = log i/u for compounds that are unionized acids and anionic bases
at pH 5.4: pH – pKa = −2 = log i/u: i/u = 1/100 (Starting point)
at pH 4.4: pH – pKa = −3 = log i/u: i/u = 1/1,000 (↑ absorption)
at pH 6.4: pH − pKa = −1 = log i/u: i/u = 1/10 (↑ excretion)
(The increased excretion at pH 6.4 is, of course, compared to the starting pH).
Now you try it. A patient is brought to the emergency room of your hospital having overdosed on heroin free base. The ionized conjugate acid of heroin has a pKa of 9.9. The patient's urinary pH is currently 5.9. As the chemist of the health care team, it is your responsibility to know what to do. Would raising urinary pH to 6.9 or decreasing pH to 4.9 best help this patient recover?
Urinary pH Modifiers
Sodium bicarbonate (NaHCO3) is commonly used to basify urine (increase pH), and ammonium chloride (NH4Cl), ascorbic acid and cranberry juice can all be used to acidify urine. A variety of antacids can be used to raise gastric pH [e.g., Al(OH)3, Mg(OH)2].
Salts of Molecular Acids and Bases
A salt is an ionic crystal comprised of multiple ions (most commonly 2) with balancing charge. A basic salt is an ionized base. An acidic salt is an ionized acid. The term “ionized salt” is redundant. Because salts are ionic, they are usually highly water soluble. Important rule: No ion goes unpaired. Every cation in a salt form must be accompanied by an anion.
Salts of Molecular Acids: There are several unionized acidic functional groups found on organic drug structures, including those drawn in Figure 5 (with an arrow pointing to the acidic hydrogen). All are relatively weak, and usually have pKa values between 3 (carboxylic acid) and 11 (phenol). While exceptions are known, sulfonamides and imides most commonly have pKa values between 5 and 8. Since all of these functional groups are weak acids, they reluctantly relinquish their proton in aqueous media. In order to form their ionized conjugate bases (their salt forms), they must be induced to relinquish proton by reaction with a strong base. The strong bases used to form basic salts from molecular acids are the hydroxides of the alkali and alkaline earth metals [NaOH, KOH, Ca(OH)2, Mg(OH)2 and Al(OH)3].
Figure 5.
General structure of molecular acids and bases.
In general, the formation of basic salts occurs in the fashion shown in Figure 6. The hydroxide from the metal hydroxide plucks the ionizable hydrogen (proton) from the molecular acid, forming water. The anionic conjugate base of the original acid is generated (:B-), and its charge is balanced by the metallic cation (M+). The anionic conjugate base and the metallic cation go together to form the salt crystal. This is shown in Figure 6 in general terms, and using fenoprofen as a specific molecular acid.
Figure 6.
Formation of salts from molecular acids (A) and bases (B).
Note the equilibrium established between conjugate forms and the balance of charge in the equations shown in Figure 6. Because it is ionized, calcium fenoprofen is water soluble. Because it is anionic, it can accept proton and, therefore, it is basic. It is a water soluble, basic salt.
As mentioned, salts are most commonly composed of two ions and, therefore, have two words in their name. To name basic salts, you simply couple the name of the metal ion that came from the ionizing strong base with the name of the original molecular acid (e.g., calcium fenoprofen). All drugs named this way will be ionized, water soluble, and basic in this form. The metal is your big clue in identifying basic salts…look for it!
Here are some examples of marketed basic salts. Can you identify what metal hydroxide formed them and the name of the original molecular acid drug?
Sodium sulfisoxazole (made from sulfisoxazole free acid and NaOH)
Potassium PenV (made from Penicillin V free acid and KOH)
Aluminum aspirin (made from aspirin free acid and Al(OH)3)
The Henderson-Hasselbalch equation for all basic salts takes the same form (and you should know what that is):
Salts of Molecular Bases: There are quite a few unionized acid functional groups but only one unionized basic functional group…the amine (Figure 5). Amines contain a nitrogen atom with a lone pair of electrons, and have pKa values that generally range between 9 and 11. They willingly accept proton from both inorganic and organic “feeder acids” to form their ionized acid conjugates (i.e., acid salts).
The inorganic acids used to make acid salts include HCl, HBr, H2SO4, H3PO4, and HNO3. Organic acids commonly used to make acid salts include maleic, fumaric, tartaric, succinic, and citric (the structures of these acids are provided in the lesson handout).
When making acid salts, the lone pair of electrons on the unionized base takes the proton from the “feeder acid” to make the salt. When the unionized amine accepts H+ it becomes cationic (remember…unionized bases have cationic conjugate acids), and this positive charge is balanced by the negatively charged counterion of the feeder acid that remains after the proton transfer. Unlike with the preparation of basic salts, no water is formed during this reaction. See Figure 6 for an example using pseudoephedrine as the unionized base.
Again, note the equilibrium established between conjugate forms and the balance of charge in the equations shown in Figure 6. For obvious reasons, the cationic conjugate acids of unionized amines are often referred to as “protonated amines.” Because it is ionized, pseudoephedrine hydrochloride is water soluble. Because it is cationic and contains an ionizable hydrogen, it can donate proton (H+) and, therefore, it is acidic. It is a water soluble, acidic salt.
To name acid salts, combine the name of the original unionized base with a modified name of the “feeder acid” used to make the salt. The modified name is the name of the anionic counterion....in other words, the piece of the original “feeder acid” that is left after it donates its proton to the molecular base. For example:
Morphine sulfate (made from morphine free base and sulfuric acid)
Triplennamine citrate (made from triplennamine free base and citric acid)
Physostigmine maleate (made from physostigmine free base and maleic acid)
Levorphanol tartrate (made from levorphanol free base and tartaric acid)
Other common counterion names include hydrobromide, nitrate, phosphate, fumarate, and succinate. Compounds named like pseudoephedrine hydrochloride or the other acid salts above will be ionized, water soluble, and acidic in this form.
The Henderson-Hasselbalch equation for all acidic salts takes the same form (and you should know what that is):
Neutral Functional Groups
Some functional groups on drug structures can neither donate nor accept proton; in other words, they have no acid-base character. We call these functional groups neutral. Most neutral functional groups are unionized, for example aldehydes, ketones, esters, amides, alcohols, tertiary sulfonamides, and tertiary imides (structures provided in the lesson handout). Only one, the quaternary ammonium salt, is ionized (as you could probably tell from the fact that the word “salt” is in its name). Because they lack an ionizable hydrogen atom or the chemical features needed to take it on, all neutral functional groups remain constant in aqueous media regardless of what we do to the pH. There are some exceptions to this last statement, but those exceptions do not relate to proton transfer and the acid-base equilibrium we've been discussing.
Cationic Neutral Functional Groups
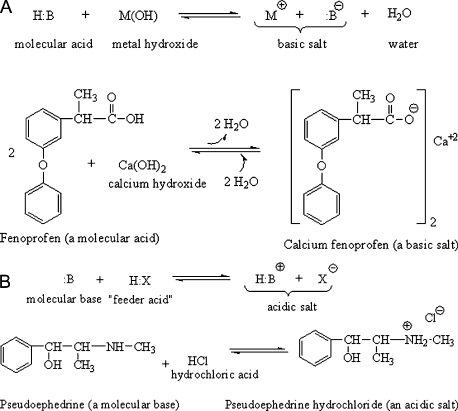
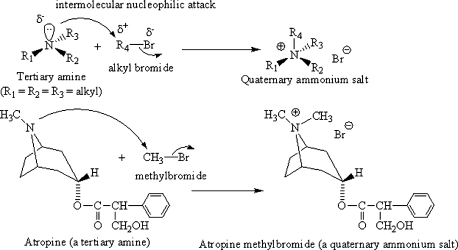
The quaternary ammonium salt is made by reacting a tertiary amine (which has three non-hydrogen groups attached to the nitrogen) with an alkyl halide, which provides the fourth alkyl group. The halogen most commonly utilized to make quaternary ammonium salts is bromine (Figure 7).
Figure 7.
Synthesis of quaternary ammonium salts.
The bromine atom is electron withdrawing and pulls electrons away from the alkyl group it is attached to, leaving the halogen with a partial negative charge (δ−) and the alkyl group with a partial positive charge (δ+). The lone pair of electrons on the tertiary amine is, of course, δ−, and it is attracted to the δ+ alkyl group. The lone pair attacks the alkyl group of the alkyl halide in a process called intermolecular nucleophilic attack. This term refers to the fact that the nucleophilic (positive charge loving) lone pair of electrons on one molecule (the amine) aggressively seeks out the partial positive charge of the alkyl group on a second molecule (the alkyl halide). The nucleophilic attack forms a new covalent bond between the nitrogen of the amine and the alkyl group of the alkyl halide. Remember that carbon can only tolerate four covalent bonds. Important rule: When you make a bond, you break a bond.
As the rule states, when the new covalent bond forms, one must break, and the one that is vulnerable is the bond between the alkyl group R4 and the halogen (Br in this case). Remember that electronegative bromine was pulling strongly on the pair of electrons it was sharing with the alkyl group. Because Br is large and electron withdrawing, it can handle the full negative charge it acquires when the bond breaks and both of those electrons leave with it. Since R4 loses its electron, it attaches to the amine with a positive charge; hence the entire quaternary ammonium group becomes positive. The halide anion remains as a counterion to balance this charge and form the salt (an ionic crystal of balancing charge).
Convince yourself that quaternary ammonium salts are neither acidic nor basic. To be acidic, the nitrogen would have to be: 1) cationic with four bonds attached to it, and 2) attached directly to at least one hydrogen atom. While the quaternary ammonium salts drawn below certainly meet the first criterium, they do not meet the second. All groups directly attached to the nitrogen atoms are alkyl, so there is no ionizable hydrogen capable of leaving as proton. Since they are not proton donors, they are not acids. Likewise, the lone pair that made the original tertiary amines basic has been given to the new alkyl group. These two electrons are now the shared pair of electrons between nitrogen and R4 (in the general example) or CH3 (in the atropine example). Without a lone pair of electrons, the compounds cannot accept proton. Therefore, they are not bases. And if a compound is not an acid and is not a base, it is neutral.
Unlike acidic or basic salts, which exist in solution in equilibrium with their conjugate species, there is no equilibrium between a quaternary ammonium salt and the tertiary amine it came from. We cannot draw a reverse arrow in the above reaction as we did in the formation of acidic and basic salts. Carbon does not dissociate from nitrogen like hydrogen does, so once the quaternary ammonium salt forms, that's it…end of story. It remains in ionized, water-soluble, quaternary salt form at all pH values, and you can take that chemical fact to the bank!
It is easy to distinguish neutral quaternary ammonium salts from acidic protonated amines by both structure and by name. By structure, both have cationic nitrogen atoms with four bonds but, in a protonated amine, one or more of those bonds will involve a hydrogen atom. In a quaternary ammonium salt, all of the bonds will be directly attached to carbon atoms.
By name, the quaternary ammonium salts have the name of the original tertiary amine coupled with the name of the alkyl halide used to make the salt. Protonated amines, on the other hand, couple the name of the original amine (which can be primary, secondary or tertiary) with the name of the “feeder acid” used to make the salt. If a halogen-containing acid was used as the feeder acid (e.g., HCl, HBr) the phrase hydro appears in the name of the acid salt, indicating that a hydrogen was picked up by the basic amino nitrogen…a hydrogen that can be donated back again as proton (e.g., the salt is acidic). If a halogen-containing alkyl halide was used to make the quaternary ammonium salt, the name of the alkyl group (e.g., methyl) that was picked up the by basic amino nitrogen is named. For example:
Atropine methylbromide (a quaternary ammonium salt: neutral)
Atropine hydrobromide (a protonated amine salt: acidic)
Atropine sulfate (a protonated amine salt: acidic)
The generic names of many quaternary ammonium salts have a clue in them…they end with “ium” or “onium”. For example, decamethonium bromide, demecarium bromide, clindinium bromide, and hexamethonium bromide are all quaternary ammonium salts. They are all neutral, ionized, and water soluble at all pH values.
Caution: Don't confuse the “ium” ending of some quaternary amines with the “ium” found at the end of the names of metallic elements like sodium, potassium, calcium, magnesium, and aluminum. If you see a metal in the name of a salt, it is a basic salt for the reasons we've already discussed.
Acid-Base Incompatibility in Solution
The aqueous solubility of salts is dependent on such factors as solution temperature, pH, saturation, and crystal structure polymorphism.5 Therapeutic salts marketed for aqueous injection are commonly made by reacting weak unionized organic acidic or basic drugs with strong bases (e.g., a metal hydroxide) or strong acids (e.g., mineral acids), respectively, and are formulated to be water soluble at room temperature within a narrow, physiologically compatible pH range. The science of solubility can be complex, however, and molecules with multiple acid-base and/or hydrogen-bonding functional groups, or which exist in several distinct polymorphic forms, can present a challenging picture to pharmaceutical scientists designing injectable formulations with desirable solubility and stability properties.
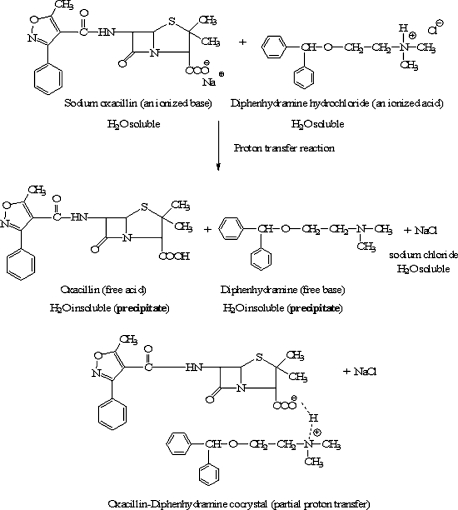
While recognizing the inherent complexity of intra- and intermolecular acid-base interactions in aqueous media, the acidic or basic properties of therapeutic salts can still be a predictable source of potential drug incompatibility in solution. Ionized acids and bases, if mixed together in water, have the capacity to interact in a proton transfer reaction. If the ionized acid donates proton to the base, it would convert to its conjugate base. Since this conjugate base would be unionized and water insoluble, it would precipitate out of solution. Likewise, if the ionized base in solution accepts a donated proton, it would form its unionized conjugate acid, which also is water insoluble and would precipitate out of solution. If the acidic and basic salts interact in a partial transfer of proton, a species called a cocrytal would be generated.6 In either case, the two counterions left behind would associate with each other, but stay ionized and water soluble. [A demonstration to exemplify compatibility, or lack thereof, when drug salts are mixed together in aqueous solution is done in class]
This salt incompatibility concept is best illustrated with a structurally-based example (Figure 8). Sodium oxacillin (an injectable antibiotic) and diphenhydramine hydrochloride (an injectable antihistamine) could conceivably be mixed in the same syringe or placed in the same solution for IV administration. However, since sodium oxacillin is readily recognized both by structure and by name as an ionized base, and diphenhydramine hydrochloride is readily recognized as an ionized acid, you would predict a potential acid-base compatibility problem and hold back from co-administering these two drug salts. Specifically, the acidic proton from the antihistamine could be donated to the solution and taken up by the anionic carboxylate group of the antibiotic. The conjugate species of each would then form and, since these species are unionized, they would be water insoluble and precipitate out in the syringe or in the IV line. Either way, that's bad news for the patient, as no drug will be delivered. In addition, there would be a risk of injecting particulate matter into the bloodstream if the drugs were being given IV. The sodium counterion from the antibiotic and the chloride counterion from the antihistamine would now associate with each other to form the common salt sodium chloride, and this would remain in solution.
Figure 8.
Complete and partial proton transfer reactions.
There is no equilibrium arrow for this reaction because, given time, the proton transfer reaction would go to completion. The products precipitate out of solution and, in solid form, cannot react with each other to re-form the original ionized species. Remember from your Organic Chemistry courses that removal of the products from any chemical reaction (including by precipitation) drives the reaction forward.
As the chemists of the health care team, it is your responsibility to know when an acid-base incompatibility is possible, and to advise against inappropriate or dangerous admixtures until you've had a chance to check on the clinical significance of a possible interaction. There are times when a predicted incompatibility does not occur in practice. The lack of clinical significance of a predicted proton transfer reaction may relate to the following:
1. The unionized conjugates have many polar functional groups capable of hydrogen bonding with water, which would allow the unionized forms to remain soluble. For example, aminoglycoside antibiotics like gentamicin are water soluble in both free base and acidic salt forms.
2. The concentration of the drugs in solution is very dilute. In this case, even though a proton transfer reaction is possible, the reaction does not occur in the time it takes to administer the agent and/or the amount of unionized conjugate that forms is so low that it remains soluble.
3. The solution is buffered to maintain a pH that allows the agent(s) to remain in ionized, water soluble form.
To wrap up this lesson, predict whether the following pairs of water soluble drugs would be compatible (able to be delivered safely) or incompatible (potentially engage in a proton transfer reaction and precipitate) if mixed together in solution. You should be able to identify the components of any predicated precipitate and identify the salt that stays soluble in the water.
buprenorphine sulfate and carphenazine maleate (Compatible or Incompatible?)
decamethonium bromide and sodium phenytoin (Compatible or Incompatible?)
calcium leucovorin and bisoprolol fumarate (Compatible or Incompatible?)
loxepin succinate and magnesium salicylate (Compatible or Incompatible?)
REFERENCES
- 1.Roche VF. The chemically elegant proton pump inhibitors: A self-contained, clinically relevant medicinal chemistry lesson. Am J Pharm Educ. 2006;70((5)) doi: 10.5688/aj7005101. Article 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roche VF. Antihyperlipidemic Statins: A self-contained, clinically relevant medicinal chemistry lesson. Am J Pharm Educ. 2005;69((4)) Article 77. [Google Scholar]
- 3.Roche VF, Alsharif NA. Stayin' Alive: Advancing medicinal chemistry by enhancing student responsibility for learning. Am J Pharm Educ. 2002;66((3)):319–28. [Google Scholar]
- 4.Alsharif NZ, Roche VF, Destache C. Teaching medicinal chemistry to meet outcome objectives for pharmacy graduates. Am J Pharm Educ. 1999;63((1)):34–40. [Google Scholar]
- 5.Jones HP, Davey RJ, Cox BG. Crystallization of a salt of a weak organic acid and base: Solubility relations, supersaturation control and polymorphic behavior. J Phys Chem. 2005;109((11)):5273–8. doi: 10.1021/jp045000q. [DOI] [PubMed] [Google Scholar]
- 6.Li ZJ, Abramov Y, Bordner J, Leonard J, Medek A, Trask AV. Solid-state acid-base interactions in complexes of heterocyclic bases with dicarboxylic acids: Crystallography, hydrogen bond analysis, and 15N NMR spectroscopy. J Am Chem Assoc. 2006;128:8199–210. doi: 10.1021/ja0541332. [DOI] [PubMed] [Google Scholar]