Abstract
Although the reticulospinal tract is a major descending motor pathway in mammals, its contribution to upper limb control in primates has received relatively little attention. Reticulospinal connections are widely assumed to be responsible for coordinated gross movements primarily of proximal muscles, whereas the corticospinal tract mediates fine movements, particularly of the hand. In this study, we used intracellular recording in anesthetized monkeys to examine the synaptic connections between the reticulospinal tract and antidromically identified cervical ventral horn motoneurons, focusing in particular on motoneurons projecting distally to wrist and digit muscles. We found that motoneurons receive monosynaptic and disynaptic reticulospinal inputs, including monosynaptic excitatory connections to motoneurons that innervate intrinsic hand muscles, a connection not previously known to exist. We show that excitatory reticulomotoneuronal connections are as common and as strong in hand motoneuron groups as in forearm or upper arm motoneurons. These data suggest that the primate reticulospinal system may form a parallel pathway to distal muscles, alongside the corticospinal tract. Reticulospinal neurons are therefore in a position to influence upper limb muscle activity after damage to the corticospinal system as may occur in stroke or spinal cord injury, and may be a target site for therapeutic interventions.
Introduction
The reticulospinal tract is a major descending pathway by which the brain controls spinal motor output in all vertebrates. Studies in cat, rodents, and lamprey have established that its functions include locomotor control (Grillner et al., 1997; Mori et al., 2001), postural and gait adjustments during locomotion (Orlovskiı̆, 1970; Drew et al., 1986; Mori, 1987; Prentice and Drew, 2001; Schepens and Drew, 2004), and contributing to posture and movement during targeted reaching (Schepens and Drew, 2004, 2006, Davidson and Buford, 2004). In primates, corticospinal pathways are the focus of most study. Their importance in man is highlighted by the impairments following stroke. The prevailing view is that the reticulospinal and other medial descending systems control coordinated whole-body postural and orienting movements, while the phylogenetically younger corticospinal tract fractionates movements of individual limbs (Kuypers, 1981). The corticospinal tract is especially well developed in primates and is thought to be critically important for dexterous hand and finger movements (Lawrence and Kuypers, 1968a; Porter and Lemon, 1993).
Consistent with this idea, within the spinal cord reticulospinal terminals are distributed mainly among intermediate zone interneurons, or medial motoneurons innervating axial and proximal limb muscles (Kuypers, 1981; Holstege and Kuypers, 1982, 1987; Jones and Yang, 1985; Martin et al., 1985). In cats, reticulospinal axons make monosynaptic excitatory and disynaptic excitatory and/or inhibitory connections with limb motoneurons (Grillner et al., 1968; Jankowska et al., 1968; Lund and Pompeiano, 1968; Wilson and Yoshida, 1969; Shapovalov and Gurevitch, 1970; Peterson et al., 1979; Takakusaki et al., 2001). The single intracellular study of primate reticulospinal tract (Shapovalov, 1972) identified monosynaptic excitation in proximal, but not distal, hindlimb motoneurons. Microstimulation experiments also emphasize responses in proximal muscles (Perreault et al., 1994), although recent studies in monkey did find reticulospinal effects as far distal as the wrist (Davidson and Buford, 2004, 2006).
Here we made intracellular recordings from antidromically identified cervical spinal motoneurons to examine the actions of reticulospinal fibers in macaque monkeys. We stimulated descending fibers in the region of the medial longitudinal fasciculus (MLF) of the medulla, a location where many descending reticulospinal axons can be activated (Jankowska et al., 2003; Edgley et al., 2004). While it is known that fibers of the medial vestibulospinal (Nyberg-Hansen, 1964; Wilson et al., 1968) and tectospinal tracts (Kuypers, 1981) also descend in this region, we believe that our data are most consistent with the activation of the reticulospinal system (see Discussion).
Our results show that significant numbers of motoneurons projecting throughout the upper limb receive short latency synaptic input from the reticulospinal tract. This includes monosynaptic connections to motoneurons projecting to hand muscles, indicating that the reticulospinal system can influence the control of finger movements.
Materials and Methods
General.
All animal procedures were performed under UK Home Office regulations in accordance with the Animals (Scientific Procedures) Act, 1986, and were approved by the Local Research Ethics Committee of Newcastle University. Recordings were made from three terminally anesthetized adult rhesus macaque monkeys (M. mulatta; 2 female; monkeys A, B, and F).
Surgical preparation.
All procedures were performed in a nonrecovery setting. Deep anesthesia was induced using sevoflurane (3–5% in 100% O2). After tracheal intubation, a carotid artery and external jugular vein were cannulated. Flexible bipolar stimulating nerve cuffs were implanted around the following nerves of the right arm: the radial nerve just below the shoulder (supplying extensor muscles of the proximal arm, forearm, and digits), the deep radial nerve at the elbow (supplying forearm and digit extensors), the median and the ulnar nerve at the elbow (supplying forearm flexors and intrinsic hand muscles), the median and the ulnar nerves at the wrist (supplying intrinsic hand muscles). A laminectomy was performed exposing cervical spinal segments C5-T1. The anesthetic regimen was then switched to an intravenous infusion of propofol (5–14 mg · kg−1 · h−1) and alfentanil (7–23 μg · kg−1 · h−1). The vertebral column was clamped at high thoracic and midlumbar levels. The head was fixed stereotaxically and angled to produce 60° neck flexion. A tungsten stimulating electrode (LF501G, Microprobe), insulated except for its tip, was implanted in the left medullary pyramidal tract (PT) using a double angle stereotaxic technique (Soteropoulos and Baker 2006). Correct location was verified using antidromic volleys evoked in the motor cortex following stimulation through the electrode, and orthodromic spinal volleys. The indifferent was a silver wire electrode inserted under the scalp.
A second tungsten stimulating electrode was implanted in the right medial longitudinal fasciculus (MLF) in the upper medulla to allow stimulation of the reticulospinal tract. This again used the double angle technique, targeting stereotaxic coordinates AP0, R0.5, DV0. Electrode positioning was guided by recording descending volleys evoked by stimulation through the electrode in the spinal cord. The indifferent was another silver wire electrode inserted under the scalp. The low thresholds to evoke a spinal volley from the MLF electrode (25–35 μA at the site where the electrode was finally fixed in place) suggested involvement of axons close to the electrode tip.
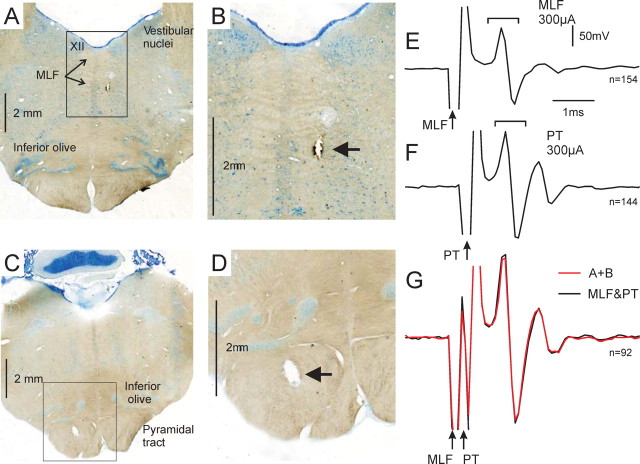
At the end of the experiments, anesthesia was increased to a lethal level (60 mg · kg−1 pentobarbitone intraperitoneal, or anesthetic regimen described above) and the animals were perfused through the heart with PBS followed by 4% paraformaldehyde. Brains were removed and, after cryoprotection in sucrose (30%), sectioned at 75 μm on a microtome. Sections were mounted and stained with cresyl violet before reconstruction of the location of stimulating electrode tips. Example sections are shown in Figure 1A–D.
Figure 1.
Positioning of PT and MLF stimulating electrodes. A–D show histological verification of stimulation sites from one animal. A and C show whole brainstem sections with lesions at the tips of the MLF and PT electrodes, respectively. XII, Hypoglossal nucleus. The tip positions are visible in B and D, which show the regions marked by boxes from A and C at higher magnification. E–G show an example of the occlusion test used to aid positioning of MLF stimulating electrodes. All panels show epidural recordings from the dorsal surface of the cervical spinal cord. Brackets enclose volley responses. Arrows indicate point of stimulus delivery. E, Following a 300 μA stimulus delivered through MLF electrode. F, Following a 300 μA stimulus delivered through PT electrode. G, Following combined stimulation of PT and MLF with 400 μs separation (MLF first). The red trace shows arithmetic sum of E and F, while the black trace shows the recorded volley. The near perfect overlay indicates an absence of occlusion, strongly implying that the responses were mediated by independent fiber tracts. Calibrations in E apply to E–G. N values indicate number of stimuli.
To ensure that the MLF electrode did not activate corticospinal fibers, occlusion tests were performed. Spinal volleys evoked by stimuli (300 μA) delivered through the PT and MLF electrodes were recorded both independently and in combination with a variable delay (300–600 μs) between the stimuli. Off-line, averaged volleys were examined for evidence of occlusion in the volleys evoked by combined stimuli, which would occur if both stimuli activated the same axon. Occlusion was not seen; an example is illustrated in Figure 1E–G. Stimuli delivered separately through PT and MLF electrodes elicited large, clear volley responses in the cervical spinal cord (Fig. 1E,F). When both stimuli were delivered together (400 μs delay, MLF first), a larger volley was recorded which matched almost exactly the arithmetic sum of the individual volleys (Fig. 1G). The close match strongly implies that the responses were mediated by independent fiber tracts.
After checking motor thresholds for each implanted nerve cuff, paralysis was induced (atracuronium, 0.7 mg · kg−1 · h−1) and ventilation commenced. A bilateral pneumothorax was made to minimize respiratory movements. A mineral oil pool was constructed to prevent cooling or desiccation of the exposed cord. Continuous monitoring of a broad range of physiological parameters (including blood pressure, oxygen saturation, heart rate, end-tidal CO2, and core temperature) ensured deep anesthesia and physiological stability throughout.
Recordings.
Intracellular recordings were made from spinal motoneurons using sharp glass micropipettes (tip impedance 5–25 MΩ) filled with 2 m potassium acetate, inserted into the dorsolateral funiculus through small holes made in the dura and arachnoid. Motoneurons were antidromically identified from the implanted nerve cuffs (stimuli delivered at 3× motor threshold) allowing them to be assigned to muscle groups. Responses were recorded to single stimuli and trains of up to four stimuli (300 μA biphasic pulses, 0.2 ms per phase, train frequency 300 Hz, 1 Hz repetition rate) applied to the PT and MLF electrodes in turn. Isolated constant-current stimulators were used to deliver all stimuli. A silver ball electrode on the cord dorsum close to the electrode penetration point (C6-Th1 segments) recorded surface volleys simultaneously with intracellular potentials. Intracellular waveforms were sampled at 25 kHz (gain 100, 20 Hz to 9 kHz bandpass) via a Power1401 interface (Cambridge Electronic Design) together with epidural waveforms (12.5 kHz sampling rate, gain 10,000, 10 Hz to 4 kHz bandpass) and stimulus markers. Onset latencies of small EPSPs were made easier to measure by membrane hyperpolarization (injection of up to 20 nA) if possible.
Analysis.
Postsynaptic responses in motoneurons were identified from superimposed single sweep and averaged records. Segmental latencies (SLs) of EPSPs were measured from the first inflection of the corresponding epidural volley to the onset of the postsynaptic response. Latencies of <1 ms were considered to be monosynaptic (Jankowska et al., 2003). In all cases responses to trains of stimuli were also recorded. Response amplitudes were measured from the onset to peak of the EPSP.
Results
Reticulospinal tract forms direct and indirect synaptic connections with cervical spinal motoneurons
In cats and rats, reticulospinal neurons have been shown to make monosynaptic connections with spinal motoneurons, but there is no direct information on projections of this type to primate distal upper limb motoneurons. We found that both monosynaptic and disynaptic EPSPs were evoked by MLF stimulation in primate cervical motoneurons. We report here intracellular recordings from 140 antidromically identified motoneurons obtained over three experiments; Table 1 lists the target muscle groups.
Table 1.
Target muscle groups innervated by motoneurons recorded in this study
| Antidromic from | Number recorded (over 3 experiments) | Principal muscle groups |
|---|---|---|
| Radial nerve in axilla but not radial nerve at the elbow | 15 | Upper arm extensors (triceps) |
| Radial nerve in axilla and radial nerve at the elbow | 16 | Forearm (wrist and digit) extensors |
| Median nerve at the elbow but not median nerve at the wrist | 61 | Forearm (wrist and digit) flexors |
| Median nerve at the elbow and median nerve at the wrist | 10 | Intrinsic hand muscles: thenar eminence and lumbricals 1 and 2 |
| Ulnar nerve at the elbow but not ulnar nerve at the wrist | 10 | Forearm (wrist and digit) flexors |
| Ulnar nerve at the elbow and ulnar nerve at the wrist | 28 | Intrinsic hand muscles: interosseous, lumbricals, and hypothenar eminence |
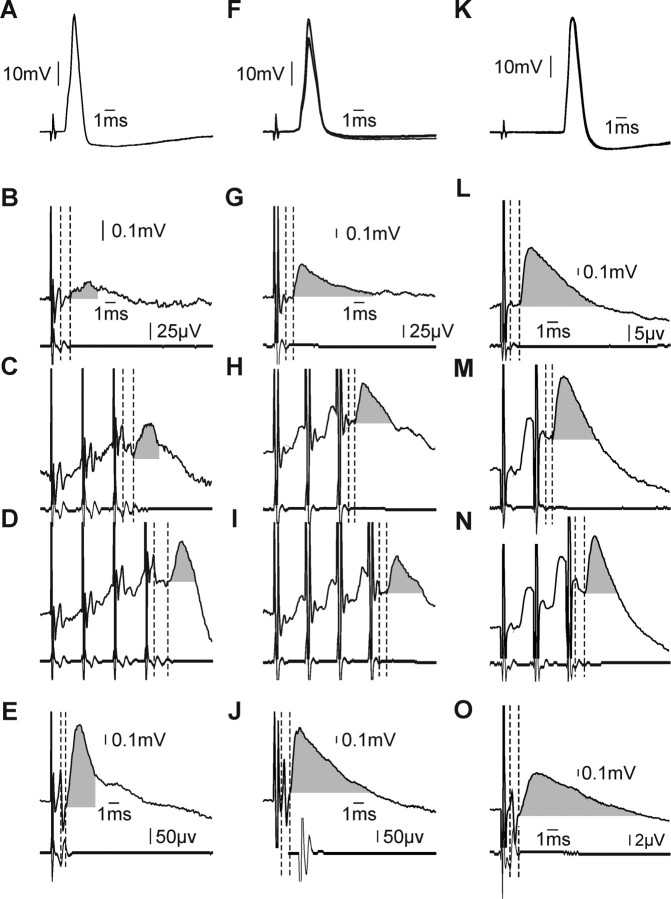
An example disynaptic reticulospinal EPSP is shown in Figure 2A–E, from a forearm flexor motoneuron antidromically activated from the median nerve above the elbow (Fig. 2A), but not at the wrist. A single MLF stimulus evoked a small EPSP with SL ∼1 ms (vertical dashed lines, Fig. 2B) and amplitude ∼70 μV (gray shading). A larger EPSP followed the third stimulus of a train (Fig. 2C) (SL 1.3 ms, amplitude 0.13 mV), and adding a fourth stimulus further augmented the EPSP (Fig. 2D) (amplitude 0.21 mV, SL 1.5 ms). Segmental latencies >1 ms and temporal facilitation with multiple stimuli are characteristic features of disynaptic responses. For comparison, Figure 2E shows a monosynaptic EPSP evoked in this cell by single pulse PT stimulation (SL 0.6 ms). At 0.9 mV (gray shading), the corticospinal EPSP was 4.3 times larger than the largest disynaptic effect from the RST.
Figure 2.
Cervical spinal motoneurons receive monosynaptic and disynaptic reticulospinal input. A–E, Example motoneuron projecting to forearm flexors that received disynaptic reticulospinal contacts. A, Antidromic activation from median nerve above the elbow (overlain single sweeps). B–D, Disynaptic reticulospinal EPSPs following single 300 μA MLF stimulus (B), train of three stimuli (C), and train of four stimuli (D). Each panel shows averaged intracellular records (top) with simultaneously recorded epidural volleys below. Vertical dashed lines highlight the segmental latency of the response. Calibrations in B apply to B–D. E, EPSP evoked in this cell following single 300 μA stimulus to PT. F–J, Example monosynaptic EPSPs evoked following reticulospinal activation in a spinal motoneuron also projecting to forearm flexors. Panels and plotting conventions are as in A–E. K–O, Example motoneuron projecting to thenar muscles which received powerful monosynaptic input from the reticulospinal tract. Panels and plotting conventions are as in A–E, except that responses to a train of two and three stimuli are shown in M and N, respectively.
Figure 2F–J illustrates responses in a different forearm flexor motoneuron, again antidromically identified as projecting to forearm flexors (Fig. 2F shows antidromic activation following stimulation of the median nerve at the elbow; there was no response to median nerve at the wrist). In this case there was monosynaptic excitation from the MLF: a single MLF stimulus produced a clear EPSP (Fig. 2G, vertical dashed lines and gray shading) (SL 0.97 ms, amplitude 0.43 mV). As expected for a monosynaptic response, each subsequent stimulus of a train evoked EPSPs of similar shape and amplitude: (Fig. 2H,I). The segmental latencies were similar for each stimulus, as were the shapes of the initial rising phases of the EPSPs. The declining phases showed differences, most likely due to superimposed disynaptic or polysynaptic inhibition. The corresponding monosynaptic corticospinal EPSP for this cell is shown in Figure 2J (SL 0.8 ms). At 1.0 mV, this response was 2.3 times larger than the reticulospinal EPSP.
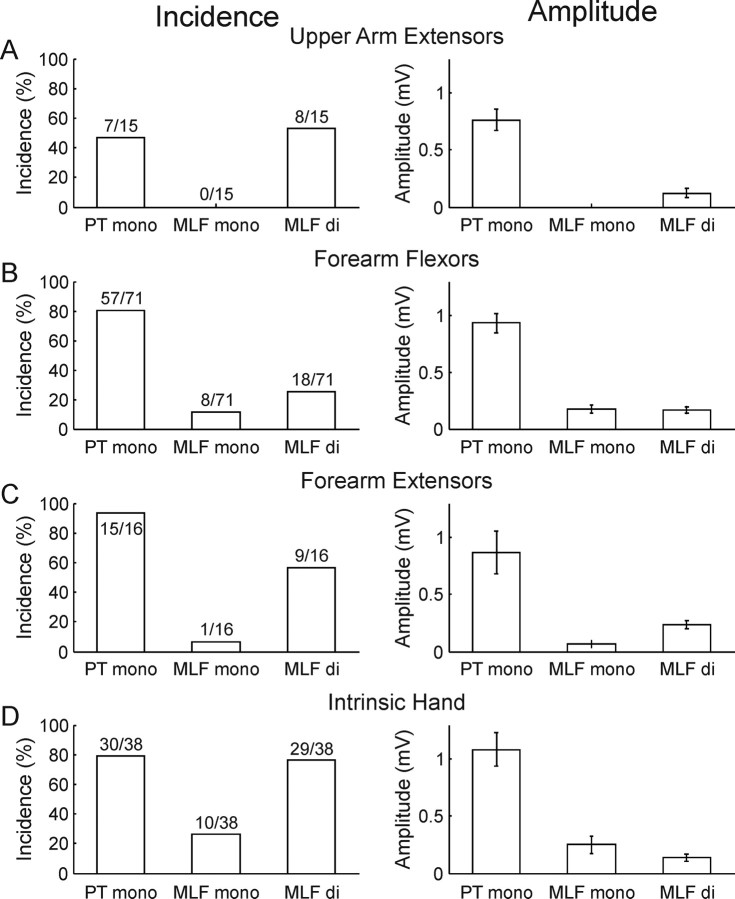
Population data are illustrated in Figure 3, for all 140 antidromically identified upper limb motoneurons, divided by muscle group. For all groups tested, the majority of cells responded to single PT stimuli with monosynaptic EPSPs (Fig. 3A–D). Disynaptic reticulospinal EPSPs were found in between 25 and 76% of motoneurons. Monosynaptic reticulospinal EPSPs occurred less frequently: they were seen in none of the 15 radial nerve motoneurons that innervated triceps, ∼10% of motoneurons that innervated the forearm flexors and extensors, and 26% of intrinsic hand muscles. While we required a segmental latency <1 ms to classify an EPSP as monosynaptic, these latencies were slightly shorter for EPSPs following PT compared with MLF stimuli (mean ± SEM 0.689 ± 0.018 ms vs 0.892 ± 0.03 ms, p < 0.001, Mann–Whitney U test). In total, 48% of the population (67 cells) had monosynaptic and/or disynaptic excitation evoked by MLF stimulation. Almost all of these received convergent input from both corticospinal and reticulospinal tracts (61/67 cells, 91%). The average monosynaptic corticospinal EPSP was more than five times larger than either the average monosynaptic or disynaptic reticulospinal EPSP. Clear inhibitory effects without concomitant EPSPs from MLF stimulation were not observed in any motoneurons, although late hyperpolarizations superimposed on the EPSPs suggest that IPSPs were evoked when trains of stimuli were used (see Fig. 2C,D,M,N for examples). Because they were superimposed on EPSPs, reliable identification and characterization of these IPSPs was difficult and they could not be analyzed further.
Figure 3.
Population data. A–D, Histograms of incidence (left) and mean amplitude (right) of monosynaptic EPSPs from the PT (PT mono), monosynaptic EPSPs evoked from the MLF (MLF mono), and disynaptic EPSPs from the MLF (MLF di). The numbers above each column in the incidence plots give the raw numbers of motoneurons. Error bars in amplitude plots are SEM. Amplitudes of disynaptic EPSPs are measured from the response to the last of a train of three or four shocks.
Reticulospinal connections to motoneurons controlling hand and wrist muscles
It is of special interest to know whether the reticulospinal tract makes direct or indirect connections to spinal motoneurons innervating intrinsic hand muscles: strong corticospinal connections to these motoneurons are usually assumed to underlie the unique dexterity of the primate hand.
Figure 2K–O illustrates an example motoneuron antidromically activated from the median nerve at the wrist (Fig. 2K), indicating that it innervated intrinsic hand muscles (most likely thenar muscles controlling the thumb). Stimuli delivered to the MLF elicited powerful, short-latency monosynaptic EPSPs (amplitude 0.81 mV, SL 0.9 ms) (vertical dashed lines and gray shading, Fig. 2L). Trains of MLF stimuli elicited responses with similar rising phases and amplitudes (Fig. 2M,N). In this cell the monosynaptic reticulospinal response exceeded its corticospinal counterpart: a single stimulus to PT resulted in a monosynaptic EPSP 0.6 mV in amplitude, SL 0.9 ms (Fig. 2O).
As previously reported (Fritz et al., 1985; Porter and Lemon, 1993), the corticospinal EPSPs were more frequent and exhibited a trend toward larger effects in motoneurons innervating forearm or hand muscles compared with those projecting to the upper arm (Fig. 3). Surprisingly, trends in incidence of EPSPs evoked both monosynaptically and disynaptically by MLF stimuli were not unlike the trends seen for corticospinal EPSPs: the frequency was at least as large in intrinsic hand motoneurons as in more proximally projecting forearm or upper arm motoneurons and, where connections were present, the amplitudes of MLF-evoked EPSPs (monosynaptic and disynaptic) were generally similar in the different muscle groups.
Discussion
The present results force a reevaluation of the role of the reticulospinal tract in primate motor control. They show that reticulospinal descending pathways are in a position to influence motoneurons projecting both to proximal and distal limb muscles, including intrinsic hand muscles, and can do so via both direct monosynaptic and disynaptic pathways. In our data reticulospinal connections were as common in intrinsic hand muscle motoneurons as in forearm and upper arm groups. Reticulospinal pathways may thus provide a parallel to the corticospinal pathway for supraspinal control.
The finding that fibers activated by stimulation near the MLF influence forearm and hand muscles was surprising. Lesions that involve these fiber tracts (reticulospinal, vestibulospinal, and tectospinal) have a powerful effect on the control of axial and postural muscles (Lawrence and Kuypers, 1968b; Kuypers, 1981). Microstimulation studies of the reticular formation found the most potent effects in proximal muscles (Perreault et al., 1994; Davidson and Buford, 2004, 2006). Given the novelty of our findings, it is important to consider the possibility that MLF stimulation activated other descending pathways. Activation of corticospinal fibers, by either axon reflex or current spread to the pyramidal tract, was excluded by our occlusion test (Fig. 1E–G). Rubrospinal fibers originate far rostral to the medulla where the MLF stimulating electrode was located; the axons pass laterally (Kuypers et al., 1962), whereas the electrode tips were close to the midline (Fig. 1). Current would have to spread several millimeters to have activated the rubrospinal tract, making this possibility unlikely. Primate tectospinal neurons are few (Harting, 1977) and reported not to make monosynaptic connections with motoneurons (Anderson et al., 1972). Studies of the medial vestibulospinal tract in cat suggest that it is purely inhibitory to motoneurons (Wilson et al., 1970). Some premotor interneurons such as the C3/C4 propriospinal neurons have axons ascending to the medulla. However, these are less common in primate and their terminals in the lateral reticular nucleus are also laterally positioned, several millimeters from the MLF electrodes. The large MLF electrode volleys suggest that the major fiber pathway activated was reticulospinal.
Our classification of effects evoked by MLF stimulation as monosynaptic or disynaptic was based on the properties and the latency of the evoked EPSPs. However, it is likely that we underestimated the prevalence of monosynaptic connections: stimulation in the MLF activates some reticulospinal fibers directly, and others transsynaptically (especially after trains of stimuli) (Jankowska et al., 2003; Edgley et al., 2004). Our criterion for a monosynaptic connection was segmental latency <1 ms, relative to the descending volleys of directly activated reticulospinal fibers; by measuring the latency relative to the first segmental volley, we automatically take account of utilization time and axonal conduction delay to the cord. However, any actions evoked by transsynaptically activated reticulospinal fibers would be classified as disynaptic in motoneurons by this criterion, although they could be mediated by direct reticulomotoneuronal contacts.
The corticospinal and reticulospinal actions in upper limb motoneurons were similarly distributed but had some key differences, most notably amplitude. On average, monosynaptic corticospinal EPSPs were five times larger than monosynaptic or disynaptic reticulospinal EPSPs. Part of this difference could be the activation of different fiber numbers. The stimuli we used activated large descending volleys (Fig. 1E–G) but were unlikely to have activated all reticulospinal axons that descend in the region of the MLF. Large stimulating currents would risk stimulus spread and a possible mixed descending volley. Instead, we used a fixed stimulus intensity (300 μA), yielding robust but pure spinal volleys. Reticulospinal axons arise from many locations including the medullary gigantocellular reticular formation level with and caudal to the MLF stimulating electrodes. These fibers may not have been activated.
In all species studied the reticulospinal tract influences motoneurons by a mixture of direct (monosynaptic) and indirect effects. From studies in nonprimates, reticulospinal output is considered to control movement predominantly via spinal interneurons [i.e., at least disynaptically (Baldissera et al., 1981)]. However, monosynaptic connections to limb motoneurons, including some acting distally have been consistently found. Wilson and Yoshida (1969) reported monosynaptic EPSPs in most motoneurons of cat ankle and foot digit muscles, including 2/5 motoneurons projecting in the plantar nerve innervating intrinsic foot muscles. Peterson et al. (1979) found monosynaptic connections to cat upper limb motoneurons, including those projecting distally. The significance of these connections is harder to assess. Microstimulation studies in the MLF or reticular formation focus on muscles above the elbow, with little reference to more distal muscles, presumably because obvious responses were not seen (Drew and Rossignol, 1990; Perreault et al., 1994; Davidson and Buford, 2004, 2006; Davidson et al., 2007). Important findings in relation to this are the spike-triggered averaging results between reticulospinal neurons and limb EMG (Schepens and Drew, 2006; Davidson et al., 2007). Davidson et al. (2007) demonstrated that microstimulation within the primate reticular formation produced bilateral activation of forearm and shoulder muscle groups as far distal as the wrist. Spike- and stimulus-triggered averaging yielded similar activity patterns, implying individual reticular neurons are capable of motoneuronal activation. In cat, postspike depression (reflecting inhibition that is at least disynaptic) was particularly common. The paucity of postspike facilitations in cat, despite the evidence from acute studies that monosynaptic connections with motoneurons exist, suggests that interneuronally mediated effects dominate. Critically, Schepens and Drew (2006) found that while the reticulospinal neuron activity was similar during reaching movements of either forelimb, postspike depression in the EMG occurred selectively when only one limb was moving. This implies a movement-specific gating at the level of spinal interneurons. A possible interpretation is that the monosynaptic connections are relatively weak and unable to evoke overt movement in response to stimulation or postspike effects in spike-triggered averaging, but that the disynaptic connections are more potent and more easily produce movements.
In primates, the forelimb is critical for fine manipulation. An important function of the reticular formation identified in cats (Schepens and Drew, 2004, 2006) and primates (Davidson and Buford, 2004) is the control of forelimb reaching. Our data suggest that this extends to control of the hand movements that complete a complex goal directed reach. In contrast to reticulospinal outflow, monosynaptic corticospinal EPSPs in macaque monkeys are common; it is unclear how functionally important disynaptic inputs are in the awake behaving state (Maier et al., 1997; Alstermark et al., 1999; Nakajima et al., 2000; Olivier et al., 2001; Sasaki et al., 2004). Primate corticomotoneuronal connections may underlie the ability to produce relatively independent finger movements. Species that lack dexterous hands, such as cats, have only disynaptic connections from the corticospinal tract to motoneurons (Kuypers, 1981). New World primates have an intermediate pattern, with mainly disynaptic connections but some weak, slowly rising monosynaptic corticospinal EPSPs (Maier et al., 1997).
Corticomotoneuronal cells in macaques project to multiple motoneuron pools (Fetz and Cheney, 1978; Shinoda et al., 1981; Buys et al., 1986). By addressing muscles not individually, but as functional groups of synergists, the formidable degrees of freedom involved in controlling the hand may be reduced, permitting effective independent finger control (Schieber, 2001). Although the reticulospinal tract has some features required for dexterous hand movement control, it is unclear whether all details of its organization are suitable to subserve this function. From the anatomy, microstimulation, and spike-triggered averaging data, it is unlikely that single reticulospinal neurons control small groups of muscles in a fractionated way. Individual RST axons exhibit extensive collateralization throughout the cervical and lumbar enlargements of cats (Peterson et al., 1975; Matsuyama et al., 1997, 1999). In addition there is evidence for bilateral effects from reticulospinal neurons (Jankowska et al., 2003; Schepens and Drew, 2006; Davidson et al., 2007). This may represent the necessary anatomical substrate for the control of larger functional muscle groups.
Lawrence and Kuypers (1968b) made combined lesions of the pyramids and the lateral medulla, interrupting rubrospinal fibers. In these monkeys the hand was not completely paralyzed but could still be used in whole-arm, whole-body movements, e.g., holding a support while climbing or grasping. Reticulospinal fibers are prime candidates for mediating this type of distal muscle activity. This fits with a general theme for reticulospinal function: descending fibers have widespread connections with diverse muscles that act in synergy during whole-body movement (Lawrence and Kuypers, 1968b; Drew and Rossignol, 1990; Schepens and Drew, 2006). For example, microstimulation in the cat usually evokes responses in multiple muscles, frequently multiple limbs and/or limb and head (Drew and Rossignol, 1990). In cats, there is considerable recovery of basic locomotor function after lesions of corticospinal and rubrospinal tracts that can be attributed to medially descending pathways (Drew et al., 2002), although movements of the most distal muscles do not fully recover.
The demonstration that both monosynaptic and disynaptic connections link reticulospinal tract fibers to distal limb motoneurons may have significance for repair or rehabilitation. This could form the substrate for the recovery of hand use following loss of corticospinal control in nonhuman primates (Lawrence and Kuypers, 1968a; Sasaki et al., 2004), and thus present a target for rehabilitation after stroke in man.
Footnotes
This work was funded by The Wellcome Trust and Medical Research Council (UK). C.N.R. was supported by a studentship from Merck Sharp & Dohme. S.A.E. was supported by National Institutes of Health Grant 5R01NS040863-07.
References
- Alstermark B, Isa T, Ohki Y, Saito Y. Disynaptic pyramidal excitation in forelimb motoneurons mediated via C(3)-C(4) propriospinal neurons in the Macaca fuscata. J Neurophysiol. 1999;82:3580–3585. doi: 10.1152/jn.1999.82.6.3580. [DOI] [PubMed] [Google Scholar]
- Anderson ME, Yoshida M, Wilson VJ. Tectal and tegmental influences on cat forelimb and hindlimb motoneurons. J Neurophysiol. 1972;35:462–470. doi: 10.1152/jn.1972.35.4.462. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Hultborn H, Illert M. Integration in spinal neuronal systems. In: Brookhart JM, Mountcastle VB, editors. Handbook of physiology—the nervous system II. Bethesda, MD: American Physiological Society; 1981. pp. 509–595. [Google Scholar]
- Buys EJ, Lemon RN, Mantel GW, Muir RB. Selective facilitation of different hand muscles by single corticospinal neurones in the conscious monkey. J Physiol. 1986;381:529–549. doi: 10.1113/jphysiol.1986.sp016342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Motor outputs from the primate reticular formation to shoulder muscles as revealed by stimulus triggered averaging. J Neurophysiol. 2004;92:83–95. doi: 10.1152/jn.00083.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Bilateral actions of the reticulospinal tract on arm and shoulder muscles in the monkey: stimulus triggered averaging. Exp Brain Res. 2006;173:25–39. doi: 10.1007/s00221-006-0374-1. [DOI] [PubMed] [Google Scholar]
- Davidson AG, Schieber MH, Buford JA. Bilateral spike-triggered average effects in arm and shoulder muscles from the monkey pontomedullary reticular formation. J Neurosci. 2007;27:8053–8058. doi: 10.1523/JNEUROSCI.0040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew T, Rossignol S. Functional organization within the medullary reticular formation of intact unanesthetized cat. I. Movements evoked by microstimulation. J Neurophysiol. 1990;64:767–781. doi: 10.1152/jn.1990.64.3.767. [DOI] [PubMed] [Google Scholar]
- Drew T, Dubuc R, Rossignol S. Discharge patterns of reticulospinal and other reticular neurons in chronic, unrestrained cats walking on a treadmill. J Neurophysiol. 1986;55:375–401. doi: 10.1152/jn.1986.55.2.375. [DOI] [PubMed] [Google Scholar]
- Drew T, Jiang W, Widajewicz W. Contributions of the motor cortex to the control of the hindlimbs during locomotion in the cat. Brain Res Brain Res Rev. 2002;40:178–191. doi: 10.1016/s0165-0173(02)00200-x. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Hammar I. Ipsilateral actions of feline corticospinal tract neurons on limb motoneurons. J Neurosci. 2004;24:7804–7813. doi: 10.1523/JNEUROSCI.1941-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz EE, Cheney PD. Muscle fields of primate corticomotoneuronal cells. J Physiol (Paris) 1978;74:239–245. [PubMed] [Google Scholar]
- Fritz N, Illert M, Kolb FP, Lemon RN, Muir RB, Van der Burg J, Wiedemann E, Yamaguchi T. The cortico-motoneuronal input to hand and forearm motoneurones in the anaesthetized monkey. J Physiol. 1985;366:20. [Google Scholar]
- Grillner S, Hongo T, Lund S. Reciprocal effects between two descending bulbospinal systems with monosynaptic connections to spinal motoneurones. Brain Res. 1968;10:477–480. doi: 10.1016/0006-8993(68)90221-7. [DOI] [PubMed] [Google Scholar]
- Grillner S, Georgopoulos AP, Jordan LM. Selection and initiation of motor behavior. In: Stein PSG, Grillner S, Selverston AI, Stuart DG, editors. Neurons, networks, and motor behavior. Cambridge, MA: MIT; 1997. pp. 3–19. [Google Scholar]
- Harting JK. Descending pathways from the superior collicullus: an autoradiographic analysis in the rhesus monkey (Macaca mulatta) J Comp Neurol. 1977;173:583–612. doi: 10.1002/cne.901730311. [DOI] [PubMed] [Google Scholar]
- Holstege G, Kuypers HG. The anatomy of brain stem pathways to the spinal cord in cat. A labeled amino acid tracing study. Prog Brain Res. 1982;57:145–175. doi: 10.1016/S0079-6123(08)64128-X. [DOI] [PubMed] [Google Scholar]
- Holstege JC, Kuypers HG. Brainstem projections to spinal motoneurons: an update. Neuroscience. 1987;23:809–821. doi: 10.1016/0306-4522(87)90160-6. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Lund S, Lundberg A, Pompeiano O. Inhibitory effects evoked through ventral reticulospinal pathways. Arch Ital Biol. 1968;106:124–140. [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Slawinska U, Maleszak K, Edgley SA. Neuronal basis of crossed actions from the reticular formation upon feline hindlimb motoneurones. J Neurosci. 2003;23:1867–1878. doi: 10.1523/JNEUROSCI.23-05-01867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE, Yang TZ. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J Comp Neurol. 1985;242:56–92. doi: 10.1002/cne.902420105. [DOI] [PubMed] [Google Scholar]
- Kuypers HG, Fleming WR, Farinholt JW. Subcorticospinal projections in the rhesus monkey. J Comp Neurol. 1962;118:107–137. doi: 10.1002/cne.901180109. [DOI] [PubMed] [Google Scholar]
- Kuypers HG. Anatomy of the descending pathways. In: Brookhart JM, Mountcastle VB, editors. Handbook of physiology—the nervous system II. Bethesda, MD: American Physiological Society; 1981. pp. 597–666. [Google Scholar]
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain. 1968a;91:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathways. Brain. 1968b;91:15–36. doi: 10.1093/brain/91.1.15. [DOI] [PubMed] [Google Scholar]
- Lund S, Pompeiano O. Monosynaptic excitation of alpha motoneurones from supraspinal structures in the cat. Acta Physiol Scand. 1968;73:1–21. doi: 10.1111/j.1748-1716.1968.tb04075.x. [DOI] [PubMed] [Google Scholar]
- Maier MA, Olivier E, Baker SN, Kirkwood PA, Morris T, Lemon RN. Direct and indirect corticospinal control of arm and hand motoneurons in the squirrel monkey (Saimiri sciureus) J Neurophysiol. 1997;78:721–733. doi: 10.1152/jn.1997.78.2.721. [DOI] [PubMed] [Google Scholar]
- Martin GF, Vertes RP, Waltzer R. Spinal projections of the gigantocellular reticular formation in the rat. Evidence for projections from different areas to laminae I and II and lamina IX. Exp Brain Res. 1985;58:154–162. doi: 10.1007/BF00238963. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Takakusaki K, Nakajima K, Mori S. Multi-segmental innervation of single pontine reticulospinal axons in the cervico-thoracic region of the cat: anterograde PHA-L tracing study. J Comp Neurol. 1997;377:234–250. [PubMed] [Google Scholar]
- Matsuyama K, Mori F, Kuze B, Mori S. Morphology of single pontine reticulospinal axons in the lumbar enlargement of the cat: a study using the anterograde tracer PHA-L. J Comp Neurol. 1999;410:413–430. [PubMed] [Google Scholar]
- Mori S. Integration of posture and locomotion in acute decerebrate cats and in awake, freely moving cats. Prog Neurobiol. 1987;28:161–195. doi: 10.1016/0301-0082(87)90010-4. [DOI] [PubMed] [Google Scholar]
- Mori S, Matsuyama K, Mori F, Nakajima K. Supraspinal sites that induce locomotion in the vertebrate central nervous system. Adv Neurol. 2001;87:25–40. [PubMed] [Google Scholar]
- Nakajima K, Maier MA, Kirkwood PA, Lemon RN. Striking differences in transmission of corticospinal excitation to upper limb motoneurons in two primate species. J Neurophysiol. 2000;84:698–709. doi: 10.1152/jn.2000.84.2.698. [DOI] [PubMed] [Google Scholar]
- Nyberg-Hansen R. The location and termination of tecto-spinal fibres in the cat. Exp Neurol. 1964;9:212–227. doi: 10.1016/0014-4886(64)90018-4. [DOI] [PubMed] [Google Scholar]
- Olivier E, Baker SN, Nakajima K, Brochier T, Lemon RN. Investigation into non-monosynaptic corticospinal excitation of macaque upper limb single motor units. J Neurophysiol. 2001;86:1573–1586. doi: 10.1152/jn.2001.86.4.1573. [DOI] [PubMed] [Google Scholar]
- Orlovskiı̆ GN. Connections of the reticulo-spinal neurons with the “locomotor sections” of the brainstem. Biofizika. 1970;15:171–178. [PubMed] [Google Scholar]
- Perreault MC, Rossignol S, Drew T. Microstimulation of the medullary reticular formation during fictive locomotion. J Neurophysiol. 1994;71:229–245. doi: 10.1152/jn.1994.71.1.229. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Maunz RA, Pitts NG, Mackel RG. Patterns of projection and branching of reticulospinal neurons. Exp Brain Res. 1975;23:333–351. doi: 10.1007/BF00238019. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Pitts NG, Fukushima K. Reticulospinal connections with limb and axial motoneurones. Exp Brain Res. 1979;36:1–20. doi: 10.1007/BF00238464. [DOI] [PubMed] [Google Scholar]
- Porter R, Lemon RN. Corticospinal function and voluntary movement. Oxford: Oxford UP; 1993. [Google Scholar]
- Prentice SD, Drew T. Contributions of the reticulospinal system to the postural adjustments occurring during voluntary gait modifications. J Neurophysiol. 2001;85:679–698. doi: 10.1152/jn.2001.85.2.679. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Isa T, Pettersson LG, Alstermark B, Naito K, Yoshimura K, Seki K, Ohki Y. Dexterous finger movements in primate without monosynaptic corticomotoneuronal excitation. J Neurophysiol. 2004;92:3142–3147. doi: 10.1152/jn.00342.2004. [DOI] [PubMed] [Google Scholar]
- Schepens B, Drew T. Independent and convergent signals from the pontomedullary reticular formation contribute to the control of posture and movement during reaching in the cat. J Neurophysiol. 2004;92:2217–2238. doi: 10.1152/jn.01189.2003. [DOI] [PubMed] [Google Scholar]
- Schepens B, Drew T. Descending signals from the pontomedullary reticular formation are bilateral, asymmetric, and gated during reaching movements in the cat. J Neurophysiol. 2006;96:2229–2252. doi: 10.1152/jn.00342.2006. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Constraints on somatotopic organization in the primary motor cortex. J Neurophysiol. 2001;86:2125–2143. doi: 10.1152/jn.2001.86.5.2125. [DOI] [PubMed] [Google Scholar]
- Shapovalov AI. Extrapyramidal monosynaptic and disynaptic control of mammalian alpha-motoneurons. Brain Res. 1972;40:105–115. doi: 10.1016/0006-8993(72)90114-x. [DOI] [PubMed] [Google Scholar]
- Shapovalov AI, Gurevitch NR. Monosynaptic and disynaptic reticulospinal actions on lumbar motoneurones of the rat. Brain Res. 1970;21:249–263. doi: 10.1016/0006-8993(70)90366-5. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Yokota J, Futami T. Divergent projection of individual corticospinal axons to motoneurons of multiple muscles in the monkey. Neurosci Lett. 1981;23:7–12. doi: 10.1016/0304-3940(81)90182-8. [DOI] [PubMed] [Google Scholar]
- Soteropoulos DS, Baker SN. Cortico-cerebellar coherence during a precision grip task in the monkey. J Neurophysiol. 2006;95:1194–1206. doi: 10.1152/jn.00935.2005. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Kohyama J, Matsuyama K, Mori S. Medullary reticulospinal tract mediating the generalised motor inhibition in cats: parallel inhibitory mechanisms acting on motoneurones and on interneuronal transmission in reflex pathways. Neuroscience. 2001;103:511–527. doi: 10.1016/s0306-4522(00)00586-8. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Yoshida M. Comparison of effects of stimulation of Deiters' nucleus and medial longitudinal fasciculus on neck, forelimb and hindlimb motoneurons. J Neurophysiol. 1969;32:743–758. doi: 10.1152/jn.1969.32.5.743. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Wylie RM, Marco LA. Organisation of the medial vestibular nucleus. J Neurophysiol. 1968;31:166–175. doi: 10.1152/jn.1968.31.2.166. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Yoshida M, Schor RH. Supraspinal monosynaptic excitation and inhibition of thoracic back motoneurons. Exp Brain Res. 1970;11:282–295. doi: 10.1007/BF01474387. [DOI] [PubMed] [Google Scholar]