Abstract
For more than 20 years, tamoxifen has been the gold standard for the adjuvant treatment of postmenopausal women with hormone-responsive early breast cancer. However, recent randomized trials have shown efficacy and tolerability benefits with the third-generation aromatase inhibitor anastrozole, resulting in an increased use of this agent in the adjuvant setting. Data on anastrozole’s long-term efficacy and tolerability are therefore of interest in clinical practice and will be reviewed here, especially in the light of the 100-month analysis of the ATAC (Anastrozole, Tamoxifen Alone or in Combination) trial.
Keywords: anastrozole, aromatase inhibitors, breast cancer, adjuvant therapy
Introduction
Breast cancer is the single most commonly diagnosed cancer in women worldwide and a leading cause of cancer-related death. In Western countries, nearly 75% of all breast cancers occur in postmenopausal women, of which about 80% are hormone-receptor positive. A recent retrospective analysis of 3614 postmenopausal patients with estrogen receptor-positive (ER+) early breast cancer treated with adjuvant tamoxifen revealed that 476 (13.2%) had developed a recurrence during the 5-year median follow-up.1 The annual hazard ratio (HR) of recurrence peaked at 2 years (4.3% per annum); the majority of this peak represented distant recurrence. In another retrospective cohort study of US patients (n = 1616) with early breast cancer, the risk of dying was found to be over 3 times greater for patients with distant recurrence as compared with loco-regional or contralateral recurrence.2
Given the potential adverse impact on overall survival associated with recurrence, adjuvant therapies that reduce the risk of early distant metastasis are also most likely to have a significant beneficial effect on overall survival. Tamoxifen has been for a long time the adjuvant treatment of choice for pre- and postmenopausal women with hormone receptor positive early breast cancer. The results of the Oxford meta-analyses3,4 have demonstrated significant reduction in both disease recurrence (41%) and breast cancer specific mortality (34%) for patients treated with 5 years of tamoxifen therapy. Although most of the divergence between treatments in disease recurrence occurs during the first 5 years on treatment, the effect on breast cancer specific mortality is not manifest until the period between 5 and 15 years. The relapse pattern for low-risk and intermediate risk tumors indicates that there is a 1.5% to 2% yearly risk of recurrence of breast cancer in years 5 to 15 after initial diagnosis. A small proportion of women treated with tamoxifen experience serious side effects including increased incidence of endometrial cancer, thromboembolism and cerebrovascular events, which limits the long-term use of tamoxifen. Recent data from the ATAC (Arimidex, Tamoxifen, Alone or in Combination)5 and BIG (Breast International Group)1–986 clinical trials demonstrate that third-generation non-steroidal aromatase inhibitors (AIs) anastrozole and letrozole are, respectively, more effective than tamoxifen in reducing recurrences and also offer tolerability advantages over tamoxifen. A technology assessment from the American Society of Clinical Oncology (ASCO)7 recommends that the optimum adjuvant treatment for postmenopausal women with hormone-receptor-positive breast cancer should include the use of an AI, either as initial treatment or sequentially after 2 to 5 years treatment with tamoxifen. Considerable debate remains over the most effective treatment strategy (upfront or sequential) and the extent to which benefits and side effects continue after treatment is completed.
In this report we review the 100-month analysis of the ATAC trial,8 which provides long-term efficacy and safety data on anastrozole compared with tamoxifen as initial adjuvant treatment for postmenopausal women with hormone-sensitive breast cancer. Similarly the combined analyses of data from two prospective, multicenter, randomized, open label trials (ABCSG trial 8 and ARNO 95) lend support to a switch from tamoxifen to anastrozole in patients who have completed 2 years of adjuvant tamoxifen.9 The results of the Italian Tamoxifen Anastrozole Trial (ITA trial)10 also confirm that switching to anastrozole after the first 2 to 3 years of treatment with tamoxifen is well tolerated and significantly improves event-free and recurrence-free survival in postmenopausal patients with early breast cancer.
Pharmacology
The basic pharmacological differences between tamoxifen and anastrozole explain their mode of action, varied side effect profile and efficacy.
Tamoxifen is a selective estrogen receptor modulator (SERM), which affects different organ systems including the endometrium (endometrial cancer and hypertrophy), the coagulation system (thrombosis), bone (modulation of mineral density) and liver (alterations of blood lipid profile). In these organ systems tamoxifen generally acts as an agonist, mimicking the effect of estrogen, in contrast to its action on breast epithelial cells, where it generally acts as an antagonist.11 Flare reactions, withdrawal responses, and the experimental demonstration of breast tumor growth stimulated by tamoxifen are evidence that tamoxifen can operate as an agonist in breast tissue under certain circumstances. Tamoxifen treatment is usually limited to 5 years because of concerns on the development of de novo and acquired resistance, and an ongoing risk of adverse events, including endometrial cancer, thromboembolic events, and gynecological symptoms with long-term use.
The therapeutic option of reducing estrogen levels in patients with breast cancer was originally restricted to patients with functioning ovaries. However, postmenopausal women still produce significant amounts of estrogen through aromatization of circulating adrenal androgens in peripheral normal tissues, such as fat, muscle, liver, and the epithelial and stromal components of the breast.11 The relative proportion of estrogens synthesized in extragonadal sites increases with age, and eventually non-ovarian estrogens predominate in the circulation. The pivotal role of aromatase in the development of breast cancer led to the successful introduction into clinical practice of potent and specific AIs. Anastrozole is a competitive AI with high potency and was the first selective AI approved in North America and Europe. Pharmacodynamic studies reveal that subjects receiving anastrozole 1 mg per day orally achieved 96.7% aromatase inhibition.11
Efficacy
The ATAC Trialists Group8 published in 2008 the findings of an analysis of data at a median follow-up of 100 months (range 0–126), which is the longest follow-up to date of an adjuvant trial of upfront treatment with AIs. In ATAC, 9366 postmenopausal women with localized invasive breast cancer treated with surgery ± radiotherapy ± chemotherapy were randomized on a 1:1:1 basis to anastrozole (n = 3125), tamoxifen (n = 3116) or a combination of anastrozole and tamoxifen (n = 3125). The combination treatment arm was discontinued after interim analysis because it showed no efficacy or tolerability benefits over tamoxifen alone. The primary end point of the trial was disease-free survival (DFS) defined as the time from randomization to the earliest occurrence of local or distant recurrence, new primary breast cancer, or death from any cause, and the secondary endpoints included time to recurrence (TTR), incidence of new contralateral breast cancer (CLBC), time to distant recurrence (TTDR), overall survival (OS) and death after recurrence. The primary and secondary end points were assessed in the total population (intention to treat; ITT: anastrozole, n = 3125; tamoxifen, n = 3116: total 6241) and the hormone receptor positive subpopulation (84% of ITT: anastrozole, n = 2618; tamoxifen, n = 2598; total 5216). Post-treatment completion, fractures and serious adverse events continued to be collected blindly. A total of 46202 women-years of follow-up for patients receiving monotherapy were included in this trial.
The results of this trial show that at median follow-up of 100 months, DFS, TTR, TTDR, and the incidence of new CLBC were improved significantly in the intention to treat and hormone-receptor-positive populations in women assigned anastrozole compared to tamoxifen (Table 1). Lower recurrence rates for anastrozole were maintained after treatment completion, especially for the hormone-receptor-positive population where the absolute benefit of 2.8% at 5 years increased to 4.8% at 9 years (Figure 1).
Table 1.
ATAC trial: Efficacy endpoints for all patients and hormone-receptor-positive patients
Reproduced with permission from The Arimidex, Tamoxifen Alone or in Combination (ATAC) Trialist’s Group (ATAC). Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53.8 Copyright © 2008 Elsevier.
Abbreviations: A, anastrozole; T, tamoxifen; ITT, intention-to-treat.
Figure 1.
ATAC trial: Curves for time to recurrence (TTR) in hormone-receptor-positive patients. A) Kaplan-Meier prevalence curves and B) smoothed hazard rate curves for time to recurrence. Plots are smoothed with an Epanechinikov kernel with bandwidth chosen by cross validation.
Reproduced with permission from The Arimidex, Tamoxifen Alone or in Combination (ATAC) Trialist’s Group (ATAC). Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53.8 Copyright © 2008 Elsevier.
The improvement in disease control with anastrozole was maintained beyond 8 years and the graphs continued to diverge even at 9 years, suggesting that the therapeutic effect of 5 years’ treatment with AIs can be prolonged after treatment cessation. The Early Breast Cancer Trialists Collaborative Group (EBCTCG) showed a carryover effect for 5 years’ treatment with tamoxifen, with decreased recurrence rates maintained after cessation of treatment in years 5 to 9.4 Therefore, given the long-term findings from the ATAC trial, anastrozole might have a larger carryover effect after cessation of treatment than tamoxifen, which may have a significant impact on clinical practice. The additional significant reduction in recurrence noted with anastrozole compared with tamoxifen after completion of treatment suggests that anastrozole would decrease recurrence by 50% in the post-treatment period compared with no treatment. The hazard ratio for recurrence favored anastrozole for all subgroups based on baseline and treatment characteristics. The benefit in favor of anastrozole was larger for the subgroup of estrogen-receptor-positive and progesterone-receptor-negative compared to estrogen-receptor-positive and progesterone-receptor-positive subgroup (p = 0.001). However, this finding according to progesterone receptor status has not been confirmed either in a similar trial comparing letrozole with tamoxifen12 or by the Trans-ATAC investigators who investigated a subset of patients from whom tissue was able to assess a link between quantitative ER and PgR expression and HER2 status with recurrence in the ATAC.13
The distant recurrence rates also continued to diverge over longer follow-up intervals, being 1.3% lower for anastrozole compared with tamoxifen at year 5 and 2.4% lower at year 9. Similarly a lower number of recurrences of new contra-lateral breast cancers after anastrozole compared with tamoxifen were also maintained in the latest findings from the ATAC trial.
However, despite the above findings, no differences were noted in overall survival. The reasons for this are not entirely clear but may partly be because of an excess of deaths from other causes without recurrence (although not statistically significant, about 44% of total deaths in the anastrozole group and 39% in the tamoxifen group were non-breast cancer deaths). The other important factor to be noted is that the mean age at last follow-up was 72 years in this analysis. The risk of major co-morbidities can increase with age, therefore deaths from causes other than breast cancer can be a major component of overall survival. In this trial no specific cause of death was increased significantly in patients assigned to anastrozole. Further analyses of ATAC, which are currently planned for 2010 when all patients will be more than 10 years post randomization, are eagerly awaited.
The sequential trials in which patients switched to anastrozole after 2 to 3 years on tamoxifen also show superiority of anastrozole over tamoxifen with better tolerability and safety profile. A recent meta-analysis14 has included data from three clinical trials (ABCSG 8, ARNO, and ITA) in which postmenopausal women with histologically confirmed, hormone-sensitive early-stage breast cancer were randomized to either 1 mg/day anastrozole (n = 2009) after 2 to 3 years of tamoxifen treatment or continued on 20 or 30 mg/day tamoxifen (n = 1997). Median follow-up for the meta-analysis was 30 months (0–89.5), with a total duration of follow-up of 5389 person-years for the anastrozole group and 5339 person-years for the tamoxifen group. Patients switched to anastrozole had fewer disease recurrences (92 vs 159) and deaths (66 vs 90) than did those who remained on tamoxifen, resulting in significant improvements in disease-free survival (hazard ratio 0.59 [95% CI 0.48–0.74]; p < 0.0001), event-free survival (0.55 [0.42–0.71]; p < 0.0001), distant recurrence-free survival (0.61 [0.45–0.83]; p = 0.002), and overall survival (0.71 [0.52–0.98]; p = 0.04). The meta-analysis only included data for the time of switched treatment: its results therefore may not be relevant to a prospective treatment strategy of starting with tamoxifen with the intention of changing to anastrozole.
The efficacy and tolerability of extended adjuvant therapy with anastrozole for 3 years among women who had completed 5 years of adjuvant therapy were evaluated in the Austrian Breast and Colorectal Cancer Study Group (ABCSG) Trial 6a.15 In ABCSG Trial 6a, patients who were disease free at the end of ABCSG Trial 6 were randomly assigned to receive either 3 years of anastrozole or no further treatment. At a median follow-up of 62.3 months, women who received anastrozole (n = 387) had a statistically significant reduced risk of recurrence (loco-regional recurrence, contra-lateral breast cancer, or distant metastasis) compared with women who received no further treatment (n = 469; hazard ratio = 0.62; 95% CI = 0.40 to 0.96, p = 0.031).
Toxicity
Trial safety data show that the overall tolerability of AIs is similar to that of tamoxifen, with adverse events being predictably characteristic of estrogen deprivation; however, some important differences in adverse event profiles between tamoxifen and the AIs have been demonstrated. In addition to anti-estrogenic effects, tamoxifen acts as an estrogen agonist in some tissues, which can lead to serious side effects not associated with the AIs, which prevent estrogen biosynthesis. A lower incidence of gynecological and thromboembolic events is observed in patients taking AIs, and fewer cases of endometrial cancer are seen compared with tamoxifen. Adverse events that are more frequent with adjuvant AI therapy compared with tamoxifen include arthralgia and myalgia, bone loss, and effects on the cardiovascular system and blood lipids. The effects of AIs on bone are predictable and may be managed, where necessary, with bisphosphonates.
Overall, treatment-related serious adverse events were fewer in patients receiving anastrozole in the ATAC trial compared with those receiving tamoxifen during the treatment period and similar after treatment completion. The safety profiles for anastrozole and tamoxifen in the sequential studies were consistent with those previously reported in other trials,9,10,16 and no new safety issues were identified within the context of switching therapy after 2 to 3 years.
Cardiovascular and thromboembolic events
Although an increase in cardiovascular risk associated with estrogen depletion is a potential concern with AIs,17 there was no difference in the number of cardiovascular deaths between the anastrozole and tamoxifen groups (67 [2%] vs 66 [2%]) in the 100-month analysis of the ATAC trial; moreover, the incidence of myocardial infarction was similar between the two groups, both during treatment (34 [0.27%] vs 33 [0.27%]) and off treatment (26 [0.28%] vs 28 [0.30%]). Fewer cerebrovascular accidents were noted in patients receiving anastrozole during treatment (20 vs 34, odds ratio [OR] 0.59 [0.32–1.05], p = 0.056), but not afterwards (22 vs 20, OR 1.10 [0.57–2.13], p = 0.75). The incidence of thromboembolic events was also decreased with anastrozole during treatment.
Gynecological and menopausal symptoms
In the ATAC trial, patients randomized to tamoxifen had significantly more gynecologic adverse events than those in the anastrozole group (34.2% vs 20.5%; p < 0.0001), which led to more diagnostic and/or therapeutic interventions, including an increase in the number of hysterectomies (5.1% vs 1.3%; p < 0.0001).18 Decreased libido and dyspareunia were reported more frequently with anastrozole than with tamoxifen, and most of these events were in patients who had vaginal dryness. Recently Cuzick and others19 reported a retrospective analysis of the ATAC trial to investigate the association between treatment-emergent endocrine symptoms (specifically vasomotor and joint symptoms) and the risk of breast cancer recurrence. Vasomotor symptoms (eg, hot flushes, night sweats, and cold sweats) are common side effects of endocrine treatment in women with breast cancer: in the ATAC trial, around 35% of patients receiving anastrozole and 40% of those receiving tamoxifen reported hot flushes during treatment. Excluding patients who already had vasomotor symptoms and/or joint symptoms at baseline, the study by Cuzick shows that 21.7% and 25.8% of patients randomized to anastrozole or tamoxifen, respectively, reported vasomotor symptoms at the 3-month follow-up visit, and 21.1% and 14.3% reported joint symptoms. The emergence of either symptom was predictive of significantly lower recurrence risk in both tamoxifen-treated and anastrozole-treated patients, even after adjustment for age, BMI, previous HRT, nodal status, tumor size, and tumor grade. A significantly larger effect was noted for joint symptoms (adjusted HR 0.60 [0.50–0.72], p < 0.0001), which was similar in both treatment groups, whereas the effect of vasomotor symptoms was smaller and most apparent in patients who had taken HRT before study entry. A similar correlation of side-effects with response to treatment has been noted in a few other situations, notably graft-versus-host disease for allogeneic bone-marrow transplantation and skin rash for antibodies directed at the EGFR or tyrosine-kinase inhibitors. An inverse association between the occurrence of vasomotor symptoms and breast cancer recurrence was previously reported for tamoxifen,20 and extends this association to the AI anastrozole and also to the presence of joint symptoms. If confirmed, this relationship between early treatment-emergent symptoms and beneficial response to therapy might be useful when reassuring patients who present with these symptoms, and also suggests the need for effective strategies to manage patients in order to ensure compliance.
Quality of life
A subgroup of patients in the ATAC trial were assessed for quality of life21 using the FACT-B questionnaire and endocrine symptoms (ES) subscale assessment of ES at baseline and 3, 6, 12, 18 and 24 months During the 2-year assessment period most patients showed a clinically significant improvement from baseline in the aggregate of their physical well-being, functional well-being and breast cancer specific concerns, as measured by the FACT-B Trial Outcome Index. Conversely, most patients in each treatment arm experienced a worsening of endocrine-related symptoms at the 3 month assessment relative to baseline. Thereafter these symptoms appeared to stabilize or improve slightly. There were some interesting differences in the reporting of the severity of individual endocrine symptoms among treatment groups. Women taking anastrozole reported fewer cold sweats, but the same number of hot flushes. Although vaginal discharge was reported less often by women taking anastrozole than women taking tamoxifen, significant vaginal dryness was more common on anastrozole, as was dyspareunia and loss of interest in sex.
Other primary cancers
Deaths due to second primary non-breast cancers and deaths due to other causes were numerically more frequent in patients assigned anastrozole in the 100-month analysis of the ATAC trial, although differences with tamoxifen are statistically not significant. Endometrial cancers, melanomas and ovarian cancers were less frequent with anastrozole, but there were more lung and colorectal cancers: only in the case of endometrial cancer, however, was the difference with tamoxifen statistically significant.
A link between estrogens and a decreased risk of developing colorectal cancer has been postulated22. In addition, estrogen receptors α and β have been shown to inhibit the development of adenomatous polyposis coli (APC)-dependent colon cancer in mice.23 By contrast, aromatase seems to enhance disease progression in lung cancers,24 suggesting that the role of estradiol in tumor progression or tumor regression could be diverse and dependent on cancer type. Since estrogen receptor β is widely expressed in many organs, the effects of hormone manipulation on non-breast malignant disease occurrence needs to be assessed further. This highlights the need to continue collecting follow-up data in the adjuvant AI trials.
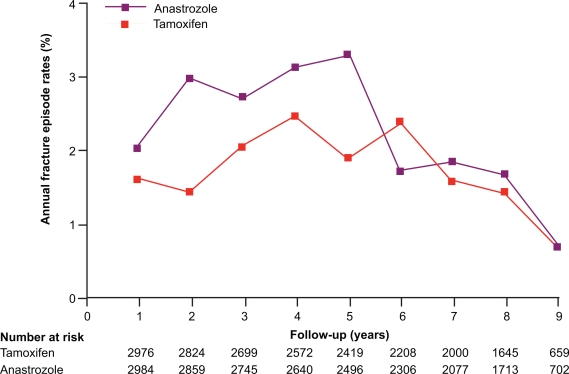
Fractures
The 68-month analysis of ATAC trial25 revealed that treatment-related adverse events occurred significantly less often with anastrozole than with tamoxifen (1884 [61%] vs 2117 [68%]; p < 0.0001), as did treatment-related serious adverse events (146 [5%] vs 277 [9%]; p < 0.0001) and adverse events leading to withdrawal (344 [11%] vs 442 [14%]; p = 0.0002). However, it was also noted that the fractures occurred more often in patients receiving anastrozole compared to the patients receiving tamoxifen (340 [11%] vs 237 [8%]; p < 0.0001). The yearly rate of fracture remained constant over the treatment period and was higher for anastrozole than for tamoxifen (22.6 vs 15.6 fractures per 1000 women-years; HR 1.43 [95% CI 1.21–1.68]; p < 0.0001). Excess fractures were recorded at several sites including the wrist, humerus or arm, and spine but hip fracture occurrence was low in both groups (37 [1%] vs 31 [1%]). The concomitant use of bisphosphonates was recorded at every follow-up visit, and overall use was low in both groups. In the 100-month ATAC analysis,8 the increased yearly rate of fracture noted in the anastrozole group compared with tamoxifen did not continue into the post-treatment period, where the rate on anastrozole was similar to that on tamoxifen. Therefore the increase in fracture rates with anastrozole persisted only in the active treatment period and did not continue after treatment completion. It is interesting to note that although the effects of anastrozole and tamoxifen on breast cancer recurrence extended beyond the cessation of treatment, the higher fracture rates on anastrozole ceased after the 5-year treatment completion (Figure 2). It has been noted that hip fractures were little affected by anastrozole in this study and although there is 6% to 7% bone loss during active treatment, no patients with normal bone density at baseline developed osteoporosis after 5 years’ treatment.26,27 This suggests that bone damage by aromatase inhibition may be reversible and manageable with the use of bisphosphonates, although the mechanisms of action of the bone damage by AIs and subsequent recovery after treatment completion are still unclear.
Figure 2.
Fracture episodea rates throughout the ATAC Trialists’ Group study.
aA fracture episode comprised one or more fractures on the same day. Fractures occurring after recurrence are not included because patients were censored after recurrence and fractures were not recorded.
Reproduced with permission from The Arimidex, Tamoxifen Alone or in Combination (ATAC) Trialist’s Group (ATAC). Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53.8 Copyright © 2008 Elsevier.
Bisphosphonate therapy reduces bone destruction and the incidence of subsequent skeletal complications in the metastatic disease setting, and recent results have demonstrated their efficacy in the prevention of AI-associated bone loss (AIBL) in patients with early breast cancer.28–30
In one large trial, premenopausal women who received tamoxifen or anastrozole, both in combination with goserelin (n = 401), were randomized to receive zoledronic acid or no additional treatment.19 Zoledronic acid (4-mg infusion every 6 months) added to both therapy combinations effectively preserved bone mineral density (BMD) and concomitant zoledronic acid not only prevented bone loss during therapy but improved BMD at 5 years. Data on fracture rates with these two treatment strategies are not yet reported. The efficacy of zoledronic acid for prevention of AIBL in postmenopausal women was evaluated by three large parallel designed trials20,21 (Z-FAST, ZO-FAST, and E-ZO-FAST) which randomized patients – who received letrozole 2.5 mg/day for up to 5 years – to zoledronic acid 4 mg every 6 months either at initiation of letrozole treatment (immediate group) or at the time of BMD decline (T-score ≥ 2 standard deviations below normal or an occurrence of fracture; delayed group).31–33. The enrolment included 602 women in the Z-FAST study (United States and Canada), 1066 women in the ZO-FAST study (approximately 30 countries outside the United States), and 527 women in the E-ZO-FAST study (66 countries in South America, the Middle East, Korea, Europe, and South Africa).34,35. The primary endpoint was the change from baseline in lumbar spine BMD after 1 year of treatment.
At 1 year in the Z-FAST study immediate zoledronic acid (n = 301) provided significant mean increases in lumbar spine and total hip BMD (p < 0.0001 for both) compared with delayed treatment (n = 301).23 Among patients in the delayed group, 8.3% met the requirement for zoledronic acid treatment at 12 months. At both 24 and 36 months, lumbar spine and total hip BMD continued to increase in the immediate zoledronic acid group, whereas BMD continued to decrease further in the delayed-treatment group.22 In the ZO-FAST, study lumbar spine BMD increased from baseline in the immediate group and decreased from baseline in the delayed group. At month 12, the differences between the treatment groups in lumbar spine and total hip BMD were 5.7% (95% CI: 5.2%–6.1%; p = 0.0001) and 3.6% (95% CI: 3.3%–4.0%; p = 0.0001), respectively.38 At month 24, the differences between the treatment groups in lumbar spine and total hip BMD increased to 8.2% and 4.7%, respectively (p < 0.0001 for both). In both studies, levels of biochemical markers of bone metabolism increased in the delayed group, but were reduced with zoledronic acid therapy. Currently, follow-up is too short for assessing fracture incidence. At 1 year in the E-ZO-FAST studies the differences between the treatment groups in lumbar spine and total hip BMD were 5.4% and 3.3%, respectively (p < 0.0001 for both).
Recently, guidance on management of cancer treatment-induced bone loss has emerged and the American Society of Clinical Oncology published an update on the role of bisphosphonates and bone health issues in women with Breast Cancer.36 Recommendations were given for regular assessments of bone health to screen for osteoporosis. Treatment for bone loss was recommended in breast cancer patients found to have osteoporosis based on BMD results (t score −2.5 or lower). Breast cancer patients found to have osteopenia based on BMD results (t score between −1 and −2.5) should have their therapy individualized according to other risk factors. Other recent guidelines have suggested a slightly more cautious level for intervention (t score ≤ 2.0) and the use of bone-targeted treatment in patients with multiple risk factors for fracture regardless of BMD and/or bone loss of more than 4% per year at either the hip or lumbar spine.37
Arthralgias
Arthralgia is an adverse class effect of AIs. Morales et al recently reported a prospective study to investigate the changes in clinical rheumatologic features and magnetic resonance imaging (MRI) of hands and wrists in AI and tamoxifen users.38 At 6 months, patients on AI had a decrease in grip strength (p = 0.0049) and an increase in tenosynovial changes (p = 0.0010) on MRI compared to only minor changes seen in patients on tamoxifen. AI users reported worsening of morning stiffness and showed an increase in intra-articular fluid on MRI.
Crew and others39 conducted a cross-sectional survey of consecutive postmenopausal women receiving adjuvant AI therapy for early-stage hormone-sensitive breast cancer at an urban academic breast oncology clinic. Among the 200 patients who completed the questionnaire assessing the presence of joint symptoms that started or worsened after initiating AIs, 94 (47%) reported having AI-related joint pain and 88 (44%) reported AI-related joint stiffness. In multiple logistic regression analysis, being overweight (BMI 25 to 30 kg/m2) and prior tamoxifen therapy were inversely associated with AI-related joint symptoms. Patients who received taxane chemotherapy were more than 4 times more likely than other patients to have AI-related joint pain and stiffness (OR = 4.08, 95% CI, 1.58–10.57 and OR = 4.76; 95% CI, 1.84–12.28, respectively). This study suggests that AI-related joint symptoms are more prevalent in the real world setting than what has been described in clinical trials, where arthralgias are reported in 20% to 35% of the patients treated with an AI. The success of AI therapy may depend on patients’ ability to be compliant with treatment recommendations; therefore, additional studies of interventions that may alleviate these symptoms are needed.
Conclusions
Long-term follow up data from the ATAC trial confirm the efficacy of anastrozole as adjuvant treatment for postmenopausal women with hormone-sensitive early breast cancer, and provide evidence of a carryover effect after completion of 5 years of treatment. The safety profile of anastrozole is also confirmed to be favorable, with a predictable pattern of estrogen-deprivation symptoms (vasomotor symptoms, joint symptoms and bone loss) which clinicians need to recognize and manage according to local guidelines. Some of the side effects, especially joint symptoms, may also predict a decreased risk of recurrence: this could help in reassuring women and improving compliance.
Footnotes
Disclosures
The authors disclose no conflicts of interest.
References
- 1.Mansell J, Monypenny IJ, Skene AI, et al. Patterns and predictors of early recurrence in postmenopausal women with estrogen receptor-positive early breast cancer. Breast Cancer Res Treat. 2008. Dec 27. [Epub ahead of print] [DOI] [PubMed]
- 2.Lamerato L, Havstad S, Gandhi S, Jones D, Chlebowski R. Breast cancer recurrence and related mortality in US pts with early breast cancer. J Clinical Oncol. 2005;23(Suppl 16s) abstract 738. [Google Scholar]
- 3.Early Breast Cancer Trialists’ Collaborative Group Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 5.Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex,Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 6.Breast International Group (BIG) 1–98 Collaborative Group A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. New Engl J Med. 2005;353:2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 7.Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol. 2005;23:619–629. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 8.The Arimidex, Tamoxifen Alone or in Combination (ATAC) Trialist’s Group (ATAC) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 9.Jakesz R, Jonat W, Gnant M, for the ABCSG and the GABG Switching of postmenopausal women with endocrine-esponsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005;366:455–462. doi: 10.1016/S0140-6736(05)67059-6. [DOI] [PubMed] [Google Scholar]
- 10.Boccardo F, Rubagotti A, Puntoni M, et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: preliminary results of the Italian Tamoxifen Anastrozole trial. J Clin Oncol. 2005;23:5138–5147. doi: 10.1200/JCO.2005.04.120. [DOI] [PubMed] [Google Scholar]
- 11.Leibowitz F, Swain SM. Hormonal therapy for breast cancer. In: Chabner B, Longo DL, editors. Cancer chemotherapy and biotherapy: principles and practices. Philadelphia: Lippincott Williams and Wilkins; [Google Scholar]
- 12.Coates AS, Keshaviah A, Thurlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol. 2007;25:486–492. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 13.Dowsett M, Allred DC, on behalf of the TransATAC Investigators Relationship between quantitative ER and PgR expression and HER2 status with recurrence in the ATAC trial. Breast Cancer Res Treat. 2006;100(suppl 1):S21. (abstract 48). [Google Scholar]
- 14.Jonat W, Gnant M, Boccardo F, et al. Effectiveness of switching from adjuvant tamoxifen to anastrozole in postmenopausal women with hormone sensitive early-stage breast cancer: a meta-analysis. Lancet Oncol. 2006;7:991–996. doi: 10.1016/S1470-2045(06)70948-2. [DOI] [PubMed] [Google Scholar]
- 15.Jakesz R, Greil R, Gnant M, et al. Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian Breast and Colorectal Cancer Study Group Trial 6a. J N Cancer Inst. 2007;99:1845–1853. doi: 10.1093/jnci/djm246. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann M, Jonat W, Hilfrich J, et al. Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated after treatment with tamoxifen compared with continued tamoxifen: the ARNO 95 study. J Clin Oncol. 2007;25:2664–2670. doi: 10.1200/JCO.2006.08.8054. [DOI] [PubMed] [Google Scholar]
- 17.Lewis S. Do endocrine treatments for breast cancer have a negative impact on lipid profiles and cardiovascular risk in postmenopausal women. Am Heart J. 2007;153:182–188. doi: 10.1016/j.ahj.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 18.Duffy SR, Distler W, Howell A, Cuzick J, Baum M. A lower incidence of gynecologic adverse events and interventions with anastrozole than with tamoxifen in the ATAC trial. Am J Obstet Gynecol. 2009;200(1):80.e1–7. doi: 10.1016/j.ajog.2008.07.062. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Cuzick J, Sestak I, Cella D, Fallowfield L, on behalf of the ATAC Trialists’ Group Treatment-emergent endocrine symptoms and the risk of breast cancer recurrence: a retrospective analysis of the ATAC trial. Lancet Oncol. 2008;9:1143–1148. doi: 10.1016/S1470-2045(08)70259-6. [DOI] [PubMed] [Google Scholar]
- 20.Mortimer JE, Flatt SW, Parker BA, et al. Tamoxifen, hot flashes and recurrence in breast cancer. Breast Cancer Res Treat. 2008;108:421–426. doi: 10.1007/s10549-007-9612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fallowfield L, Cella D, Cuzick J, et al. Quality of Life of Postmenopausal Women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) Adjuvant Breast Cancer Trial. J Clinical Oncol. 2004;22:4261–4271. doi: 10.1200/JCO.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 22.Humphries KH, Gill S. Risks and benefits of hormone replacement therapy: the evidence speaks. CMAJ. 2003;168:1001–1010. [PMC free article] [PubMed] [Google Scholar]
- 23.Cho NL, Javid SH, Carothers AM, et al. Estrogen receptors alpha and beta are inhibitory modifiers of Apc-dependent tumorigenesis in the proximal colon of Min/+ mice. Cancer Res. 2007;67:2366–2372. doi: 10.1158/0008-5472.CAN-06-3026. [DOI] [PubMed] [Google Scholar]
- 24.Mah V, Seligson DB, Li A, et al. Aromatase expression predicts survival in women with early-stage non-small cell lung cancer. Cancer Res. 2007;67:10, 484–490. doi: 10.1158/0008-5472.CAN-07-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The ATAC Trialists’ Group Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol. 2006;7:633–643. doi: 10.1016/S1470-2045(06)70767-7. [DOI] [PubMed] [Google Scholar]
- 26.Coleman RE, on behalf of the ATAC Trialists’ Group Effect of anastrozole on bone mineral density: 5-year results from the ‘Arimidex,’ Tamoxifen, Alone or in Combination (ATAC) trial. Proc Am Soc Clin Oncol. 2006;24(suppl 18) doi: 10.1200/JCO.2007.11.0726. abstract 511. [DOI] [PubMed] [Google Scholar]
- 27.Eastell R, Adams JE, Coleman R, et al. Effect of anastrozole on bone mineral density: 5-year results from the ATAC trial (18233230) J Clin Oncol. 2008;26:1051–1057. doi: 10.1200/JCO.2007.11.0726. [DOI] [PubMed] [Google Scholar]
- 28.Gnant MFX, Mlineritsch B, Luschin-Ebengreuth G, et al. Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: a report from the Austrian Breast and Colorectal Cancer study Group. J Clin Oncol. 2007;25:820–828. doi: 10.1200/JCO.2005.02.7102. [DOI] [PubMed] [Google Scholar]
- 29.Brufsky A. Management of cancer-treatment-induced bone loss in postmenopausal women undergoing adjuvant breast cancer therapy: a Z-FAST update. Semin Oncol. 2006;33(suppl 7):13–17. doi: 10.1053/j.seminoncol.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 30.Bundred N, Campbell I, Coleman R, et al. Zoledronic acid in the prevention of cancer treatment-induced bone loss in postmenopausal women receiving letrozole as adjuvant therapy for early breast cancer (ZOFAST study) Eur J Cancer Suppl. 2006;4(48) (abstract 12). [Google Scholar]
- 31.Brufsky A, Bosserman L, Caradonna R, et al. The effect of zoledronic acid on aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole: the Z-FAST study 36-month follow-up. 30th Annual San Antonio Breast Cancer Symposium; December 13–17, 2007; San Antonio, TX. (abstract 27). [Google Scholar]
- 32.Brufsky A, Harker WG, Beck JT, et al. Zoledronic acid inhibits adjuvant letrozole-induced bone loss in postmenopausal women with early breast cancer. J Clin Oncol. 2007;25:829–836. doi: 10.1200/JCO.2005.05.3744. [DOI] [PubMed] [Google Scholar]
- 33.Bundred N, Campbell I, Coleman R. Zoledronic acid in the prevention of cancer treatment induced bone loss in postmenopausal women receiving letrozole as adjuvant therapy for early breast cancer. Cancer Epidemiol Biomarkers Prev [Google Scholar]
- 34.De Boer R, Eidtmann H, Lluch A, et al. The ZO-FAST trial: Zoledronic acid effectively inhibits aromatase inhibitor associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole: 24-month BMD results. Breast Cancer Res Treat. 2007;106(suppl 1):S36. (abstract 501). [Google Scholar]
- 35.Schenk N, Llombart A, Frassoladti A, et al. The E-ZO-FAST trial: Zoledronic acid (ZA) effectively inhibits aromatase inhibitor associated bone loss (AIBL) in postmenopausal women (PMW) with early breast cancer (EBC) receiving adjuvant letrozole (Let) Eur J Cancer. 2007;5:186. (abstract 2008). [Google Scholar]
- 36.Hillner BE, Ingle JN, Chlebowski RT, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003;21:4042–4057. doi: 10.1200/JCO.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 37.Reid DM, Doughty J, Eastell R, et al. Guidance for the management of breast cancer treatment-induced bone loss: a consensus position statement from a UK Expert Group. Cancer Treat Rev. 2008;34(Suppl 1):S3–S18. doi: 10.1016/j.ctrv.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Morales L, Pans S, Verschueren K, et al. Prospective study to assess short-term intra-articular and tenosynovial changes in the aromatase inhibitor-associated arthralgia syndrome. J Clin Oncol. 2008;26:3147–3152. doi: 10.1200/JCO.2007.15.4005. [DOI] [PubMed] [Google Scholar]
- 39.Crew KD, Greenlee H, Capodice J, et al. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol. 2007;25:3877–3883. doi: 10.1200/JCO.2007.10.7573. [DOI] [PubMed] [Google Scholar]