Abstract
Objective
To examine sex differences in autonomic nervous system functioning in children and adolescents with conduct problems and to evaluate the role of aggression in predicting autonomic nervous system functioning, over and above the effects of disruptive behavior. Although deficiencies in autonomic responding among boys with oppositional defiant disorder and/or conduct disorder are well documented, it remains unclear whether such findings extend to girls or apply only to children with aggressive forms of conduct problems.
Method
Electrodermal responding, cardiac pre-ejection period, and respiratory sinus arrhythmia were recorded while boys (n = 110; 53 with conduct problems, 57 controls) and girls (n = 65; 33 with conduct problems, 32 controls) between the ages of 8 and 12 sat for an extended baseline, then played a game with conditions of reward and frustrative nonreward.
Results
Both sex effects and aggression effects were found. Aggressive boys with conduct problems demonstrated reduced autonomic functioning, consistent with previous research. In contrast, aggressive girls with conduct problems exhibited greater electrodermal responding than controls, with no differences in cardiovascular reactivity to incentives.
Conclusions
Observed sex differences in the autonomic correlates of conduct problems and aggression may suggest different etiological mechanisms of externalizing psychopathology for girls compared with boys.
Keywords: conduct disorder, aggression, electrodermal responding, cardiac pre-ejection period, respiratory sinus arrhythmia
Externalizing behaviors in children and adolescents have been linked consistently with autonomic nervous system (ANS) deficiencies.1 Reduced electrodermal responding (EDR) and lower resting heart rate (HR) predict both concurrent and future conduct problems and aggression among male preschoolers, middle schoolers, and adolescents.2,3 Deficiencies in both sympathetic and parasympathetic influences on cardiovascular functioning are also observed in males with conduct disorder (CD).4,5
Despite consistency in these findings, several limitations characterize the current literature. First, most studies have included an overwhelming preponderance of males.1,6 This limitation is likely due to the uneven sex distribution of severe CD, in which boys outnumber girls in ratios approaching 10:1.7 Such large disparities in prevalence may suggest different etiological mechanisms of externalizing behavior for girls versus boys, a conjecture that is supported by recent behavioral genetics research. For example, Rose and colleagues8 found that adding sex-specific genetic terms to their structural models of CD significantly improved model fit. In turn, sex-specific vulnerabilities to CD may be reflected in psychophysiological responding.
A second limitation of the current literature concerns the role of conduct problem severity in predicting ANS responding. Among adults, ANS deficiencies are most often observed in more serious offenders, including psychopaths.1 In contrast, most studies of ANS responding among children and adolescents have not distinguished between different forms of conduct problems. However, in a recent meta-analysis, Lorber1 reported that deficient HR responding is linked to both conduct problems and aggression. Nevertheless, in studies that differentiate between adolescent males who score high and low on aggression, larger ANS deficiencies are often observed among aggressive participants.2,4 At present, however, such studies are few in number, and no studies have explored the role of aggression in predicting ANS response deficiencies among girls.
Finally, most studies of ANS responding among youths with conduct problems have used HR as a dependent variable. Yet HR is determined by both sympathetic and parasympathetic influences, and dysregulation in the sympathetic nervous system (SNS) appears to have different implications for adjustment than dysregulation in the parasympathetic nervous system (PNS). SNS-linked cardiac activity has been associated with approach motivational processes,5,9 whereas PNS-linked cardiac activity consistently predicts emotion regulation capabilities.5,10 Accordingly, the cardiac pre-ejection period (PEP), an index of SNS-linked cardiac activity, has been used as a peripheral marker of CNS reward sensitivity during incentive conditions. Studies of autonomic activity during appetitive and aversive responding indicate that PEP reactivity is specific to conditions of reward, whereas respiratory sinus arrhythmia (RSA), a marker PNS activation, responds across a wide variety of stimulus conditions.9 This finding is consistent with the functional role of the SNS. When mobilized in response to environmental demands, the SNS increases cardiac output in preparation for approach or active avoidance behaviors.11 Studies have shown decreased PEP reactivity during conditions of reward among aggressive preschoolers, middle schoolers, and adolescents with disruptive behavior disorders, suggesting reward insensitivity among these groups.4,5,12
In contrast, RSA is often used to index vagal tone and vagal reactivity.13 Primarily of parasympathetic origin, RSA reflects both attentional and emotional processes.10,14 Deficiencies in RSA consistently mark emotional lability, a sine qua non of psychopathology. Reduced RSA is observed consistently in aggressive middle schoolers and adolescents4,15 and has been linked with trait hostility,16 depression,17 self-injury,18 anxiety,19 and panic.20 In contrast, accumulating evidence suggests that effective vagal modulation of HR can buffer children from developing internalizing and externalizing behavioral disorders in adverse familial environments.21–23
Finally, EDR, a marker of SNS activation that is independent of PEP, has been used to index behavioral inhibition. Attenuated EDR characterizes externalizing males who are low on behavioral inhibition, including those who are aggressive and those with related externalizing behavior patterns.2,4,12 In contrast, inhibited children exhibit higher skin conductance levels than uninhibited children and are at risk of anxiety disorders.24,25
Despite these findings, few studies conducted to date have included differentiated measures of ANS responding, and even fewer have examined sex differences. Our primary objectives were to evaluate group differences in ANS responding between males and females with conduct problems using measures of both sympathetic and parasympathetic responding and determine whether aggression accounts for variance in ANS responding, over and above the effects of conduct problems. Toward accomplishing the first objective, we recruited a larger sample of disruptive girls than found in most studies, which provided adequate statistical power to detect sex effects. We evaluated both SNS- and PNS-linked cardiac reactivity to reward among participants. In addition, given moderately consistent findings linking resting EDR to conduct problems,1 we compared skin conductance responses among boys and girls with and without disruptive behavioral profiles.
METHOD
Participants
All of the study procedures were approved by the University of Washington Institutional Review Board, and both parental consent and child assent were obtained. Data were collected as part of a longitudinal study examining the development of conduct problems in middle childhood. The sample included 175 children (110 males, 65 females) between the ages of 8 and 12 (mean 9.7, SD 1.5). Among males, 53 had oppositional defiant disorder (ODD) and/or conduct disorder (CD) according to parent report and 57 were nonpsychiatric controls (see below). Among females, 33 had ODD and/or CD and 32 were nonpsychiatric controls. We also assessed aggression to ensure that all of the externalizing participants had severe conduct problems and that none were merely defiant (see below).
Participants were recruited through advertisements placed in Seattle newspapers, community publications, and city buses seeking disruptive and aggressive children. Parents who responded to these ads completed a computerized structured telephone interview with a trained research assistant. Interviews lasted between 20 and 30 minutes and included portions of the Child Symptom Inventory (CSI)26 and the Child Behavior Checklist (CBCL).27 The CSI yields both dimensional scores and diagnostic cutoffs for many DSM-IV disorders. Symptoms are rated on a 4-point scale (0 = never, 1 = sometimes, 2 = often, 3 = very often), with ratings of ≥2 considered positive for each diagnostic criterion. Scales from the CSI included those assessing ODD, CD, and attention-deficit/hyperactivity disorder. In the most recent validation sample, internal consistencies (α) were.91,.91, and.79 for the ADHD, ODD, and CD scales, respectively, and 4-month test-retest reliabilities were.72,.65, and.68, respectively.28 In addition, the attention problems, aggressive behavior, and anxious/depressed subscales of the CBCL were administered.
Interviews were scored by computer immediately, and parents of children who met inclusion criteria (conduct problem or control) were invited to participate. To be included in the conduct problem group, children were required to meet DSM-IV criteria on the CSI for ODD and/or CD and to score at or above the 98th percentile (T ≥ 70) on the CBCL Aggression subscale. In total, 98% (84/86) of participants with conduct problems met criteria for ODD and 66% (57/86) met criteria for CD. In addition, 85% (73/86) met criteria for attention-deficit/hyperactivity disorder. No sex difference in the distribution of diagnoses was observed (χ22 = 2.89, p =.24). To be included in the control group, participants had to be free of psychopathology on all of the CSI-assessed scales and have a T score of ≤60 on all of the CBCL scales. Children with symptoms of psychosis, autism, or mental retardation were excluded from the study. In addition, children who were taking medications other than psychostimulants were excluded. For children who were taking stimulants (n = 18), a 48-hour washout period was required before participation in the psychophysiological protocol, a procedure used in several previous studies.4,12 In the conduct problem group, stimulant use at the time of assessment was not correlated with sex or with symptoms of ODD, CD, or aggression (r ≤ 0.19, p ≤.17). It was therefore not included as a covariate in any of the analyses. Rates of current smoking and other drug use (n = 2) were still low in this age range, so no statistical controls were used. A $75 incentive was provided to the parent, who was also told that his or her child could earn between $25 and $30 playing a video game during the laboratory visit, described below.
Procedure
Participants were seated in a sound-attenuated room that was monitored by video camera and microphone. Patterns of cardiac and electrodermal activity were measured during a 5-minute baseline, which was followed by a computerized repetitive response task that included several blocks of reward. Because this task has been described in detail elsewhere,4 it is only summarized below.
Large, single-digit numbers (1, 3, 5, 7, 9) were presented in random order on a large projection screen. Participants were required to press the matching number on a 10-key pad placed near their dominant hand, and then press the enter key to proceed to the next stimulus. After 2 minutes of practice, the task was performed across six 2-minute blocks, each separated by a 2.5-minute rest period. Intertrial baselines (see below) were recorded during the last 30 seconds of these rest periods. During the first three blocks, signal tones and a 6-cent reward followed each correct response. An incorrect response resulted in no signal tones and no reward. The fourth block included 30 seconds of reward and 90 seconds of extinction. The fifth reverted to all reward. The sixth blocked consisted of 90 seconds of reward and 30 seconds of extinction. Throughout the task, the total amount of money earned was displayed in the upper right corner of the projection screen. Participants were told they could earn more money the faster they played, that most children earn about $25, and that they would have to play through periods of extinction to advance to the next reward block. Participants were asked to sit quietly through all of the baselines. This task has been used in a series of studies to demonstrate group differences in autonomic responding among male children and adolescents with and without CD.4,5 One advantage of this task for evaluating individual differences in psychophysiological responding is that it tends not to generate behavior differences in response speed, as more complex tasks often do.4,9 Thus, psychophysiological responses are not confounded with differences in behavioral responding.
For purposes of this study, only data collected during the intertrial baselines (six) and the pure reward blocks (four) are presented because these are the conditions under which autonomic deficiencies among externalizing children have been observed consistently.4,5,9,12 Thus, data collected during extinction are omitted. However, these data were analyzed and no sex differences or aggression effects in reactivity during extinction were found.
Psychophysiological Measures
Cardiac PEP
SNS-linked cardiac activity was assessed using PEP, an index of the time elapsed between left ventricular depolarization and ejection of blood into the aorta. Shorter intervals represent greater SNS activity. Electrocardiographic and impedance cardiographic signals were obtained using a HIC 2000 Impedance Cardiograph (Chapel Hill, NC). The electrocardiographic and impedance cardiographic waveforms were sampled according to established guidelines using spot electrodes.29 PEPs were ensemble averaged in 30-second epochs using Bio-Impedance Technology’s CopWin software, version 6.0. The validity of PEP as an index of SNS-linked cardiac activity has been established via β-adrenergic blockade.11
RSA
PNS-linked cardiac activity was assessed using spectral analysis, which decomposes the HR time series into component frequencies using fast Fourier transformations. High-frequency variability (>0.15 Hz) was extracted to measure RSA. PNS influences on HR are observed in this range.13 Spectral densities were calculated in 30-second epochs, roughly the minimum length required for reliable assessment,4,13 and normalized via natural log transformations using Nevrokard software. The validity of RSA as an index of PNS-linked cardiac activity has been established via cholinergic blockade.13
EDR
Skin conductance was recorded using a Grass Model 15LT Physiodata Amplifier System and a 15A12 DC amplifier (West Warwick, RI). The EDR signal was collected using two 0.8-cm2 Ag-AgCl electrodes secured to the thenar eminence of the participant’s nondominant hand with Parker Labs Signa Gel (Fairfield, NJ). The number of nonspecific skin conductance responses exceeding 0.05 microsecond was recorded by trained research assistants using Grass PolyVIEW software.
Data Analyses
Before performing statistical analyses, all of the variables were examined to ensure that they conformed to the assumptions of multiple linear regression. Although aggression T scores were moderately kurtotic (1.3), all of the other values of skew (≤0.33) and kurtosis (≤0.64) were well within acceptable limits.
Because participants’ psychophysiological responses were nested within blocks and because participants were nested within groups, analyses were conducted using HLM 6.0.4.30 One advantage of multilevel modeling is the simultaneous estimation of both within-participant and between-participants effects. Accordingly, HLM with full maximum likelihood estimation was used to model EDR, PEP, and RSA among participants. For each psychophysiological measure, a two-level model was constructed. At level 1, repeated observations across blocks were modeled for each participant. At level 2, the effects of diagnostic status (CD/ODD versus control), aggression (CBCL T scores), and sex (male versus female) were modeled as level 1 predictors.
We used a forward-stepping procedure to determine whether diagnostic status, assessed by the CSI, and/or aggression, assessed by the CBCL, belonged in each model as a level 1 predictor. This involved testing each effect individually (in HLM) before adding sex as a predictor in the full models. In addition, because both male and female participants in the conduct problem groups reported elevated CBCL anxious/depressed T scores and because age effects are common for psychophysiological measures collected in middle childhood,31 we also forward stepped these variables into each model as level 1 predictors. Thus, when the effects of anxiety/depression or age were nonsignificant in forward stepping, they were dropped from the final models to maximize degrees of freedom. Intercepts in all of the models were mean centered, providing an index of group differences in responding across trials.
RESULTS
Descriptive Statistics
Descriptive statistics for both the psychopathology and the ANS measures are reported by group and sex in Table 1. As expected given the recruitment strategy, participants with conduct problems exceeded control participants on all measures of psychopathology. Analyses of the behavioral data indicated no group difference in response speed between participants with and without conduct problems, F1,162 = 0.13, p =.72.
TABLE 1.
Sample Descriptive Characteristics (Mean and SD) by Group and Sex
| Variable | Males |
Females |
||||
|---|---|---|---|---|---|---|
| Disruptive/Aggressive (n = 53) | Control (n = 57) | Effect Size (d) | Disruptive/Aggressive (n = 33) | Control (n = 32) | Effect Size (d) | |
| Age, y | 9.8 (1.5) | 9.8 (1.5) | 0.0 | 9.4 (1.5) | 9.3 (1.5) | 0.0 |
| CSI ODD criteria endorsed | 18.4 (3.5) | 4.1 (3.2) | 4.3 | 17.9 (3.7) | 5.5 (3.1) | 3.6 |
| CSI CD criteria endorsed | 8.1 (5.0) | 0.9 (1.5) | 2.2 | 7.8 (4.7) | 1.3 (1.7) | 2.0 |
| CSI ADHD criteria endorsed | 16.4 (6.6) | 3.8 (3.7) | 2.4 | 17.4 (6.5) | 5.9 (5.2) | 2.0 |
| CBCL attention problems T score | 76.6 (10.6) | 55.8 (8.9) | 2.1 | 75.4 (9.7) | 57.5 (9.5) | 1.9 |
| CBCL aggressive behavior T score | 81.4 (6.8) | 51.6 (3.3) | 5.9 | 82.0 (9.4) | 53.5 (6.1) | 3.7 |
| CBCL anxious/depressed T score | 77.5 (10.2) | 57.0 (8.8) | 2.2 | 80.8 (10.1) | 56.5 (10.1) | 2.4 |
| Baseline electrodermal responses | 3.3 (1.5) | 3.2 (1.7) | 0.1 | 3.6 (1.9) | 3.0 (1.5) | 0.4 |
| Baseline cardiac PEP (ms) | 104.9 (18.0) | 106.3 (15.7) | 0.0 | 95.9 (16.6) | 102.9 (17.2) | 0.4 |
| Reward cardiac PEP change (ms) | 2.0 (5.2) | −0.2 (4.3) | 0.5 | 0.6 (6.5) | 0.3 (4.5) | 0.1 |
| Baseline RSA (ln[ms2]) | 6.8 (1.0) | 7.2 (1.0) | 0.4 | 7.2 (1.0) | 7.1 (1.1) | 0.1 |
| Reward RSA change (ln[ms2]) | −2.9 (1.5) | −2.7 (1.0) | 0.2 | −3.0 (1.2) | −2.8 (1.1) | 0.2 |
Note: CSI = Child Symptom Inventory; ODD = oppositional defiant disorder; CD = conduct disorder; ADHD = attention-deficit/hyperactivity disorder; CBCL = Child Behavior Checklist; PEP = pre-ejection period; RSA = respiratory sinus arrhythmia.
Cardiac PEP
Baseline
Analyses of PEP across intertrial baselines revealed no significant main effects or interactions for either intercepts or slopes.
Reward
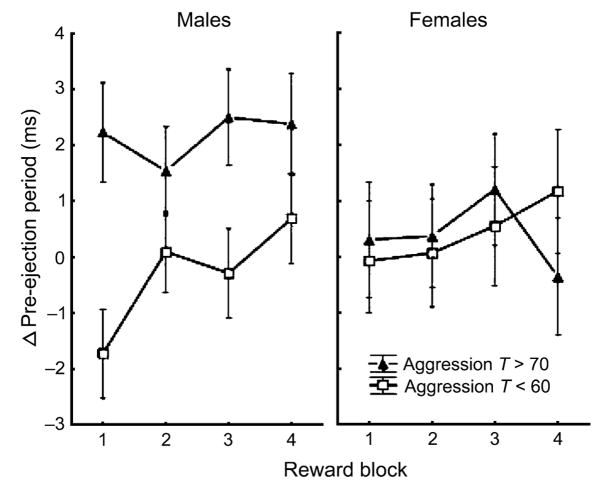
Analyses of PEP reactivity during reward were performed using change scores from baseline. Forward stepping indicated that diagnostic status, anxiety, and age did not predict either intercepts or slopes in PEP reactivity to reward. In contrast, significant effects of aggression on both intercepts and slopes were found. The significant aggression effect on intercepts (β =.06, p =.049) indicates less PEP shortening during reward among participants who were high on aggression than among participants who were low on aggression, consistent with previous research. Although the interaction effect was not significant, follow-up contrasts indicated that group differences in PEP reactivity to reward were significant for male participants, but not for female participants (Fig. 1). The significant aggression effect on slopes (β = −.01, p =.020) indicates that those who were low on aggression exhibited initial PEP reactivity to incentives, which habituated across trials. In contrast, those high on aggression exhibited no PEP reactivity to reward (Fig. 1). These findings are also consistent with previous research and were not moderated by a sex effect.
Fig. 1.
Change from baseline in cardiac pre-ejection period (ms) across reward blocks for boys (left) and girls (right) with T scores >70 on the Child Behavior Checklist (CBCL) Aggression subscale (closed triangles) and <60 on the CBCL Aggression subscale (open squares).
RSA
Baseline
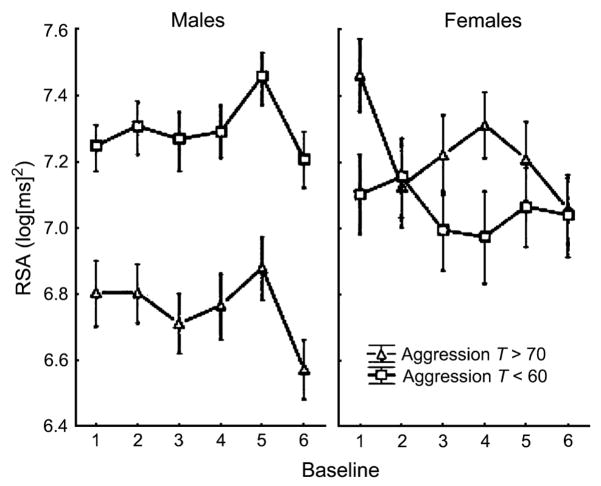
Forward stepping indicated that diagnostic status and anxiety did not predict either intercepts or slopes in baseline RSA. However, although age was not a significant predictor of intercepts, it did predict RSA slopes (β = −.02, p =.04). Thus, older participants exhibited greater reductions in RSA across baselines than younger participants. Of greater interest given the aims of this study, the final model of RSA across baselines yielded significant intercept effects for both aggression (β = −.01, p =.036) and sex (β = −1.68, p =.017), as well as a significant aggression × sex interaction (β =.03, p =.012), as depicted in Figure 2. Consistent with previous research, males who were high on aggression exhibited lower RSA across baselines than males who were low on aggression. In contrast, no difference in baseline RSA was observed for females who were high versus low on aggression.
Fig. 2.
Respiratory sinus arrhythmia values across multiple baselines for boys (left) and girls (right) with T scores >70 on the Child Behavior Checklist (CBCL) Aggression subscale (closed triangles) and <60 on the CBCL Aggression subscale (open squares).
Reward
Analyses of RSA reactivity during reward were performed using change scores from baseline. Forward stepping indicated that diagnostic status and anxiety did not predict either intercepts or slopes in RSA across reward trials. Once again, however, age predicted RSA slopes (β =.01, p =.04). Thus, older participants exhibited smaller reductions in RSA across baselines than younger participants. Furthermore, although the sex effect was nonsignificant in the final model, all of the participants exhibited reductions in RSA across reward trials (β = −.92, p <.001).
EDR
Baseline
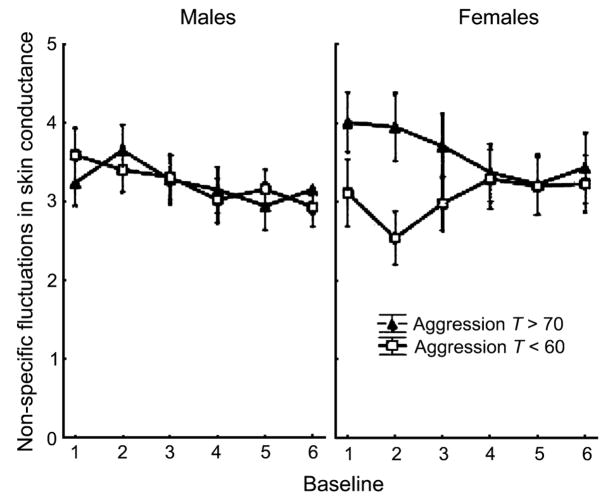
Analyses of the EDR data across baselines yielded no intercept effects for any of the predictors. Thus, no differences in mean levels of responding were observed. In the analyses of EDR slopes, both age and anxiety/depression were nonsignificant in forward stepping, so both variables were dropped from the final model. However, forward stepping indicated that both diagnostic status and aggression T scores predicted slopes in baseline EDR. Yet when both variables were included in the same model, only aggression offered independent prediction, in the form of an aggression × sex interaction (β = −.01, p =.004). Thus, aggression explained unique variance in EDR slopes, over and above the effects of conduct problems. In addition, a significant effect of sex on baseline EDR slopes was found (β =.77, p =.002). As indicated in Figure 3, boys who were high versus low on aggression did not differ in patterns of EDR across baselines. In contrast, girls who were aggressive exhibited initially high levels of EDR, which then decreased across baselines, whereas girls who were not aggressive exhibited initially low levels of EDR, which then increased across baselines.
Fig. 3.
Number of nonspecific fluctuations in skin conductance exceeding 0.05 μs across multiple baselines for boys (left) and girls (right) with T scores>70 on the Child Behavior Checklist (CBCL) Aggression subscale (closed triangles) and <60 on the CBCL Aggression subscale (open squares).
Reward
Analyses of EDR across reward trials revealed no significant main effects or interactions for either intercepts or slopes.
DISCUSSION
Three findings from this study are particularly important for discussion. First, among externalizing males, patterns of cardiovascular responding—including both low baseline RSA and PEP nonreactivity to reward—were similar to those reported in previous research. Second, these findings only emerged when aggression scores were included in the multilevel models. Thus, dichotomous disruptive behavior classifications (CD/ODD versus control) explained less variance in psychophysiological responding than aggression scores. Third, and most important, given our primary objective of examining sex differences, psycho-physiological response patterns among aggressive girls with conduct problems did not resemble those of aggressive boys with conduct problems. We discuss each of these findings in turn below.
As expected, males with conduct problems who were also aggressive exhibited lower baseline RSA and less PEP reactivity to reward than age-matched controls. These findings are consistent with a growing body of research indicating functional deficiencies in both the sympathetic and the parasympathetic branches of the ANS in similar samples.4,5,12,15 As noted in the introduction, deficient parasympathetic cardiac control, reflected in reduced RSA, has been linked repeatedly with difficulties regulating emotions.4,16–20 This replication lends further support to theories linking vagal deficiencies to dysregulated affective control.10,14
Findings of PEP nonreactivity to monetary incentives among aggressive boys with conduct problems also add to a growing literature suggesting reward insensitivity in externalizing behavior disorders.4,5,12 Among individuals without behavior disorders, PEP shortens during appetitive responding, which increases cardiac output in preparation for approach behaviors. Among boys with conduct problems, such PEP shortening is not observed.4,5,12
Among the psychophysiological measures collected, only EDR failed to differentiate between controls and aggressive boys with conduct problems. Although somewhat surprising given previous findings,2,4,12 null results for EDR as a marker of externalizing behaviors are not uncommon.1
The finding that aggression accounted for variance in psychophysiological correlates of externalizing outcomes—over and above the effects of dichotomous classifications (ODD/CD versus control)—could have resulted from at least one of two sources. One possibility is that aggression T scores offered greater statistical power because they were continuous rather than dichotomous. For sample sizes such as the one used here, dichotomizing a continuous outcome variable in multiple regression can reduce effect sizes by almost half, resulting in considerably reduced power.32 Alternatively, aggressive symptoms of CD may be more closely linked with compromised ANS functioning than nonaggressive symptoms. We suspect that the latter possibility is less likely, however, for two reasons. First, the Lorber1 meta-analysis suggested comparable effect sizes for cardiac measures when CD and aggression were used as independent variables. Second, all of the participants with conduct problems in the present study had T scores ≥70 on the Aggressive behavior subscale of the CBCL, placing them at or above the 98th percentile with respect to national norms.
Considering our primary objective, the most important findings involve observed sex differences in psychophysiological responding. Although results for the cardiovascular data among aggressive male participants were consistent with previous research, aggressive female participants exhibited different response patterns, which did not vary from those of female controls. Given that ours is the first study to examine SNS- and PNS-linked cardiac activity among aggressive girls with CD, we are reluctant to overinterpret these findings. Nevertheless, some elaboration is warranted.
If replicated, then these findings will add to the growing evidence of sex differences in vulnerability and risk for conduct problems. As outlined above, current explanations for reduced SNS- and PNS-linked cardiac activity among externalizing males focus on disruptions in motivational processes that give rise to impulsivity and on emotion dysregulation that promotes anger and emotional lability. Among males, biological markers of vulnerability for conduct problems appear to be largely heritable,33 as is genetic risk for externalizing behaviors.34 Failure to find biological markers of such vulnerabilities among aggressive girls with CD may suggest that their behaviors are being driven by different etiological mechanisms, perhaps including stronger social–environmental influences. For example, Burt et al.35 recently conducted a behavior genetics study of 708 female twin pairs, finding evidence of especially strong shared environmental influences on the development of CD among those who experienced early menarche. Although we did not collect data on the timing of menarche in this study, it is well known that early pubertal onset affects a substantial portion of girls who develop CD.36,37
There is also evidence of differential genetic effects on CD symptoms for males versus females, which could be reflected in psychophysiological response patterns. In a recent behavior genetics analysis, Rose et al.8 found evidence of significant genetic effects on CD for both boys and girls, yet the source of genetic influence differed, as indicated by improved model fit through the addition of sex-specific genetic effects. Similar findings have been reported by others.38
In contrast with the cardiovascular data, a group difference in EDR was found between controls and aggressive girls with CD. Those in the latter group exhibited higher levels of EDR in the early baselines, which gradually decreased, whereas controls exhibited initially lower levels of EDR that gradually increased. The direction of this effect was opposite to that often found among boys with and without conduct problems. We are especially reluctant to interpret these findings given that the group difference diminished and then disappeared across baselines and given that no other studies are available with which to compare our results. We therefore await future studies before speculating about potential sources of this effect.
One possible explanation for sex differences in the autonomic correlates of conduct problems is higher rates of comorbid anxiety for girls versus boys. Studies suggest that girls with CD are more likely to be diagnosed with concurrent internalizing disorders than are boys with CD.39 In turn, both trait and state anxiety have been linked with autonomic hyperresponding.25,40 Thus, symptoms of anxiety could be driving autonomic activity and reactivity among girls with conduct problems, resulting in failure to find group differences compared with controls.
Although this explanation may be valid in some samples, it is unlikely to account for findings observed in the present study, for two reasons. First, both male and female participants reported elevated CBCL anxious/depressed T scores, with no significant difference between groups. Second, anxiety scores were entered into the multilevel models using forward stepping, yet anxiety did not account for significant variance in any of the psychophysiological measures. Thus, observed sex differences in the autonomic correlates of conduct problems and aggression are more likely to be the result of an etiological factor that was not measured.
A recent meta-analysis indicated significant aggregated effects of antisocial behavior on HR for girls.41 Although these findings are not directly comparable to ours given that we measured independent SNS and PNS effects on cardiovascular functioning, one would expect some correspondence in results. Ortiz and Raine41 included eight studies in which the effects of antisocial behavior on HR were reported for girls. Among these, five studies indicated significant effects and three did not. Perhaps more important, the only clinical sample of girls—which included by far the largest number of participants (N = 206) —indicated no significant effect.42 Thus, one possible explanation for the discrepancy in findings lies in the differences in sample characteristics.
Two limitations of this study stand out as particularly important to mention. First, classification of participants into groups was not based on diagnostic interviews. Rather, participant children were assigned to conduct problem and control groups based on structured telephone interviews. Although this method has been applied successfully in similar research4,12 and yields high sensitivity and specificity vis-à-vis DSM 43 classifications,26 diagnostic interviews may have produced more homogeneous groups. Although we administered the Diagnostic Interview Schedule for Children to all of the participant children and adolescents, these data were unreliable for many of the younger children in the sample.
Second, we relied on a single informant, usually the mother, to obtain interview data. Research indicates that agreement among multiple informants in ratings of child behavior is typically modest and often identifies unique facets of child functioning.44 Yet in the age range of participant children, parent reports of externalizing behaviors—the main focus of this study—are often considered the gold standard for assessment purposes given the tendency of disruptive children to minimize their symptoms.45 Accordingly, we chose parents as a single informant. However, as noted by an anonymous reviewer, both social desirability and maternal depression are likely to influence the ability of a mother to perceive her daughter as antisocial or aggressive. Thus, this sample may not be representative of all girls with conduct problems.
In addition to these limitations, the age range of our sample (8–12 years) was restricted, which could reduce generalizability. Nevertheless, our findings are consistent with previous research conducted with both preschool and adolescent samples.4,5,12 Results from this study therefore add to a growing literature on autonomic deficiencies among children and adolescents with conduct problems. However, more studies are needed of both younger and older samples of girls, who have not been represented in previous research. Finally, most of our findings were characterized by modest effect sizes. Given the exploratory nature of the sex effects analyses in particular, we did not apply a strict correction for family wise α error rates. Thus, it will be important in future research to replicate our findings.
To our knowledge, this is the first study to compare SNS- and PNS-linked cardiac correlates of conduct problems and aggression in males versus females. When considered in conjunction with the research discussed above, our findings suggest that etiological models of externalizing psychopathology that are derived from work with males may not apply to female samples. Thus, this study highlights the need for further research on both biological vulnerabilities and risk factors for conduct problems and aggression among girls. As a growing body of research now suggests, sex differences may be the rule rather than the exception in this area of research. Increasing our understanding of the mechanisms of conduct problems among girls may be imperative if we expect to maximize treatment efficacy.
Acknowledgments
This study was funded by a grant from the National Institute of Mental Health R01 MH63699 (Theodore P. Beauchaine, PI) and by an award from the Mary Gates Endowment for Students (James Hong, recipient).
Footnotes
Disclosure: The authors report no conflicts of interest.
References
- 1.Lorber MF. Psychophysiology of aggression, psychopathy, and conduct problems: a meta-analysis. Psychol Bull. 2004;130:531–552. doi: 10.1037/0033-2909.130.4.531. [DOI] [PubMed] [Google Scholar]
- 2.Herpertz SC, Wenning B, Mueller B, Qunaibi M, Sass H, Herpertz-Dahlmann B. Psychophysiological responses in ADHD boys with and without conduct disorder: implications for adult antisocial behavior. J Am Acad Child Adolesc Psychiatry. 2001;40:1222–1230. doi: 10.1097/00004583-200110000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Raine A, Venables PH, Mednick SA. Low resting heart rate at age 3 years predisposes to aggression at age 11 years: evidence from the Mauritius Child Health Project. J Am Acad Child Psychiatry. 1997;36:1457–1464. doi: 10.1097/00004583-199710000-00029. [DOI] [PubMed] [Google Scholar]
- 4.Beauchaine TP, Katkin ES, Strassberg Z, Snarr J. Disinhibitory psychopathology in male adolescents: discriminating conduct disorder from attention-deficit/hyperactivity disorder through concurrent assessment of multiple autonomic states. J Abnorm Psychol. 2001;110:610–624. doi: 10.1037//0021-843x.110.4.610. [DOI] [PubMed] [Google Scholar]
- 5.Beauchaine TP, Gatzke-Kopp L, Mead HK. Polyvagal theory and developmental psychopathology: emotion dysregulation and conduct problems from preschool to adolescence. Biol Psychol. 2007;74:174–184. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keenan K, Loeber R, Green S. Conduct disorder in girls: a review of the literature. Clin Child Fam Psychol Rev. 1999;2:3–19. doi: 10.1023/a:1021811307364. [DOI] [PubMed] [Google Scholar]
- 7.Moffitt TE, Caspi A. Childhood predictors differentiate life-course persistent and adolescence-limited antisocial pathways among males and females. Dev Psychopathol. 2001;13:355–375. doi: 10.1017/s0954579401002097. [DOI] [PubMed] [Google Scholar]
- 8.Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Genetic and environmental effects on conduct disorder and alcohol dependence symptoms and their covariation at age 14. Alcohol Clin Exp Res. 2004;28:1541–1548. doi: 10.1097/01.alc.0000141822.36776.55. [DOI] [PubMed] [Google Scholar]
- 9.Brenner SL, Beauchaine TP, Sylvers PD. A comparison of psychophy-siological and self-report measures of BAS and BIS activation. Psychophysiology. 2005;42:108–115. doi: 10.1111/j.1469-8986.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- 10.Beauchaine TP. Vagal tone, development, and Gray’s motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Dev Psychopathol. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- 11.Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Doornen LJP. Committee report: methodological guidelines for impedance cardiography. Psychophysiology. 1990;27:1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 12.Crowell S, Beauchaine TP, Gatzke-Kopp L, Sylvers P, Mead H. Autonomic correlates of attention-deficit/hyperactivity disorder and oppositional defiant disorder in preschool children. J Abnorm Psychol. 2006;115:174–178. doi: 10.1037/0021-843X.115.1.174. [DOI] [PubMed] [Google Scholar]
- 13.Berntson GG, Bigger JT, Eckberg DL, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 14.Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. Int J Psychophysiol. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- 15.Mezzacappa E, Tremblay RE, Kindlon D, et al. Anxiety, antisocial behavior, and heart rate regulation in adolescent males. J Child Psychol Psychiatry. 1997;38:457–469. doi: 10.1111/j.1469-7610.1997.tb01531.x. [DOI] [PubMed] [Google Scholar]
- 16.Sloan RP, Shapiro PA, Bigger JT, Bagiella M, Steinman RC, Gorman JM. Cardiac autonomic control and hostility in healthy subjects. Am J Cardiol. 1994;74:298–300. doi: 10.1016/0002-9149(94)90382-4. [DOI] [PubMed] [Google Scholar]
- 17.Rechlin T, Weis M, Spitzer A, Kaschka WP. Are affective disorders associated with alterations of heart rate variability? J Affect Disord. 1994;32:271–275. doi: 10.1016/0165-0327(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 18.Crowell S, Beauchaine TP, McCauley E, Smith C, Stevens AL, Sylvers P. Psychological, autonomic, and serotonergic correlates of parasuicidal behavior in adolescent girls. Dev Psychopathol. 2005;17:1105–1127. doi: 10.1017/s0954579405050522. [DOI] [PubMed] [Google Scholar]
- 19.Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biol Psychiatry. 1996;39:255–266. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- 20.Friedman BH, Thayer JF. Anxiety and autonomic flexibility: a cardiovascular approach. Biol Psychol. 1998;49:303–323. doi: 10.1016/s0301-0511(98)00051-9. [DOI] [PubMed] [Google Scholar]
- 21.El-Sheikh M, Harger J, Whitson SM. Exposure to interparental conflict and children’s adjustment and physical health: the moderating role of vagal tone. Child Dev. 2001;72:1617–1636. doi: 10.1111/1467-8624.00369. [DOI] [PubMed] [Google Scholar]
- 22.Katz LF, Gottman JM. Vagal tone protects children from martial conflict. Dev Psychopathol. 1995;7:83–92. [Google Scholar]
- 23.Shannon KE, Beauchaine TP, Brenner SL, Neuhaus E, Gatzke-Kopp L. Familial and temperamental predictors of resilience in children at risk for conduct disorder and depression. Dev Psychopathol. 2007;19:701–727. doi: 10.1017/S0954579407000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biederman J, Roesenblaum JF, Bolduc-Murphy EA, et al. A 3-year follow-up of children with and without behavioral inhibition. J Am Acad Child Psychiatry. 1993;32:814–821. doi: 10.1097/00004583-199307000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Scarpa A, Raine A, Sarnoff MA, Venables PH. Heart rate and skin conductance in behaviorally inhibited Mauritian children. J Abnorm Psychol. 1997;106:182–190. doi: 10.1037//0021-843x.106.2.182. [DOI] [PubMed] [Google Scholar]
- 26.Gadow KD, Sprafkin J. Child Symptom Inventory 4 Screening Manual. Stony Brook, NY: Checkmate Plus; 1997. [Google Scholar]
- 27.Achenbach TM. Manual for the Child Behavior Checklist/4–18 and 1991 Profile. Burlington: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- 28.Sprafkin J, Gadow KD, Salisbury H, Schneider J, Loney J. Further evidence of reliability and validity of the Child Symptom Inventory-4: parent checklist in clinically referred boys. J Am Acad Child Adolesc Psychiatry. 2002;31:513–524. doi: 10.1207/S15374424JCCP3104_10. [DOI] [PubMed] [Google Scholar]
- 29.Qu M, Zhang Y, Webster JG, Tomkins WJ. Motion artifact from spot and band electrodes during impedance cardiography. Trans Biomed Eng. 1986;33:1029–1036. doi: 10.1109/TBME.1986.325869. [DOI] [PubMed] [Google Scholar]
- 30.Raudenbush S, Bryk A, Cheong YF, Congdon R. HLM 6: Hierarchical Linear and Nonlinear Modeling. Lincolnwood, IL: Scientific Software International; 2004. [Google Scholar]
- 31.Alkon A, Goldstein LH, Smider N, Essex MJ, Kupfer DJ, Boyce WT. Developmental and contextual influences on autonomic reactivity in young children. Dev Psychobiol. 2003;42:64–78. doi: 10.1002/dev.10082. [DOI] [PubMed] [Google Scholar]
- 32.MacCallum RC, Zhang S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychol Methods. 2002;7:19–40. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- 33.Carlson SR, Iacono WG. Heritability of P300 amplitude development from adolescence to adulthood. Psychophysiology. 2006;43:470–480. doi: 10.1111/j.1469-8986.2006.00450.x. [DOI] [PubMed] [Google Scholar]
- 34.Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J Abnorm Psychol. 2002;111:411–424. [PubMed] [Google Scholar]
- 35.Burt SA, McGue M, DeMarte JA, Krueger RF, Iacono WG. Timing of menarche and the origins of conduct disorder. Arch Gen Psychiatry. 2006;63:890–896. doi: 10.1001/archpsyc.63.8.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caspi A, Lynam D, Moffitt TE, Silva PA. Unraveling girls’ delinquency: biological, dispositional, and contextual contributions to adolescent misbehavior. Dev Psychol. 1993;29:19–30. [Google Scholar]
- 37.Obeidallah D, Brennan RT, Brooks-Gunn J, Earls F. Links between pubertal timing and neighborhood contexts: implications for girls’ violent behavior. J Am Acad Child Adolesc Psychiatry. 2004;43:1460–1468. doi: 10.1097/01.chi.0000142667.52062.1e. [DOI] [PubMed] [Google Scholar]
- 38.Silberg J, Rutter M, Meyer J, et al. Genetic and environmental influences on the covariation between hyperactivity and conduct disturbance in juvenile twins. J Child Psychol Psychiatry. 1996;37:803–816. doi: 10.1111/j.1469-7610.1996.tb01476.x. [DOI] [PubMed] [Google Scholar]
- 39.Loeber R, Keenan K. Interaction between conduct disorder and its comorbid conditions: effects of age and gender. Clin Psychol Rev. 1994;14:497–523. [Google Scholar]
- 40.Weems CF, Zakem AH, Costa NM, Cannon MF, Watts SE. Physiological response and childhood anxiety: association with symptoms of anxiety disorders and cognitive bias. J Clin Child Adolesc. 2005;34:712–723. doi: 10.1207/s15374424jccp3404_13. [DOI] [PubMed] [Google Scholar]
- 41.Ortiz J, Raine A. Heart rate level and antisocial behavior in children and adolescents: a meta-analysis. J Am Acad Child Adolesc Psychiatry. 2004;43:154–162. doi: 10.1097/00004583-200402000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Rogeness GA, Cepeda C, Macedo CA, Fischer C, Harris WR. Differences in heart rate and blood pressure in children with conduct disorder, major depression, and separation anxiety. Psychiatr Res. 1990;33:199–206. doi: 10.1016/0165-1781(90)90074-f. [DOI] [PubMed] [Google Scholar]
- 43.American Psychiatric Association. Text Revision (DSM-IV-TR) 4. Washington, DC: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 44.Youngstrom EA, Findling RL, Calabrese JR. Who are the comorbid adolescents? Agreement between psychiatric diagnosis, youth, parent, and teacher report. J Abnorm Child Psychol. 2003;31:231–245. doi: 10.1023/a:1023244512119. [DOI] [PubMed] [Google Scholar]
- 45.Loeber R, Green SN, Lahey BB, Stouthamer-Loeber M. Optimal informants on child disruptive behaviors. Dev Psychopathol. 1989;1:317–337. [Google Scholar]