Abstract
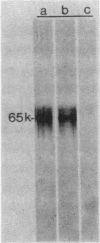
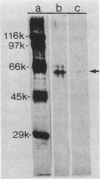
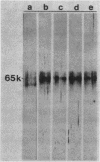
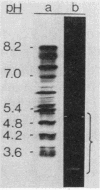
We recently reported the isolation and identification of a Giardia lamblia-specific antigen (GSA 65) that is shed in the stool of giardiasis patients. In the present study, this antigen was affinity purified from sonic extracts of axenically cultured G. lamblia trophozoites and characterized to better understand its biological function and its potential usefulness in the design of coprodiagnostic assays for giardiasis. GSA 65 was resistant to proteolytic digestion with trypsin, chymotrypsin, and protease but was sensitive to treatment with NaIO4 as assessed by Western blotting. This antigen was also stable during prolonged storage at 4 and -20 degrees C in 10% Formalin or distilled H2O as assessed by counterimmunoelectrophoresis. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and isoelectric focusing gel banding patterns, in conjunction with protein and carbohydrate assays and lectin binding studies, confirmed that this antigen is a highly glycosylated glycoprotein. The resistance of GSA 65 to proteolytic degradation, together with previous immunofluorescence data that indicate the antigen is an integral part of the G. lamblia cyst wall, suggests that this molecule may play a role in maintaining the integrity of the cyst in vivo. The ability of GSA 65 to maintain its antigenic structure under a wide variety of conditions makes it an ideal antigen around which to design sensitive immunodiagnostic assays for giardiasis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balázs R., Brooksbank B. W., Patel A. J., Johnson A. L., Wilson D. A. Incorporation of ( 35 S) sulphate into brain constituents during development and the effects of thyroid hormone on myelination. Brain Res. 1971 Jul 23;30(2):273–293. doi: 10.1016/0006-8993(71)90079-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Einfeld D. A., Stibbs H. H. Identification and characterization of a major surface antigen of Giardia lamblia. Infect Immun. 1984 Nov;46(2):377–383. doi: 10.1128/iai.46.2.377-383.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Schwarz R. T. Glycosylation is not necessary for membrane insertion and cleavage of Semliki Forest virus membrane proteins. Nature. 1978 Aug 3;274(5670):487–490. doi: 10.1038/274487a0. [DOI] [PubMed] [Google Scholar]
- Geisow M. A carbohydrate signal for intracellular transit. Nature. 1981 Mar 5;290(5801):15–15. doi: 10.1038/290015a0. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Grundy M. S. Preliminary observations using a multi-layer ELISA method for the detection of Entamoeba histolytica trophozoite antigens in stool samples. Trans R Soc Trop Med Hyg. 1982;76(3):396–400. doi: 10.1016/0035-9203(82)90199-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Nash T. E., Gillin F. D., Smith P. D. Excretory-secretory products of Giardia lamblia. J Immunol. 1983 Oct;131(4):2004–2010. [PubMed] [Google Scholar]
- Olden K., Parent J. B., White S. L. Carbohydrate moieties of glycoproteins. A re-evaluation of their function. Biochim Biophys Acta. 1982 May 12;650(4):209–232. doi: 10.1016/0304-4157(82)90017-x. [DOI] [PubMed] [Google Scholar]
- Olden K., Pratt R. M., Yamada K. M. Role of carbohydrate in biological function of the adhesive glycoprotein fibronectin. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3343–3347. doi: 10.1073/pnas.76.7.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosoff J. D., Stibbs H. H. Isolation and identification of a Giardia lamblia-specific stool antigen (GSA 65) useful in coprodiagnosis of giardiasis. J Clin Microbiol. 1986 May;23(5):905–910. doi: 10.1128/jcm.23.5.905-910.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. T., Schmidt M. F., Datema R. Inhibition of glycosylation of viral glycoproteins. Biochem Soc Trans. 1979 Apr;7(2):322–326. doi: 10.1042/bst0070322. [DOI] [PubMed] [Google Scholar]
- Sharon N. Glycoproteins. Sci Am. 1974 May;230(5):78–86. doi: 10.1038/scientificamerican0574-78. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- White D. A., Speake B. K. Protein glycosylation in animal secretory tissues. Biochem Soc Trans. 1979 Apr;7(2):326–328. doi: 10.1042/bst0070326. [DOI] [PubMed] [Google Scholar]