Abstract
Administration per os of 2 X 10(6) fluorescent cell-forming units of a human serotype 3 rotavirus (RV-3) protected all of nine gnotobiotic piglets against severe diarrheal disease when they were challenged 10 to 14 days later with 8 X 10(3) fluorescent cell-forming units of virulent wild-type porcine rotavirus (AT/76). The porcine virus was similar antigenically to porcine prototype strain OSU, previously described as antigenically distinct from all four recognized human serotypes. Administration of RV-3 was associated with the development of serum-neutralizing antibody to both RV-3 and AT/76 in piglets that excreted RV-3. Neutralizing antibody levels to RV-3 and AT/76 increased rapidly postchallenge. Vaccinated piglets were not immune to infection with AT/76 but showed no or minimal gastrointestinal symptoms after challenge. Control nonvaccinated piglets that were fed AT/76 developed severe dehydrating diarrhea and low levels of neutralizing antibody to AT/76 alone. The apparent heterologous clinical protection observed in this study could have been predicted from results of in vitro assays. Neutralization tests with reduction of fluorescence focus indicated a one-way cross-reaction between RV-3 and AT/76 such that hyperimmune antiserum to RV-3 neutralized porcine virus to moderate titer, but not vice versa. The results emphasize the importance of neutralizing antibody in protection against disease and the need to determine reciprocal cross-neutralization titers, rather than serotype alone, in order to predict the ability of rotavirus strains to cross protect.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert M. J., Bishop R. F. Cultivation of human rotaviruses in cell culture. J Med Virol. 1984;13(4):377–383. doi: 10.1002/jmv.1890130409. [DOI] [PubMed] [Google Scholar]
- Bohl E. H. Rotaviral diarrhea in pigs: brief review. J Am Vet Med Assoc. 1979 Mar 15;174(6):613–615. [PubMed] [Google Scholar]
- Bohl E. H., Theil K. W., Saif L. J. Isolation and serotyping of porcine rotaviruses and antigenic comparison with other rotaviruses. J Clin Microbiol. 1984 Feb;19(2):105–111. doi: 10.1128/jcm.19.2.105-111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridger J. C., Woode G. N., Jones J. M., Flewett T. H., Bryden A. S., Davies H. Transmission of human rotaviruses to gnotobiotic piglets. J Med Microbiol. 1975 Nov;8(4):565–569. doi: 10.1099/00222615-8-4-565. [DOI] [PubMed] [Google Scholar]
- Coulson B. S., Fowler K. J., Bishop R. F., Cotton R. G. Neutralizing monoclonal antibodies to human rotavirus and indications of antigenic drift among strains from neonates. J Virol. 1985 Apr;54(1):14–20. doi: 10.1128/jvi.54.1.14-20.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
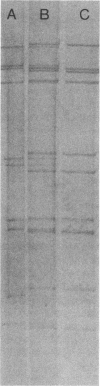
- Dyall-Smith M. L., Holmes I. H. Sequence homology between human and animal rotavirus serotype-specific glycoproteins. Nucleic Acids Res. 1984 May 11;12(9):3973–3982. doi: 10.1093/nar/12.9.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaul S. K., Simpson T. F., Woode G. N., Fulton R. W. Antigenic relationships among some animal rotaviruses: virus neutralization in vitro and cross-protection in piglets. J Clin Microbiol. 1982 Sep;16(3):495–503. doi: 10.1128/jcm.16.3.495-503.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H., McAuliffe V., Valdesuso J., Wyatt R., Flores J., Kalica A., Hoshino Y., Singh N. Serological analysis of the subgroup protein of rotavirus, using monoclonal antibodies. Infect Immun. 1983 Jan;39(1):91–99. doi: 10.1128/iai.39.1.91-99.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Lazdins I., Sonza S., Dyall-Smith M. L., Coulson B. S., Holmes I. H. Demonstration of an immunodominant neutralization site by analysis of antigenic variants of SA11 rotavirus. J Virol. 1985 Oct;56(1):317–319. doi: 10.1128/jvi.56.1.317-319.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin T. J., Tzipori S. Inexpensive techniques for the production and maintenance of gnotobiotic piglets, calves and lambs. Aust Vet J. 1980 Aug;56(8):353–358. doi: 10.1111/j.1751-0813.1980.tb09558.x. [DOI] [PubMed] [Google Scholar]
- Mebus C. A., Wyatt R. G., Sharpee R. L., Sereno M. M., Kalica A. R., Kapikian A. Z., Twiehaus M. J. Diarrhea in gnotobiotic calves caused by the reovirus-like agent of human infantile gastroenteritis. Infect Immun. 1976 Aug;14(2):471–474. doi: 10.1128/iai.14.2.471-474.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberton D. M., Harrison M., Hosking C. S., Adams L. C., Bishop R. F. Rapid diagnosis of rotavirus infection: comparison of electron microscopy and enzyme linked immunosorbent assay (Elisa). Aust Paediatr J. 1979 Dec;15(4):229–232. doi: 10.1111/j.1440-1754.1979.tb01235.x. [DOI] [PubMed] [Google Scholar]
- Rodger S. M., Schnagl R. D., Holmes I. H. Biochemical and biophysical characteristics of diarrhea viruses of human and calf origin. J Virol. 1975 Nov;16(5):1229–1235. doi: 10.1128/jvi.16.5.1229-1235.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Inaba Y., Shinozaki T., Fujii R., Matumoto M. Isolation of human rotavirus in cell cultures: brief report. Arch Virol. 1981;69(2):155–160. doi: 10.1007/BF01315159. [DOI] [PubMed] [Google Scholar]
- Snodgrass D. R., Ojeh C. K., Campbell I., Herring A. J. Bovine rotavirus serotypes and their significance for immunization. J Clin Microbiol. 1984 Sep;20(3):342–346. doi: 10.1128/jcm.20.3.342-346.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. R. Immunoperoxidase techniques: practical and theoretical aspects. Arch Pathol Lab Med. 1978 Mar;102(3):113–121. [PubMed] [Google Scholar]
- Tzipori S. R., Makin T. J., Smith M. L. The clinical response of gnotobiotic calves, pigs and lambs to inoculation with human, calf, pig and foal rotavirus isolates. Aust J Exp Biol Med Sci. 1980 Jun;58(3):309–318. doi: 10.1038/icb.1980.31. [DOI] [PubMed] [Google Scholar]
- Tzipori S., Williams I. H. Diarrhoea in piglets inoculated with rotavirus. Aust Vet J. 1978 Apr;54(4):188–192. doi: 10.1111/j.1751-0813.1978.tb02447.x. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Isolauri E., D'Hondt E., Delem A., André F. E., Zissis G. Protection of infants against rotavirus diarrhoea by RIT 4237 attenuated bovine rotavirus strain vaccine. Lancet. 1984 May 5;1(8384):977–981. doi: 10.1016/s0140-6736(84)92323-7. [DOI] [PubMed] [Google Scholar]
- Whitby H. J., Rodgers F. G. Detection of virus particles by electron microscopy with polyacrylamide hydrogel. J Clin Pathol. 1980 May;33(5):484–487. doi: 10.1136/jcp.33.5.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woode G. N., Bew M. E., Dennis M. J. Studies on cross protection induced in calves by rotaviruses of calves, children and foals. Vet Rec. 1978 Jul 8;103(2):32–34. doi: 10.1136/vr.103.2.32. [DOI] [PubMed] [Google Scholar]
- Woode G. N., Kelso N. E., Simpson T. F., Gaul S. K., Evans L. E., Babiuk L. Antigenic relationships among some bovine rotaviruses: serum neutralization and cross-protection in gnotobiotic calves. J Clin Microbiol. 1983 Aug;18(2):358–364. doi: 10.1128/jcm.18.2.358-364.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R. G., Kapikian A. Z., Mebus C. A. Induction of cross-reactive serum neutralizing antibody to human rotavirus in calves after in utero administration of bovine rotavirus. J Clin Microbiol. 1983 Sep;18(3):505–508. doi: 10.1128/jcm.18.3.505-508.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R. G., Mebus C. A., Yolken R. H., Kalica A. R., James H. D., Jr, Kapikian A. Z., Chanock R. M. Rotaviral immunity in gnotobiotic calves: heterologous resistance to human virus induced by bovine virus. Science. 1979 Feb 9;203(4380):548–550. doi: 10.1126/science.216077. [DOI] [PubMed] [Google Scholar]
- Zissis G., Lambert J. P., Marbehant P., Marissens D., Lobmann M., Charlier P., Delem A., Zygraich N. Protection studies in colostrum-deprived piglets of a bovine rotavirus vaccine candidate using human rotavirus strains for challenge. J Infect Dis. 1983 Dec;148(6):1061–1068. doi: 10.1093/infdis/148.6.1061. [DOI] [PubMed] [Google Scholar]