Abstract
Background:
Natalizumab is an antibody directed against integrin α4 that reduces disease activity in patients with multiple sclerosis (MS) by blocking migration of T and B cells into the CNS. The goal of this study was to characterize the effects of natalizumab treatment on cytokine production and expression of activation markers, costimulatory molecules, and trafficking determinants on CD4+ and CD8+ T cells.
Methods:
In a longitudinal study, we investigated the expression of surface makers and cytokine expression on peripheral blood lymphocytes from 28 patients with MS who started natalizumab treatment and were followed for 1 year. A mixed effects model was used to compare pretreatment to on-treatment measurements.
Results:
The frequency of CD4+ T cells producing interferon-γ, tumor necrosis factor, and interleukin (IL)-17 upon anti-CD3 stimulation increased 6 months after initiation of natalizumab treatment and remained elevated throughout the follow-up. The frequency of CD4+ T cells expressing CD25, HLA-DR, and CCR6 ex vivo was increased at one or more time points during treatment. Among CD8+ T cells, the frequency of cells producing IL-2 and IL-17 after stimulation was increased during natalizumab treatment, as was the frequency of CD8+ T cells expressing CD58 and CCR5 ex vivo. The increase in the frequency of activated cells could not be replicated by in vitro exposure to natalizumab.
Conclusion:
Natalizumab treatment increases the percentage of activated leukocytes producing proinflammatory cytokines in blood, presumably due to sequestration of activated cells in the peripheral circulation.
GLOSSARY
- AICD
= activation-induced cell death;
- IFN
= interferon;
- IL
= interleukin;
- mAb
= monoclonal antibody;
- MS
= multiple sclerosis;
- PBMC
= peripheral blood mononuclear cell;
- TGF
= transforming growth factor;
- TNF
= tumor necrosis factor.
Natalizumab is a humanized monoclonal antibody (mAb) against the α4 subunit of the α4β1 (VLA-4) and α4β7 integrins that was shown to reduce measures of disease activity and severity in patients with relapsing-remitting multiple sclerosis (MS).1 The effects of natalizumab have largely been attributed to inhibition of T-cell trafficking into the CNS due to its ability to block interactions between VLA-4 on leukocytes and its ligand VCAM-1 on cerebral endothelial cells.2-4 However, cross-linking of VLA-4 using either VCAM-1 or the CS-1 region of fibronectin, an alternate VLA-4 ligand, results in tyrosine phosphorylation and T-cell costimulation5 and it is likely that integrin α4 initiates a number of immune processes, potentially leading to leukocyte activation and differentiation. Interestingly, experiments in chronic-relapsing experimental autoimmune encephalomyelitis, an animal model of MS, demonstrated that while blockade of VLA-4 prior to clinically overt disease inhibited the onset and severity of disease, treatment during acute disease or in the remission phase was associated with increased antigen-specific T-cell proliferation and interferon (IFN)-γ secretion in peripheral lymph nodes.6,7 Similarly, increased expression of IFN-γ and tumor necrosis factor (TNF) was observed in total peripheral blood mononuclear cells (PBMCs) from patients with MS treated with natalizumab for 6 months.8 The full effects of integrin α4 blockade are thus not fully understood. The goal of this study was to characterize the effects of natalizumab treatment on cytokine production and expression of activation markers, costimulatory molecules, and trafficking determinants on CD4+ and CD8+ T cells in a cohort of 28 patients with MS beginning treatment with natalizumab.
METHODS
Patients.
We followed 28 patients with relapsing-remitting MS (19 women, 9 men; mean age 38.5 years, range: 22–60), who started treatment with natalizumab (Tysabri®; Biogen Idec Inc., Cambridge, MA; 300 mg IV every 4 weeks). Blood was obtained before each infusion at 0, 1, 3, 6, and 12 months of treatment. The median Expanded Disability Status Scale score at study entry was 2.5 (range: 0–6) and the median disease duration was 5 years (range: 0–20). None of the patients was treated with corticosteroids or any other immunomodulatory drugs during natalizumab treatment. Nineteen patients had been on immunomodulatory therapy during the 6-month interval preceding initiation of natalizumab treatment with a minimum washout period of 1 month (interferon-β, 12 patients, mean washout period 1.7 months; glatiramer acetate, 7 patients, 2 months; mycophenolate mofetil, 2 patients, 2.5 months; daclizumab, 1 patient, 3 months). Three patients discontinued natalizumab treatment due to allergic reactions. Blood was not obtained at 13 of the visits. Staining for surface markers was performed in 14 patients at 0, 1, and 12 months, while cytokine expression was analyzed in all patients at each time point. Blood was obtained from eight healthy donors (5 women, 3 men; mean age 34.5 years, range: 21–50) for in vitro experiments. The study was approved by the Institutional Review Board at the Brigham and Women's Hospital, Boston, MA, and all subjects provided written informed consent.
Cell isolation and stimulation.
PBMCs were isolated through density centrifugation on Ficoll-Paque (GE Healthcare, Chalfont St. Giles, UK) within 4 hours of blood collection. Staining for flow cytometry was performed directly on fresh cells ex vivo or after 16 hours stimulation with plate-bound anti-CD3 (1 μg/mL, clone UCHT1, BD Biosciences, San Jose, CA) and anti-CD28 (1 μg/mL, clone 3D10, eBioscience, San Diego, CA) mAbs. Excess PBMCs were cryopreserved in liquid nitrogen.
Staining for flow cytometry.
A total of 105 naïve or stimulated PBMCs were stained with antibodies against surface markers for 20 minutes at 4°C. Intracellular staining was performed for 30 minutes at 4°C in the presence of 0.1% saponin. The combinations of antibodies used are specified in table e-1 on the Neurology® Web site at www.neurology.org. Cells were acquired on a FACS Calibur or an LSR II flow cytometer (BD Biosciences) and analyzed using FlowJo version 8.4.3 (Tree Star). Isotype matched antibodies were used for defining background fluorescence. The results are presented as percentage of cells expressing the various markers within each leukocyte subset (CD4+ or CD8+ T cells). In order to minimize potential temporal artifacts caused by the long time span of the study, a rigorous quality control assessment was performed post hoc. All samples were analyzed by one investigator at the end of the study using stringent criteria for compensation and staining quality and all data not fulfilling these criteria were excluded from final analysis. The numbers listed in the results reflect the samples that passed the quality control criteria and that were included in the analysis.
Cell sorting and real-time PCR.
Frozen cells were thawed, stained with CD4, sorted on a FACS Aria flow cytometric cell sorter (BD Biosciences), and RNA isolated using Qiagen RNeasy micro kit (Qiagen, Valenica, CA). Total RNA was converted to complementary DNA using Taqman reverse transcription reagents (Applied Biosystems, Carlsbad, CA). Quantitative PCR was performed using a 7500 Fast Real-time PCR system (Applied Biosystems). All primers and probes were obtained from Applied Biosystems and used according to standard protocols. A comparative threshold cycle (CT) value was normalized for each sample using the following formula: ΔCT = CT(gene of interest) − CT(GAPDH), and the relative expression calculated using the formula 2−ΔCT.
In vitro proliferation assays.
CD4+ T cells were isolated from PBMCs obtained from healthy donors by negative selection using magnetic beads (Miltenyi Biotech, Bergisch Gladbach, Germany) and stimulated with plate-bound anti-CD3 (clone OKT3, 1 μg/mL) for 72 hours in the presence of increasing concentrations of natalizumab (Tysabri; 1, 15, 100 μg/mL) or equal concentrations of purified human IgG4κ (Sigma Aldrich, St. Louis, MO). Cells were pulsed with 1 μCi [3H]-thymidine (PerkinElmer, Waltham, MA) for 16 hours and incorporation of [3H]-thymidine was measured. A subset of CD4+ T cells was labeled with 5 μM CFSE (Sigma Aldrich) prior to stimulation with anti-CD3. After 5 days, cells were washed, stained with CD4 and 7-AAD (both from BD Biosciences), and immediately acquired on a BD LSR II flow cytometer. Activation-induced cell death (AICD) was calculated as percent diving cells (CFSEdim) that bound 7-AAD. Production of IFN-γ in culture supernatants was measured using a standard ELISA protocol (capture mAb: IFN-γ clone 2G-1, 1 μg/mL; detection mAb: biotinylated IFN-γ clone B133.5, 0.5 μg/mL; Pierce Biotechnology, Rockford, IL).
Statistical analysis.
The treatment effect on the expression of cytokines and surface cell markers was analyzed using a mixed effects model with a random intercept and robust standard errors. For all cytokines, the primary analysis compared each posttreatment measurement to the pretreatment measurement so that the timing of the treatment effect could be estimated. Differences in mRNA expression were tested with an extension of the Wilcoxon test that allows both paired and unpaired measurements.9 To compare IFN-γ secretion and proliferation of cultured T cells, the median expression level of triplicates stimulated using natalizumab and control was selected and compared using a Wilcoxon signed rank test. A two-tailed p value of < 0.05 was considered significant. Given the exploratory nature of this study, no corrections for multiple comparisons were completed. The data analysis for this article was generated using SAS software, version 9.1, of the SAS System for Windows (SAS Institute Inc., Cary, NC) and the statistical package R (R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org).
RESULTS
Effects of natalizumab on T-cell phenotype.
Using a mixed effects model comparing pretreatment to on-treatment phenotype, we observed an increased percentage of CD4+ T cells expressing CD25, HLA-DR, and CCR6 during natalizumab treatment (figure 1, table e-2). The percentage of CCR6+CD4+ cells was increased after 1 month of treatment (p = 0.02) and remained elevated at month 12 (p = 0.003). The percentage of HLA-DR+CD4+ was transiently increased at 1 month (p = 0.04), while the percentage of CD25+CD4+ was increased only at the 12-month visit (p = 0.01). Increased expression of CD25 was present on both naïve CD62Lhi and memory CD62Llow CD4 cell subsets (table e-2).
Figure 1 Surface markers with changed expression on CD4+ (A) or CD8+ (B) T cells during natalizumab treatment
Graph shows the frequency of T cells expressing the different markers before treatment and after 1 and 12 months of treatment in 14 patients followed longitudinally. Box and whiskers plot showing median, 25th/75th percentile, and range. p Values represent comparison between an individual time point and baseline using a mixed effects model. *p = 0.05–0.005; **p = 0.005–0.0005; *****p < 0.00005.
Among CD8+ T cells, natalizumab treatment increased the percentage of cells expressing CD58 and CCR5 (figure 1, table e-2). The percentage of CD58+CD8+ cells was increased after 1 month (p = 0.008) and remained elevated at month 12 (p = 0.01). CCR5+CD8+ was not significantly increased after 1 month of treatment, but was elevated at month 12 (p = 0.0004). The effects of natalizumab on CCR5+ cells were primarily observed on CD45RA−CD27+ and CD45RA−CD27− memory CD8+ T cells (table e-2). The only surface marker for which the frequency was decreased during natalizumab treatment was CXCR3+CD8+ (p = 0.04 at 1 month, p = 0.00004 at 12 months). The decrease in CXCR3 frequency affected CD45RAhiCD27+ and CD45RA−CD27−, but not CD45RA−CD27+ CD8+ T cells (table e-2).
Natalizumab treatment results in an increased frequency of cytokine-producing CD4+ and CD8+ T cells.
Next, we examined the effect of natalizumab treatment on the frequency of cytokine-producing T cells after in vitro stimulation with anti-CD3 and -CD28. The percentage of CD4+ T cells producing IFN-γ, TNF, and IL-17 was increased twofold to threefold at 6 and 12 months compared to pretreatment levels (figure 2, table 1). In addition, the percentage of IL-2-producing cells was transiently increased at 6 months.

Figure 2 Intracellular staining for interferon-γ, tumor necrosis factor, and interleukin-17 on CD4+ T cells
Figure shows representative plots from the same individual before (baseline) and after 12 months of natalizumab treatment.
Table 1 Percentages of CD4+ and CD8+ T cells expressing the different cytokines under study during natalizumab treatment
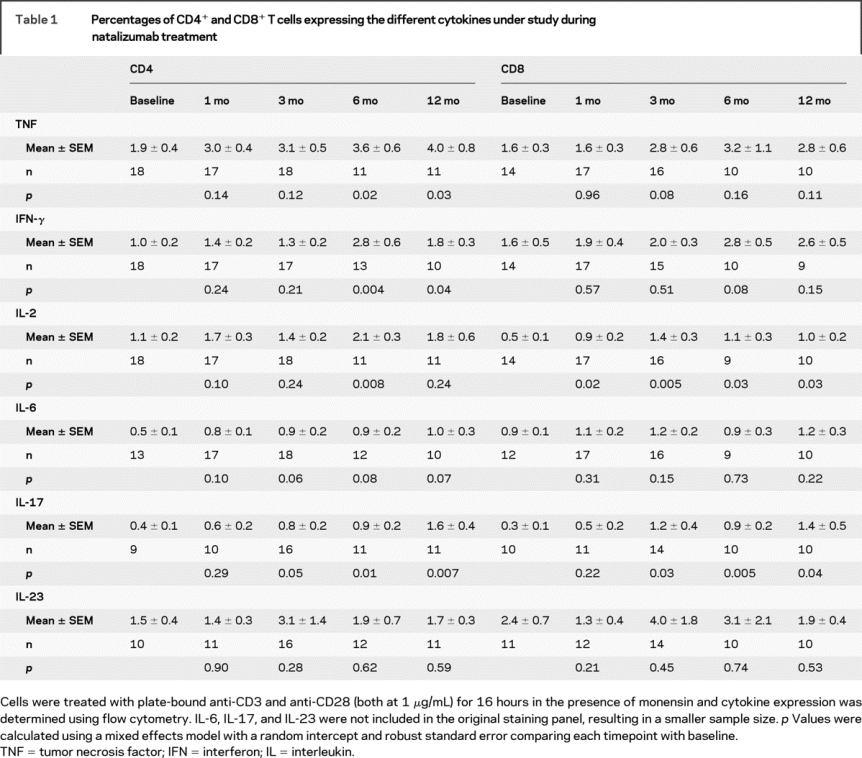
The frequency of IL-2-expressing CD8+ T cells increased after 1 month of natalizumab treatment (table 1) and remained high throughout the follow-up (6 and 12 months). Natalizumab treatment also resulted in increased percentage of IL-17+CD8+ T cells after 6 and 12 months of treatment.
Effects of natalizumab on cytokine mRNA expression on purified CD4+ cells.
To verify some of the findings obtained by flow cytometry, we purified CD4+ T cells from frozen PBMCs, which were available from a subset of patients at baseline, 1 month, and 12 months, and used quantitative rt-PCR to analyze mRNA expression of cytokines and transcription factors associated with a Th1, Th2, or Th17 phenotype. Consistent with the increases in protein levels, treatment with natalizumab resulted in increased expression of IFN-γ and CCR6 mRNA (table 2). IFN-γ mRNA expression increased early after initiation of natalizumab treatment (1 month) and persisted at 12 months, while increased CCR6 mRNA expression was detected only after 1 month of treatment. In addition, we observed decreased mRNA expression of IL-23 and the three transcription factors IRF-1 (related to Th1 cells), GATA3, and STAT6 (both related to Th2 cells) after 1 month of natalizumab treatment.
Table 2 Effects of natalizumab treatment on mRNA expression of cytokines and transcription factors in purified CD4+ T cells
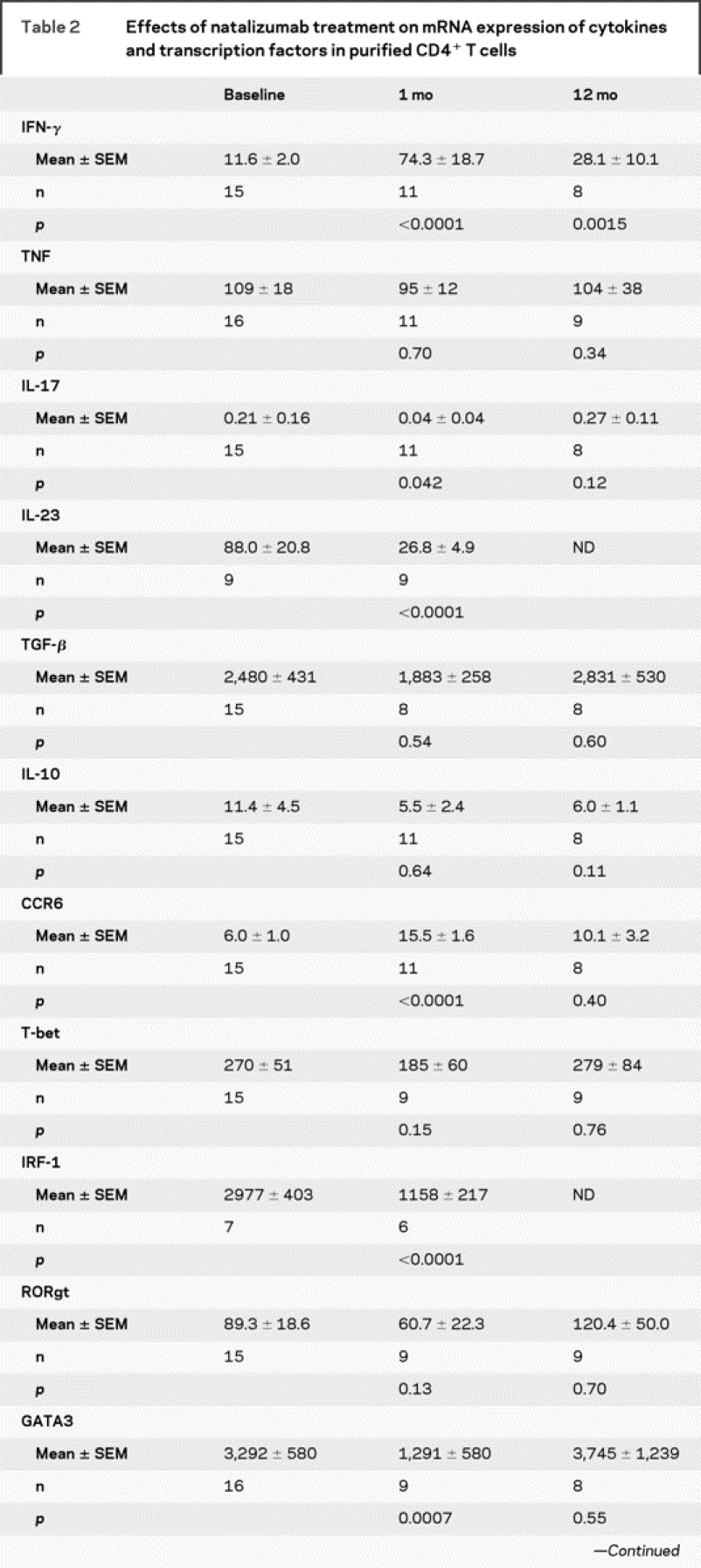
Table 2 Continued

In vitro exposure to natalizumab does not increase proliferation or IFN-γ secretion of cultured CD4+ T cells.
To address whether the observed increase in IFN-γ-producing cells during natalizumab treatment was through direct effects of natalizumab on T cells, we stimulated purified CD4+ T cells with anti-CD3 in the presence of physiologic concentrations of natalizumab. This analysis did not support the hypothesis that natalizumab induces T-cell proliferation. Natalizumab at 15 μg/mL (corresponding to steady-state trough concentration10) and 100 μg/mL (corresponding to maximum serum concentration) reduced thymidine incorporation by 2/3 (p = 0.016 for both comparisons; figure 3A). Natalizumab also caused a dose-dependent reduction in IFN-γ levels with maximum suppression at 15 μg/mL (p = 0.023 for both concentrations; figure 3B).
Figure 3 In vitro effects of natalizumab on proliferation and interferon-γ secretion of purified CD4+ T cells
(A, B) CD4+ T cells were isolated from peripheral blood mononuclear cells from healthy donors and stimulated with plate-bound anti-CD3 (1 μg/mL) in the presence of physiologic concentrations of natalizumab or isotype matched control antibody for 72 hours. The figure shows percent reduction (mean ± SD) in [3H]-thymidine incorporation (A) and interferon-γ secretion (B) in cultures with natalizumab compared to control antibody. (C–F) CD4+ T cells were labeled with 5 μM CFSE, stimulated with anti-CD3 for 5 days, and stained with CD4 and 7-AAD to measure proliferation and activation-induced cell-death (AICD). The figure shows percent CD4+ cells with at least one cell division in cultures treated with 15 μg/mL natalizumab (C) or control immunoglobulin (D). AICD was calculated as percent dead cells stained with 7-AAD in the population of dividing cells (as gated in C and D) in cultures treated with 15 μg/mL natalizumab (E) or control immunoglobulin G (F). The figure shows one representative donor of 10.
We used a CFSE dilution assay to rule out that the observed reduction in proliferation and IFN-γ secretion was due to increased cell death. This showed that 3.6 ± 3.0% (mean ± SD) of CD4+ T cells had divided in cultures treated with 15 μg/mL of natalizumab compared to 14.5 ± 11.3% in control cultures (p = 0.008; figure 3, C and D). Treatment with 100 μg/mL of natalizumab reduced the frequency of dividing cells from 14.1 ± 11.5% to 6.2 ± 5.6% (p = 0.008). The reduction in proliferation was associated with a slight increase in AICD in cultures treated with 15 μg/mL of natalizumab (24.0 ± 10.1% compared to 14.9 ± 11.2% in controls, p = 0.02; figure 3, E and F), but not in cultures treated with 100 μg/mL of natalizumab (20.5 ± 7.2% compared to 13.7 ± 8.3%; p = 0.15).
DISCUSSION
In this study, we observed that patients treated with natalizumab had increased percentage of activated CD4+ and CD8+ T cells expressing proinflammatory cytokines in peripheral blood. This finding could be explained by either inhibited leukocyte extravasation,11 resulting in sequestration of T cells in the peripheral circulation, or by natalizumab-induced T-cell activation through direct or indirect pathways. Ligation of VLA-4 is known to provide costimulatory signals to T cells in vitro5,12 and it is possible that natalizumab treatment could lead to T-cell activation. However, we could not find any evidence for increased proliferation or enhanced IFN-γ secretion when treating CD4+ T cells from healthy donors with natalizumab at concentrations corresponding to serum levels observed in treated patients. This lack of costimulatory effects of natalizumab is consistent with a recent study using immature or mature allogeneic DC to stimulate CD4+ T cells in vitro.13 In contrast to other anti-VLA-4 mAbs, natalizumab was not designed to have biologic effects upon binding, but to physically interfere with VLA-4 interactions with the endothelium,10 which may explain the lack of costimulatory activity observed. In addition, natalizumab may indirectly cause T-cell activation through reactivation of latent viral infections, either by reduced immune surveillance of the CNS or by mobilization of virus-infected cells from the bone marrow, as evidenced by the occurrence of progressive multifocal leukoencephalopathy in a small number of natalizumab-treated patients.14,15 It is possible that natalizumab-treated patients have a higher incidence of low-grade subclinical infections associated with prolonged T-cell activation and high numbers of activated T cells expressing proinflammatory cytokines in peripheral blood in spite of normal infection rates. Another explanation to our findings is that CD4+ T cells are sequestered in the peripheral circulation. While there are no major changes in total leukocyte counts in patients receiving natalizumab,11,16 it is clear that integrin α4 is differentially expressed on various leukocyte subsets, suggesting that natalizumab may preferentially affect trafficking of certain cell types to the CNS.12,13,17 It would have been interesting to study expression of integrin α4 on the different T-cell subsets in our study, but instrument setup was not optimized to accurately reflect changes in fluorescence intensity over the long period of the follow-up.
Interestingly, all T-cell subsets that were increased during natalizumab treatment have been associated with activated cells exerting predominantly proinflammatory effects. Very few resting T cells express CD25, the α-chain of the IL-2 receptor complex, but its expression is quickly upregulated upon activation through the T-cell receptor complex.18 We did not observe increased frequencies of CD25hiCD4+ cells, which in contrast to CD25intCD4+ cells have a regulatory function,19 consistent with recent findings demonstrating that Foxp3+ T regulatory cells were unaffected by natalizumab treatment.13 CD58, found increased during natalizumab treatment, is a costimulatory molecule, which mediates cell adhesion by binding to CD2, resulting in enhanced antigen-specific T-cell activation.20 Single nucleotide polymorphisms in the genes encoding CD25 and CD58 have been linked to MS in a genome-wide association study.21
It is believed that activated antigen-specific T cells produce proinflammatory cytokines, including IFN-γ and TNF, upon reactivation in the CNS, leading to activation of resident cells such as microglia and astrocytes.22 Much interest has recently been focused on IL-17, a cytokine with profound proinflammatory effects, which induces tissue damage during the course of various autoimmune diseases.23 Increased expression of IL-17 has been detected in blood and CSF from patients with active MS and IL-17 mRNA was also present in MS lesions.24-26 Here we report increased frequency of IL-17+ T cells during natalizumab treatment in both CD4+ and CD8+ subsets. CCR6 is a chemokine receptor expressed by memory T cells capable of high IL-17 production,27,28 and we observed an increase in CCR6+ cells in this study. Another interesting subpopulation that was increased during treatment was the CCR5+ population. Circulating CCR5+ T cells isolated from patients with MS are characterized by high expression of IFN-γ and CCR5+ leukocytes were identified in inflammatory MS lesions.29,30 A role for CCR5 in T-cell recruitment to the CNS was suggested by the observation that individuals carrying the nonfunctional CCR5 Δ32 mutation display increased susceptibility to West Nile virus encephalitis, due to failure of leukocyte trafficking to the CNS.31
The observed decrease in CXCR3+CD8+ frequency during natalizumab treatment is more intriguing. CXCR3 is preferentially expressed by Th1 cells expressing high levels of IFN-γ27 and virtually all T cells within perivascular MS lesions are CXCR3 positive.30 Furthermore, CXCR3 expression on CD8+ T cells correlated with measures of MS disease activity on MRI.32 Although CXCR3 initially was believed to be important for T-cell recruitment to the CNS, more recent data suggest that CXCR3-deficient T cells are capable of entering the brain and that CXCR3 may modulate effector functions of T cells, including IFN-γ production, rather than directing migration.33
We did not find any evidence for increased expression of Th2-related cytokines. On the contrary, mRNA expression of GATA3 and STAT6, two transcription factors related to Th2 cytokine expression,34,35 was decreased during natalizumab treatment. This is likely to reflect a predominance of Th1 cells and an increased Th1/Th2 ratio in blood, rather than increased migration of Th2 cells into the CNS during natalizumab treatment.
The aim of this study was to analyze the effects of natalizumab on a panel of markers reflecting various immunologic functions to detect if any of the markers were affected. Given the exploratory nature of this study, we elected not to perform any corrections for multiple comparisons. Although we cannot exclude that some of the observed findings are caused by chance, the consistent increase in frequencies of activated T cells supports a biologic significance of the findings.
The increased frequency of activated T cells in the periphery during natalizumab treatment raises concerns about rebound disease activity once treatment is discontinued. Exacerbated disease activity upon cessation of treatment was observed in mice treated with the small-molecule VLA-4 antagonist BIO 5192.7 Interestingly, treated mice had increased antigen-specific proliferative T-cell responses in spleen and lymph nodes, while delayed-type hypersensitivity responses, an in vivo measure of Th1 trafficking, were significantly decreased. Upon cessation of treatment, enhanced disease was caused by the release of encephalitogenic cells from the periphery leading to rapid accumulation of T cells in the CNS. In patients with MS, two smaller studies have shown increased relapse rates or numbers of active T2 lesions post natalizumab withdrawal.16,36 In contrast, other studies have not found evidence of rebound disease activity after termination of treatment37-39 or in patients no longer responding to natalizumab treatment due to development of neutralizing antibodies.40 The reasons for these discrepancies are unclear, but might be related to length of follow-up and differences in treatment after natalizumab termination.
AUTHOR CONTRIBUTIONS
B.C.H. performed the statistical analysis.
Supplementary Material
Address correspondence and reprint requests to Dr. Samia J. Khoury, Center for Neurological Diseases, Brigham and Women's Hospital, Harvard Institutes of Medicine, Room 710, 77 Avenue Louis Pasteur, Boston, MA 02115 skhoury@rics.bwh.harvard.edu.
Supplemental data at www.neurology.org.
Supported by NIH grants U19 AI46130 and N01 AI05411.
Disclosure: Dr. Viglietta is currently an employee of EMD Serono. Dr. Weiner has received consulting or lecture fees from AutoImmune, Biogen Idec, EMD Serono, Enzo, Genentech, Teva Neuroscience, and Vascular Biogenics, and received grant support from Millennium Pharmaceuticals. Dr. Khoury has received consulting or lecture fees from Argos Therapeutics, Daiichi-Suntory Pharma, EpiVax, LifeCycle Pharmaceutical, PDL BioPharma, Repligen, and Wyeth Pharmaceuticals, and received educational grant support to fund a yearly symposium from Biogen Idec, EMD Serono, and Teva Neuroscience.
Medications: Natalizumab (Tysabri®; Biogen Idec Inc., Cambridge, MA).
Received November 21, 2008. Accepted in final form March 2, 2009.
REFERENCES
- 1.Goodin DS, Cohen BA, O'Connor P, Kappos L, Stevens JC. Assessment: the use of natalizumab (Tysabri) for the treatment of multiple sclerosis (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2008;71:766–773. [DOI] [PubMed] [Google Scholar]
- 2.Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway CA Jr. Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med 1993;177:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brocke S, Piercy C, Steinman L, Weissman IL, Veromaa T. Antibodies to CD44 and integrin alpha4, but not L-selectin, prevent central nervous system inflammation and experimental encephalomyelitis by blocking secondary leukocyte recruitment. Proc Natl Acad Sci USA 1999;96:6896–6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature 1992;356:63–66. [DOI] [PubMed] [Google Scholar]
- 5.Sato T, Tachibana K, Nojima Y, D'Avirro N, Morimoto C. Role of the VLA-4 molecule in T cell costimulation: identification of the tyrosine phosphorylation pattern induced by the ligation of VLA-4. J Immunol 1995;155:2938–2947. [PubMed] [Google Scholar]
- 6.Theien BE, Vanderlugt CL, Eagar TN, et al. Discordant effects of anti-VLA-4 treatment before and after onset of relapsing experimental autoimmune encephalomyelitis. J Clin Invest 2001;107:995–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theien BE, Vanderlugt CL, Nickerson-Nutter C, et al. Differential effects of treatment with a small-molecule VLA-4 antagonist before and after onset of relapsing EAE. Blood 2003;102:4464–4471. [DOI] [PubMed] [Google Scholar]
- 8.Khademi M, Stol D, Olsson T, Wallstrom E. Induction of systemic TNFalpha in natalizumab-treated multiple sclerosis. Eur J Neurol 2008;15:309–312. [DOI] [PubMed] [Google Scholar]
- 9.Brunner E, Puri ML. Nonparametric methods in design and analysis of experiments. In: Ghosh S, Rao CR, eds. Handbook of Statistics, vol. 13. Amsterdam: North-Holland; 1996:81–87. [Google Scholar]
- 10.Stuve O, Bennett JL. Pharmacological properties, toxicology and scientific rationale for the use of natalizumab (Tysabri) in inflammatory diseases. CNS Drug Rev 2007;13:79–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stuve O, Marra CM, Jerome KR, et al. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol 2006;59:743–747. [DOI] [PubMed] [Google Scholar]
- 12.Niino M, Bodner C, Simard ML, et al. Natalizumab effects on immune cell responses in multiple sclerosis. Ann Neurol 2006;59:748–754. [DOI] [PubMed] [Google Scholar]
- 13.Stenner MP, Waschbisch A, Buck D, et al. Effects of natalizumab treatment on Foxp3+ T regulatory cells. PLoS ONE 2008;3:e3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med 2005;353:369–374. [DOI] [PubMed] [Google Scholar]
- 15.Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med 2005;353:375–381. [DOI] [PubMed] [Google Scholar]
- 16.Tubridy N, Behan PO, Capildeo R, et al. The effect of anti-alpha4 integrin antibody on brain lesion activity in MS: The UK Antegren Study Group. Neurology 1999;53:466–472. [DOI] [PubMed] [Google Scholar]
- 17.Stuve O, Marra CM, Bar-Or A, et al. Altered CD4+/CD8+ T-cell ratios in cerebrospinal fluid of natalizumab-treated patients with multiple sclerosis. Arch Neurol 2006;63:1383–1387. [DOI] [PubMed] [Google Scholar]
- 18.Waldmann TA. Anti-Tac (daclizumab, Zenapax) in the treatment of leukemia, autoimmune diseases, and in the prevention of allograft rejection: a 25-year personal odyssey. J Clin Immunol 2007;27:1–18. [DOI] [PubMed] [Google Scholar]
- 19.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol 2001;167:1245–1253. [DOI] [PubMed] [Google Scholar]
- 20.Selvaraj P, Plunkett ML, Dustin M, Sanders ME, Shaw S, Springer TA. The T lymphocyte glycoprotein CD2 binds the cell surface ligand LFA-3. Nature 1987;326:400–403. [DOI] [PubMed] [Google Scholar]
- 21.Hafler DA, Compston A, Sawcer S, et al. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med 2007;357:851–862. [DOI] [PubMed] [Google Scholar]
- 22.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol 2005;23:683–747. [DOI] [PubMed] [Google Scholar]
- 23.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature 2008;453:1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matusevicius D, Kivisäkk P, He B, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler 1999;5:101–104. [DOI] [PubMed] [Google Scholar]
- 25.Lock C, Hermans G, Pedotti R, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med 2002;8:500–508. [DOI] [PubMed] [Google Scholar]
- 26.Graber JJ, Allie SR, Mullen KM, et al. Interleukin-17 in transverse myelitis and multiple sclerosis. J Neuroimmunol 2008;196:124–132. [DOI] [PubMed] [Google Scholar]
- 27.Acosta-Rodriguez EV, Rivino L, Geginat J, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 2007;8:639–646. [DOI] [PubMed] [Google Scholar]
- 28.Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J Exp Med 2007;204:1849–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balashov KE, Rottman JB, Weiner HL, Hancock WW. CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci USA 1999;96:6873–6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorensen TL, Tani M, Jensen J, et al. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Invest 1999;103:807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glass WG, Lim JK, Cholera R, Pletnev AG, Gao JL, Murphy PM. Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J Exp Med 2005;202:1087–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fox RJ, Kivisäkk P, Fisher E, et al. Multiple sclerosis: chemokine receptor expression on circulating lymphocytes in correlation with radiographic measures of tissue injury. Mult Scler 2008;14:1036–1043. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, Huang D, Matsui M, et al. Severe disease, unaltered leukocyte migration, and reduced IFN-gamma production in CXCR3−/− mice with experimental autoimmune encephalomyelitis. J Immunol 2006;176:4399–4409. [DOI] [PubMed] [Google Scholar]
- 34.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 1997;89:587–596. [DOI] [PubMed] [Google Scholar]
- 35.Hou J, Schindler U, Henzel WJ, Ho TC, Brasseur M, McKnight SL. An interleukin-4-induced transcription factor: IL-4 Stat. Science 1994;265:1701–1706. [DOI] [PubMed] [Google Scholar]
- 36.Vellinga MM, Castelijns JA, Barkhof F, Uitdehaag BM, Polman CH. Postwithdrawal rebound increase in T2 lesional activity in natalizumab-treated MS patients. Neurology 2008;70:1150–1151. [DOI] [PubMed] [Google Scholar]
- 37.O'Connor PW, Goodman A, Willmer-Hulme AJ, et al. Randomized multicenter trial of natalizumab in acute MS relapses: clinical and MRI effects. Neurology 2004;62:2038–2043. [DOI] [PubMed] [Google Scholar]
- 38.Miller DH, Khan OA, Sheremata WA, et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2003;348:15–23. [DOI] [PubMed] [Google Scholar]
- 39.Stuve O, Cravens PD, Frohman EM, et al. Immunologic and clinical status 14 months after cessation of natalizumab therapy. Neurology 2009;72:396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calabresi PA, Giovannoni G, Confavreux C, et al. The incidence and significance of anti-natalizumab antibodies: results from AFFIRM and SENTINEL. Neurology 2007;69:1391–1403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




