Abstract
Background:
Hypoxic ischemic brain injury secondary to pediatric cardiac arrest (CA) may result in acute symptomatic seizures. A high proportion of seizures may be nonconvulsive, so accurate diagnosis requires continuous EEG monitoring. We aimed to determine the safety and feasibility of long-term EEG monitoring, to describe electroencephalographic background and seizure characteristics, and to identify background features predictive of seizures in children undergoing therapeutic hypothermia (TH) after CA.
Methods:
Nineteen children underwent TH after CA. Continuous EEG monitoring was performed during hypothermia (24 hours), rewarming (12–24 hours), and then an additional 24 hours of normothermia. The tolerability of these prolonged studies and the EEG background classification and seizure characteristics were described in a standardized manner.
Results:
No complications of EEG monitoring were reported or observed. Electrographic seizures occurred in 47% (9/19), and 32% (6/19) developed status epilepticus. Seizures were nonconvulsive in 67% (6/9) and electrographically generalized in 78% (7/9). Seizures commenced during the late hypothermic or rewarming periods (8/9). Factors predictive of electrographic seizures were burst suppression or excessively discontinuous EEG background patterns, interictal epileptiform discharges, or an absence of the expected pharmacologically induced beta activity. Background features evolved over time. Patients with slowing and attenuation tended to improve, whereas those with burst suppression tended to worsen.
Conclusions:
EEG monitoring in children undergoing therapeutic hypothermia after cardiac arrest is safe and feasible. Electrographic seizures and status epilepticus are common in this setting but are often not detectable by clinical observation alone. The EEG background often evolves over time, with milder abnormalities improving and more severe abnormalities worsening.
GLOSSARY
- BS
= burst suppression;
- CA
= cardiac arrest;
- CPR
= cardiopulmonary resuscitation;
- DD
= developmental delay;
- FEN
= fentanyl;
- FOS
= fosphenytoin;
- HIE
= hypoxic ischemic encephalopathy;
- LEV
= levetiracetam;
- LZP
= lorazepam;
- MDZ
= midazolam;
- NCS
= nonconvulsive seizures;
- NCSE
= nonconvulsive status epilepticus;
- NPV
= negative predictive value;
- PB
= phenobarbital;
- PED
= periodic epileptiform discharge;
- PICU
= pediatric intensive care unit;
- PPV
= positive predictive value;
- SE
= status epilepticus;
- SIDS
= sudden infant death syndrome;
- sz
= seizures;
- TH
= therapeutic hypothermia;
- VEC
= vecuronium;
- VPA
= valproic acid;
- VT
= ventricular tachycardia.
Mortality and neurologic morbidity are high after cardiac arrest (CA) in children.1 Therapeutic hypothermia (TH) may mitigate some of this injury and has been shown to improve outcome in multiple laboratory models, in adults after CA,2,3 and in neonates with hypoxic ischemic encephalopathy.4,5 As a result of these studies, TH is now being used as a neuroprotective strategy in children after CA, although rigorous efficacy data are not yet available.
Recent retrospective studies have demonstrated the high incidence of nonconvulsive seizures (NCS) and nonconvulsive status epilepticus (NCSE) in a variety of critically ill children with acute encephalopathies, including hypoxic ischemic.6–11 Further, patients treated with TH may require pharmacologic paralysis to manage shivering, clinically masking all electrographic seizures. Consequently, EEG monitoring is required for seizure detection. Accurate identification of NCS is important because they are associated with worse outcome,12 may contribute to the burden of brain injury, and may be treated with antiseizure medications.
Formulating an early prognosis in children after CA is important in counseling families and in making thoughtful treatment decisions. In acutely ill normothermic children, the EEG is a good indicator of cerebral cortical function and thalamocortical connectivity. Several EEG features are useful in prognostication.13,14 However, it is unclear whether moderate TH (target core body temperature of 34°C) alters the timing or prognostic significance of these EEG background features. Thus, both the EEG features used for prognosis and their timing may differ in children undergoing TH compared with normothermic children.
The present study was conducted to assess whether EEG monitoring was safe and feasible after CA in children undergoing TH. The second aim was to prospectively determine the incidence of their clinical and electrographic seizures. The third aim was to characterize the sequential evolution of the EEG background during hypothermia, during rewarming, and beyond. Collectively, these data could suggest the possible roles of long-term EEG monitoring.
METHODS
Infants and children treated in the pediatric intensive care unit (PICU) at a tertiary care referral hospital with TH after successful resuscitation from CA were eligible for this study. TH was initiated as clinical treatment at the discretion of the attending PICU physicians. Children treated with TH all experienced in-hospital or out-of-hospital CA, defined as cardiopulmonary resuscitation (CPR) for greater than 60 seconds with return of spontaneous circulation; were encephalopathic (obtunded or comatose) at presentation; and received care in the PICU within several hours of the hypoxic–ischemic event. Patients with traumatic brain injury were excluded. Our PICU's standard hypothermia protocol was used, in which children were surface cooled by a cooling blanket to 34°C for 24 hours and then slowly rewarmed over 12 to 24 hours. This study was approved by the hospital's institutional review board.
Data describing the use of paralytic, sedative, and antiseizure medications were tracked. Medication administration was classified as continuous infusions or intermittent boluses.
Long-term monitoring using a Grass-Telefactor (West Warwick, RI) video-EEG system was initiated as soon as the patient was stabilized in the PICU. On-call registered EEG technologists initiated monitoring within a few hours of the onset of cooling. EEG monitoring was continued with the goal of obtaining at least 72 hours of recording (24 hours of hypothermia, 12–24 hours of rewarming, and 24 hours of normothermia). Twenty-one gold-over-silver scalp surface electrodes were positioned according to the international 10-20 system and affixed with collodion adhesive. EEG data were acquired on a portable bedside monitor networked to the main EEG server, allowing review from multiple sites in the hospital and remotely. Full video-EEG files were stored for later re-review and analysis. Potential complications of EEG monitoring, including disruption to bedside intensive care, skin breakdown, and infections, were assessed daily.
After clinical interpretation during the acute hospitalization, EEG recordings were reinterpreted in a standardized manner by two pediatric neurophysiologists blind to outcome and clinical information, except for patient age. Special attention was directed to the character of the EEG background, the presence of interictal epileptiform discharges (spike/sharp-and-slow waves), and electrographic seizure characteristics. Clinical seizures were recorded by bedside caretakers and by the investigators' review of the coincident video recordings. Clinical seizures were defined as abnormal movements associated with EEG change. Electrographic seizures were defined as abnormal, ictal EEG events lasting longer than 10 seconds with evolution of morphology, frequency, and amplitude, and a plausible electrographic field. EEG seizures were categorized by location of onset, duration, and coincident clinical manifestations. Seizures were considered nonconvulsive if there was no clinical change on simultaneous video. NCSE was defined as a state of impaired consciousness with a single 30-minute electroencephalographic seizure or recurrent independent electroencephalographic seizures totaling more than 30 minutes in a 1-hour period. Timing of seizures was categorized in 12-hour epochs defined as early hypothermia (0–12 hours), late hypothermia (>12–24 hours), early rewarming (>24–36 hours), late rewarming (>36–48 hours), or after rewarming (>48–72 hours). The EEG background was also classified for each epoch as 1) normal for age, 2) mild/moderately abnormal (attenuation, slowing), or 3) severely abnormal (burst suppression, excessive discontinuity).
Descriptive statistics are reported. The Fisher exact test was used to determine the association between interictal EEG descriptors and the presence or absence of electrographic seizures.
RESULTS
Patient population.
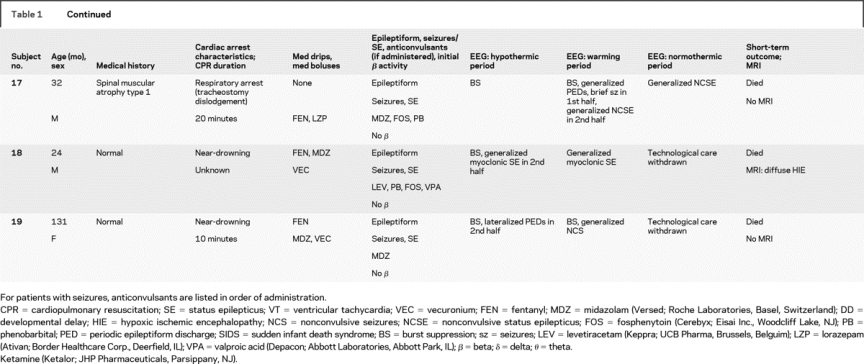
Nineteen patients were studied from March 2007 to September 2008 (table 1). Their median age was 10.7 ± 50 months (range 2.2 months to 16 years). There were 12 boys and 7 girls. Twelve patients were neurologically normal before CA, and 7 had preexisting neurodevelopmental disabilities. Arrest etiologies and CPR durations are listed in table 1. Etiologies included respiratory arrest in 8, near-drowning in 5, near–sudden infant death syndrome in 3, primary cardiac in 2, and anaphylaxis in 1. All patients underwent acute TH, with a mean duration between return of spontaneous circulation and hypothermia initiation of 5.5 ± 2.2 hours (range 3–11 hours).
Table 1 Subject medical history, event description, medications, EEG description, and short-term outcome
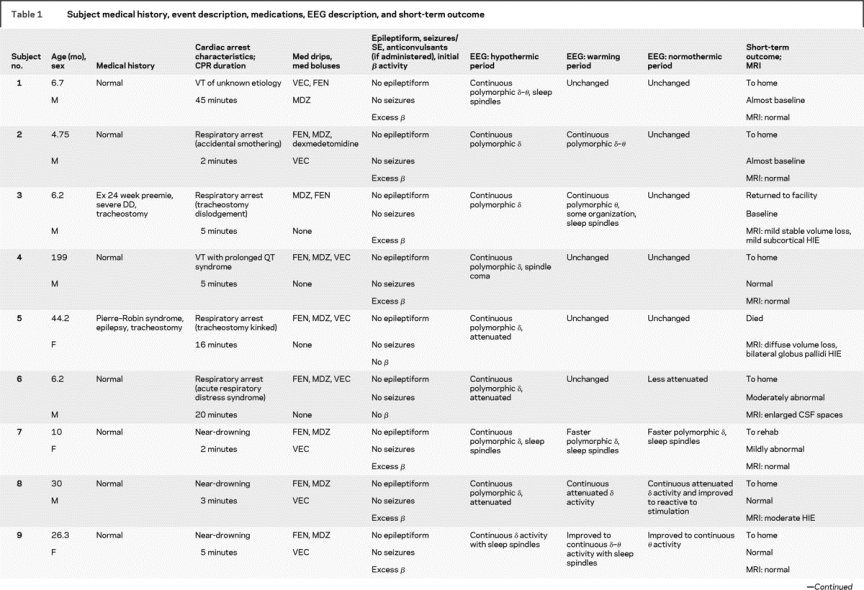
Table 1 Continued
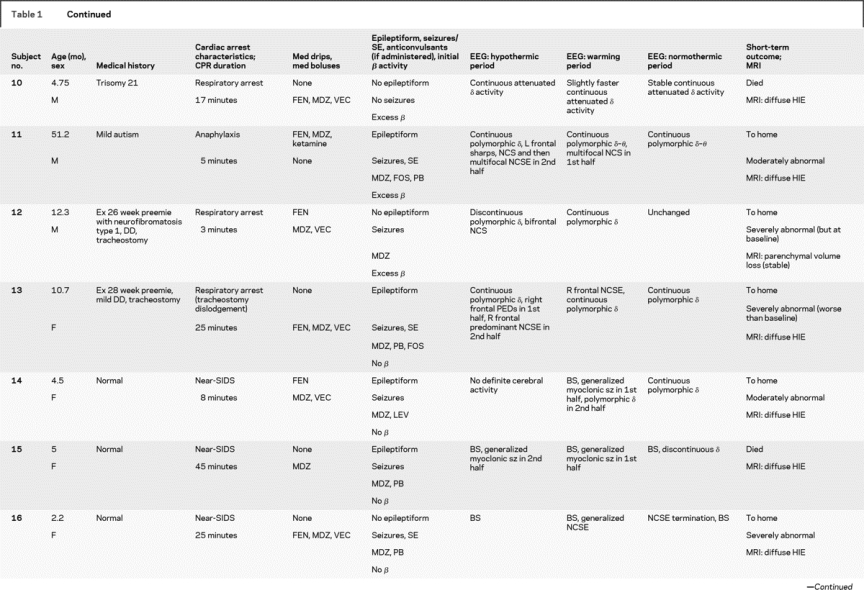
Table 1 Continued
Medication administration.
All patients received benzodiazepines (continuous in 12, boluses in 7). Fifteen underwent therapeutic paralysis with vecuronium (continuous in 4, boluses in 8). Eighteen received fentanyl for sedation.
Video-EEG monitoring.
EEG monitoring began within 5 hours of initiating hypothermia in all children and was continued without difficulty through the entire hypothermia protocol in 16 patients. EEG monitoring lasted a mean of 68 ± 20 hours (range 37–134 hours). A total of 1,291 hours of EEG monitoring were reviewed. Monitoring was discontinued early in 1 patient who seemed to quickly recover and required neuroimaging, and in 2 others after a decision to withdraw technological support. EEG monitoring did not interfere with care provided by bedside personnel. No patients had evidence of skin breakdown, infection, or any other complication related to electrode placement.
Clinical and EEG seizures.
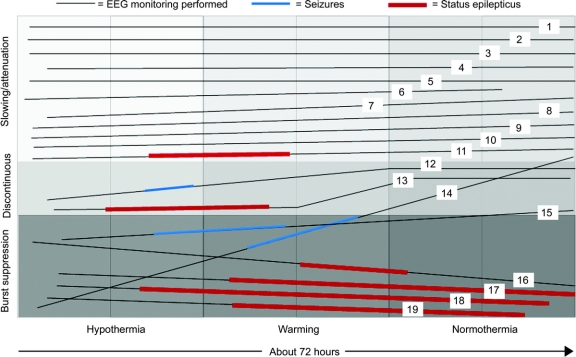
Electrographic seizures arose in 47% (9/19) of patients. Seizures began during the first half of hypothermia in 1, the second half of hypothermia in 4, and during rewarming in 4 patients. No patient had seizure onset in the first 6 hours of monitoring (figure). Electrographic status epilepticus occurred in 32% (6/19). Six patients (32%) had only NCS, and 2 of these had not received neuromuscular blockage. One had myoclonic seizures, and 2 had myoclonic status epilepticus. Electrographically, seizures appeared generalized (either at onset or secondarily after focal onset) in 7 patients. Seizures were focal or multifocal without generalization in 2 patients.
Figure Background evolution and seizure occurrence by subject
Time extends from left to right, demonstrating hypothermic, rewarming, and normothermic periods. EEG background characteristics are most abnormal on the bottom (burst suppression) and least abnormal on the top (attenuation, slowing). Each subject is shown as a line that demonstrates background evolution over time. Dark line segments demonstrate the time during which recurrent independent seizures occurred, and thick dark line segments represent status epilepticus.
EEG background abnormalities.
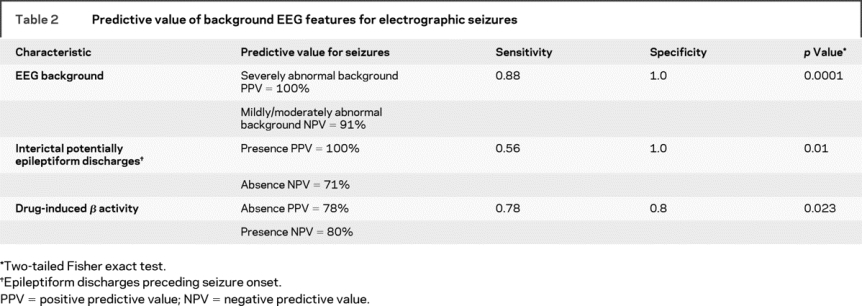
The background EEG patterns during TH consisted of burst suppression in 6 patients or excessive discontinuity in 2, and mild/moderately abnormal slowing/attenuation in 11. Seizures occurred in all 8 patients with a severely abnormal background and in 1 patient with a mildly/moderately abnormal background (figure). The positive predictive value (PPV) and negative predictive value (NPV) of a severely abnormal background (burst suppression or excessive discontinuity) are summarized in table 2. The background pattern evolved during the course of treatment in most patients. None of the 11 patients with an initial slow/attenuated background deteriorated to a discontinuous pattern. Five of 11 remained stable and 6 of 11 improved with subsequent higher voltage and faster frequencies. Of patients with initial burst suppression or excessive discontinuity, 4 of 8 worsened over time and 4 improved, but never to a normal tracing.
Table 2 Predictive value of background EEG features for electrographic seizures
Interictal discharges.
Potentially epileptogenic discharges (focal spikes or sharp waves, periodic lateralized epileptiform discharges, or bilateral periodic epileptiform discharges) occurred in 7 patients, all of whom also had electrographic seizures. Interictal potentially epileptiform discharges preceded the electrographic seizures in 5 of the 7 patients. Seizures occurred in 2 patients without any interictal potentially epileptiform discharges. The PPV and NPV of epileptiform discharges, before seizure occurrence, are summarized in table 2.
Benzodiazepine-induced beta activity.
In most healthy children, it is expected that the administration of benzodiazepines induces fast (beta frequency) EEG activity. All patients received per protocol benzodiazepines during the initial induction of hypothermia. The expected EEG-induced beta activity in the initial 12 hours was seen in 10 patients but was absent in 9. Seizures occurred in 78% (7/9) of those without drug-induced beta activity but only in 20% (2/10) of those with beta activity. The PPV and NPV of drug induced beta activity for seizure occurrence are summarized in table 2.
Short-term outcome.
Five patients died, including 3 with severely abnormal backgrounds and electrographic seizures and 2 with mild/moderately abnormal backgrounds and without seizures. Of the 14 survivors, 4 had severe neurologic morbidity and 10 had mild/moderate/no neurologic disability. The 4 with worse outcome had severe background abnormalities initially. Of the 10 with better outcomes, 1 had an initially severely abnormal background that improved during TH and 9 had mild/moderate background abnormalities initially.
DISCUSSION
This study demonstrates that long-term video-EEG monitoring is feasible and safe in children undergoing TH after CA. It can be initiated quickly, and there were no complications such as skin breakdown or infection related to electrode application or interference with bedside care providers.
Electrographic seizures and status epilepticus are common in children undergoing TH after CA. Most electrographic seizures were not clinically apparent and would have been missed without EEG monitoring. The 47% incidence of seizures is higher than that found in prior reports of EEG monitoring in other critically ill patients. Retrospective studies have demonstrated NCS or NCSE in 14% to 39% of children who underwent long-term EEG monitoring in PICU or emergency departments for a variety of etiologies of acute encephalopathies.6,7,9,10 Retrospective studies may not identify all patients who had seizures, because not all patients underwent EEG monitoring. The current study may have identified a higher incidence of seizures because EEG was performed prospectively on all children undergoing TH. Additionally, these children all experienced hypoxic ischemic brain injury, a known high-risk trigger for NCSE in children,8,10 whereas prior studies combined children with multiple etiologies for brain injury. Most (four of six) patients with exclusively NCS had received some doses of paralytics, but two of the six had not received any suggesting a bona fide physiologic uncoupling between electrographic seizures and clinical seizure manifestations. Further studies with closer attention to the timing and extent of deliberate neuromuscular blockage might reveal its true role in reducing some electrographic seizures to be “subclinical.” No children received cisatracurium, and thus none were exposed to laudanosine, a potential proconvulsant.15
None of the patients had seizure onset in the first 6 hours of TH, and only one had seizure onset in the initial 12 hours. If this finding is replicated in future larger studies, it suggests that in clinical practice initiation of EEG may not be needed immediately after CA and could be delayed several hours. This would make EEG monitoring implementation more feasible. However, time of seizure onset may also relate to the duration of time elapsed between spontaneous return of circulation and TH initiation, which was a mean of 5.5 hours in this study. Seizure onset timing may change as protocols improve and TH is initiated more rapidly. The fact that most seizures were electrographically generalized suggests that limited electrode montages (e.g., only two recording electrodes available on cerebral function monitors) may have a role in screening for NCS in children undergoing TH. These limited montages could be placed by bedside caregivers rather than EEG technologists.
Often, NCS and NCSE cannot be detected by a routine 30-minute EEG recording, and more prolonged monitoring is required.7,8,10 Some prior studies have reported that 95% of NCSE8 and 80% of NCS7 are detected within 24 hours of monitoring. This study suggests that in children undergoing TH, EEG monitoring may be indicated for a longer duration, because seizures often began after the initial 24-hour hypothermia period. Patients with severely abnormal background patterns all had seizures, suggesting that more prolonged monitoring may be needed if these patterns are identified initially. The presence of interictal potentially epileptiform discharges and the absence of expected pharmacologically induced beta activity also predicted seizures. Beta activity is expected when benzodiazepines are administered, so lack of expected beta activity could suggest a greater degree of dysfunction and higher risk of seizures.
Prior reports have also described the occurrence of electrographic and clinical seizures after discontinuation of hypothermia in adults16,17 and neonates.18 This could be due to a TH-induced deferment in some components of brain injury, leading to later seizure occurrence. Alternatively, hypothermia itself could act as a seizure-suppressing therapy such that seizures are postponed until rewarming. An animal model of status epilepticus demonstrated that hypothermia reduced seizure frequency and intensity and that hypothermia with diazepam reduced the amplitude and frequency of epileptiform discharges.19 In four adults, hypothermia to 31° to 35°C for 20 to 61 hours using an endovascular cooling system was reported to terminate status epilepticus refractory to multiple agents, allowing withdrawal of benzodiazepine infusions. Two of the four adults remained seizure free after controlled rewarming over 1.5 to 50 hours.20 A recent study in adults with refractory epilepsy demonstrated a reduction in seizures with head and neck cooling.21 One study demonstrated control of refractory status epilepticus in three children with a combination of hypothermia to 30° to 31°C combined with thiopental.22
Detecting NCS and NCSE is important so that appropriate treatment can be initiated. Clinical studies are consistent with the hypothesis that continuous electrographic discharges, even without clinical seizures, can be harmful. Studies in adults have demonstrated that NCSE duration and time to detection predict outcome in patients with NCSE.12 NCS have been associated with mortality of 32% to 57%, and NCSE has been associated with mortality of 51% to 57%.12,23,24 In adults with traumatic brain injury, intracranial pressure and lactate-to-pyruvate ratios were higher during NCS than between NCS.25 Serum neuron-specific enolase, a marker of neuron injury, has been demonstrated to be elevated in adults with NCSE26 and children with continuous epileptiform discharges.27 Studies in neonates have demonstrated an association between neonatal seizures and poor outcome, especially with more prolonged28–30 and harder-to-control seizures,31 although the confounding interplay between etiology, seizures, and outcome remains unclear32 and studies have not proven that treatment improves outcome.
Differentiating between electrographic seizures and brief or periodic runs of rhythmic sharp waves may sometimes prove difficult. This study defined electrographic seizures as lasting longer than 10 seconds, consistent with other of pediatric7,11 and neonatal33 studies. Still, it is recognized that this definition, although commonly cited, is arbitrary. Terminology is being developed to better describe rhythmic discharges.34
Early prognostic information is important in making treatment decisions, so identifying prognostic EEG patterns early in TH is clinically important. This study demonstrates that the background EEG features do evolve during the course of TH, either improving or worsening, but rarely change from markedly abnormal to normal/mildly abnormal. The stability of the background suggests that prognostication may be possible based on EEG findings early in the course of TH, or possibly even before TH is started to identify patients most likely to benefit. The stability of the background as the patients are rewarmed to normothermia suggests that TH alone is not responsible for the background abnormalities. In future studies of EEG monitoring during TH, it might be possible to initiate a limited array EEG montage in some patients before and during initial cooling, which would help to differentiate between the electrographic features attributable to TH and anoxic brain injury. This study did not use a standardized sedative protocol, and therefore the effect of different sedatives, doses, and combinations on the background could not be determined.
Patients with more severely abnormal EEG backgrounds tended to have a worse short-term outcome than patients with only mild/moderate background abnormalities. However, outcome determination was made by clinical neurologic examination and not more detailed neuropsychological evaluation. Extended follow-up was not performed. Future studies of prognosis will need more detailed outcome evaluation over a longer period. In patients not treated with TH, some background features are known to have prognostic significance in adults14 and children.13,35,36 In adults, postanoxic status epilepticus is associated with worse outcome, with or without TH.37 Further, at extremely low temperatures, as used in deep hypothermic circulatory arrest for vascular surgery, the EEG develops a discontinuous and then isoelectric pattern, but these severe EEG abnormalities are not seen with the moderate hypothermia temperatures used in TH.38 This suggests that EEG features may be useful in prognostication, but revalidation of specific features is required in children being treated with TH. Prospective studies with multivariate analysis of EEG and other factors predictive of outcome will be needed to fully assess the role of EEG in prognosis.
Development and implementation of continuous long-term video-EEG monitoring may allow improvement in neurocritical care. EEG monitoring is noninvasive, provides data on the entire cortex, and can be performed in real time at the bedside.39 Severely abnormal early EEG findings might suggest futility of treatment or indicate the need for deeper or more prolonged hypothermia. In adults treated with deep hypothermic circulatory CA, outcome is better when the EEG becomes continuous at lower temperatures40 (i.e., after less rewarming has occurred). If a similar finding is identified in children being treated with TH after CA, an EEG that remains discontinuous during warming might suggest more severe brain injury and the need for longer TH or slower warming. With further development, EEG monitoring might guide TH management in individual patients.
AUTHOR CONTRIBUTIONS
Statistical analyses were performed by N.S. Abend, R.R. Clancy, and D.J. Dlugos.
DISCLOSURE
N.S.A. has received a Young Investigators Award from UCB Pharmaceuticals (not related to this study). N.S.A. has received grants from the National EpiFellows Foundation and the University of Pennsylvania Clinical Neuroscience Center. A.T. has received a grant from the University of Pennsylvania Clinical Trials Center. R.I. has received a grant from the NIH (Validation of the NIH Stroke Scale in Children) and a consultation fee for participation in Clinical Event Committee for Berlin Heart ventricular assist device trial. S.T.H. has received consulting fees from GlaxoSmithKline. M.H. has received grants from the NIH (Validation of the NIH Stroke Scale in Children) and Genzyme. M.D. reports no disclosures. V.N. has received grants from the NIH, Agency for Healthcare Research and Quality, National Highway Safety and Traffic Administration, and Laerdal Foundation for Acute Care Medicine. D.J.D. has received a grant from UCB Pharma (investigator initiated grant). R.R.C. has received a grant from the University of Pennsylvania Pediatric Pharmacologic Research Unit and an honorarium from Abbott Lab.
Received December 10, 2008. Accepted in final form February 27, 2009.
Address correspondence and reprint requests to Dr. Nicholas S. Abend, Division of Neurology, The Children's Hospital of Philadelphia, 34th Street and Civic Center Boulevard, Philadelphia, PA 19104 abend@email.chop.edu
Supported by grants from the Penn Alliance for Therapeutic Hypothermia (University of Pennsylvania Neuroscience Center), the National EpiFellows Foundation (N.S.A.), the University of Pennsylvania Clinical Trials Research Center Grant (UL1-RR-024134; A.T. and V.N.), and the Pediatric Pharmacologic Research Unit (R.R.C.).
Disclosures: Author disclosures are provided at the end of the article.
Medications and Devices: Fosphenytoin (Cerebyx; Eisai Inc., Woodcliff Lake, NJ); ketamine (Ketalor; JHP Pharmaceuticals, Parsippany, NJ); levetiracetam (Keppra; UCB Pharma, Brussels, Belguim); lorazepam (Ativan; Border Healthcare Corp., Deerfield, IL); midazolam (Versed; Roche Laboratories, Basel, Switzerland); valproic acid (Depacon; Abbott Laboratories, Abbott Park, IL); video-EEG system (Grass-Telefactor, West Warwick, RI).
REFERENCES
- 1.Samson RA, Nadkarni VM, Meaney PA, Carey SM, Berg MD, Berg RA. Outcomes of in-hospital ventricular fibrillation in children. N Engl J Med 2006;354:2328–2339. [DOI] [PubMed] [Google Scholar]
- 2.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002;346:557–563. [DOI] [PubMed] [Google Scholar]
- 3.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002;346:549–556. [DOI] [PubMed] [Google Scholar]
- 4.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 2005;353:1574–1584. [DOI] [PubMed] [Google Scholar]
- 5.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365:663–670. [DOI] [PubMed] [Google Scholar]
- 6.Hosain SA, Solomon GE, Kobylarz EJ. Electroencephalographic patterns in unresponsive pediatric patients. Pediatr Neurol 2005;32:162–165. [DOI] [PubMed] [Google Scholar]
- 7.Jette N, Claassen J, Emerson RG, Hirsch LJ. Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol 2006;63:1750–1755. [DOI] [PubMed] [Google Scholar]
- 8.Abend NS, Dlugos DJ. Nonconvulsive status epilepticus in a pediatric intensive care unit. Pediatr Neurol 2007;37:165–170. [DOI] [PubMed] [Google Scholar]
- 9.Alehan FK, Morton LD, Pellock JM. Utility of electroencephalography in the pediatric emergency department. J Child Neurol 2001;16:484–487. [DOI] [PubMed] [Google Scholar]
- 10.Tay SK, Hirsch LJ, Leary L, Jette N, Wittman J, Akman CI. Nonconvulsive status epilepticus in children: clinical and EEG characteristics. Epilepsia 2006;47:1504–1509. [DOI] [PubMed] [Google Scholar]
- 11.Saengpattrachai M, Sharma R, Hunjan A, et al. Nonconvulsive seizures in the pediatric intensive care unit: etiology, EEG, and brain imaging findings. Epilepsia 2006;47:1510–1518. [DOI] [PubMed] [Google Scholar]
- 12.Young GB, Jordan KG, Doig GS. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology 1996;47:83–89. [DOI] [PubMed] [Google Scholar]
- 13.Abend NS, Licht DJ. Predicting outcome in children with hypoxic ischemic encephalopathy. Pediatr Crit Care Med 2008;9:32–39. [DOI] [PubMed] [Google Scholar]
- 14.Wijdicks EF, Hijdra A, Young GB, Bassetti CL, Wiebe S. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;67:203–210. [DOI] [PubMed] [Google Scholar]
- 15.Fodale V, Santamaria LB. Laudanosine, an atracurium and cisatracurium metabolite. Eur J Anaesthesiol 2002;19:466–473. [DOI] [PubMed] [Google Scholar]
- 16.Legriel S, Troche G, Bedos JP. Status epilepticus after discontinuation of induced hypothermia: an incidental association? Resuscitation 2006;70:159–160. [DOI] [PubMed] [Google Scholar]
- 17.Hovland A, Nielsen EW, Kluver J, Salvesen R. EEG should be performed during induced hypothermia. Resuscitation 2006;68:143–146. [DOI] [PubMed] [Google Scholar]
- 18.Battin M, Bennet L, Gunn AJ. Rebound seizures during rewarming. Pediatrics 2004;114:1369. [DOI] [PubMed] [Google Scholar]
- 19.Schmitt FC, Buchheim K, Meierkord H, Holtkamp M. Anticonvulsant properties of hypothermia in experimental status epilepticus. Neurobiol Dis 2006;23:689–696. [DOI] [PubMed] [Google Scholar]
- 20.Corry JJ, Dhar R, Murphy T, Diringer MN. Hypothermia for refractory status epilepticus. Neurocrit Care 2008;9:189–197. [DOI] [PubMed] [Google Scholar]
- 21.Bagic A, Theodore WH, Boudreau EA, et al. Towards a non-invasive interictal application of hypothermia for treating seizures: a feasibility and pilot study. Acta Neurol Scand 2008;118:240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orlowski JP, Erenberg G, Lueders H, Cruse RP. Hypothermia and barbiturate coma for refractory status epilepticus. Crit Care Med 1984;12:367–372. [DOI] [PubMed] [Google Scholar]
- 23.DeLorenzo RJ, Waterhouse EJ, Towne AR, et al. Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia 1998;39:833–840. [DOI] [PubMed] [Google Scholar]
- 24.Jaitly R, Sgro JA, Towne AR, Ko D, DeLorenzo RJ. Prognostic value of EEG monitoring after status epilepticus: a prospective adult study. J Clin Neurophysiol 1997;14:326–334. [DOI] [PubMed] [Google Scholar]
- 25.Vespa PM, Miller C, McArthur D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med 2007;35:2830–2836. [PMC free article] [PubMed] [Google Scholar]
- 26.DeGiorgio CM, Heck CN, Rabinowicz AL, Gott PS, Smith T, Correale J. Serum neuron-specific enolase in the major subtypes of status epilepticus. Neurology 1999;52:746–749. [DOI] [PubMed] [Google Scholar]
- 27.O'Regan ME, Brown JK. Serum neuron specific enolase: a marker for neuronal dysfunction in children with continuous EEG epileptiform activity. Eur J Paediatr Neurol 1998;2:193–197. [DOI] [PubMed] [Google Scholar]
- 28.Pisani F, Cerminara C, Fusco C, Sisti L. Neonatal status epilepticus vs recurrent neonatal seizures: clinical findings and outcome. Neurology 2007;69:2177–2185. [DOI] [PubMed] [Google Scholar]
- 29.Coen RW, McCutchen CB, Wermer D, Snyder J, Gluck FE. Continuous monitoring of the electroencephalogram following perinatal asphyxia. J Pediatr 1982;100:628–630. [DOI] [PubMed] [Google Scholar]
- 30.McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology 2000;55:506–513. [DOI] [PubMed] [Google Scholar]
- 31.Ronen GM, Buckley D, Penney S, Streiner DL. Long-term prognosis in children with neonatal seizures: a population-based study. Neurology 2007;69:1816–1822. [DOI] [PubMed] [Google Scholar]
- 32.Dlugos D, Sirven JI. Prognosis of neonatal seizures: “It's the etiology, stupid”—or is it? Neurology 2007;69:1812–1813. [DOI] [PubMed] [Google Scholar]
- 33.Clancy RR, Legido A. The exact ictal and interictal duration of electroencephalographic neonatal seizures. Epilepsia 1987;28:537–541. [DOI] [PubMed] [Google Scholar]
- 34.Hirsch LJ, Brenner RP, Drislane FW, et al. The ACNS Subcommittee on Research Terminology for Continuous EEG Monitoring: proposed standardized terminology for rhythmic and periodic EEG patterns encountered in critically ill patients. J Clin Neurophysiol 2005;22:128–135. [DOI] [PubMed] [Google Scholar]
- 35.Mandel R, Martinot A, Delepoulle F, et al. Prediction of outcome after hypoxic-ischemic encephalopathy: a prospective clinical and electrophysiologic study. J Pediatr 2002;141:45–50. [DOI] [PubMed] [Google Scholar]
- 36.Nishisaki A, Sullivan J III, Steger B, et al. Retrospective analysis of the prognostic value of electroencephalography patterns obtained in pediatric in-hospital cardiac arrest survivors during three years. Pediatr Crit Care Med 2007;8:10–17. [DOI] [PubMed] [Google Scholar]
- 37.Rossetti AO, Logroscino G, Liaudet L, et al. Status epilepticus: an independent outcome predictor after cerebral anoxia. Neurology 2007;69:255–260. [DOI] [PubMed] [Google Scholar]
- 38.Stecker MM, Cheung AT, Pochettino A, et al. Deep hypothermic circulatory arrest, I: effects of cooling on electroencephalogram and evoked potentials. Ann Thorac Surg 2001;71:14–21. [DOI] [PubMed] [Google Scholar]
- 39.Jette N, Hirsch LJ. Continuous electroencephalogram monitoring in critically ill patients. Curr Neurol Neurosci Rep 2005;5:312–321. [DOI] [PubMed] [Google Scholar]
- 40.Stecker MM, Cheung AT, Pochettino A, et al. Deep hypothermic circulatory arrest, II: changes in electroencephalogram and evoked potentials during rewarming. Ann Thorac Surg 2001;71:22–28. [DOI] [PubMed] [Google Scholar]