Abstract
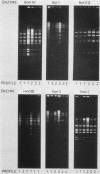
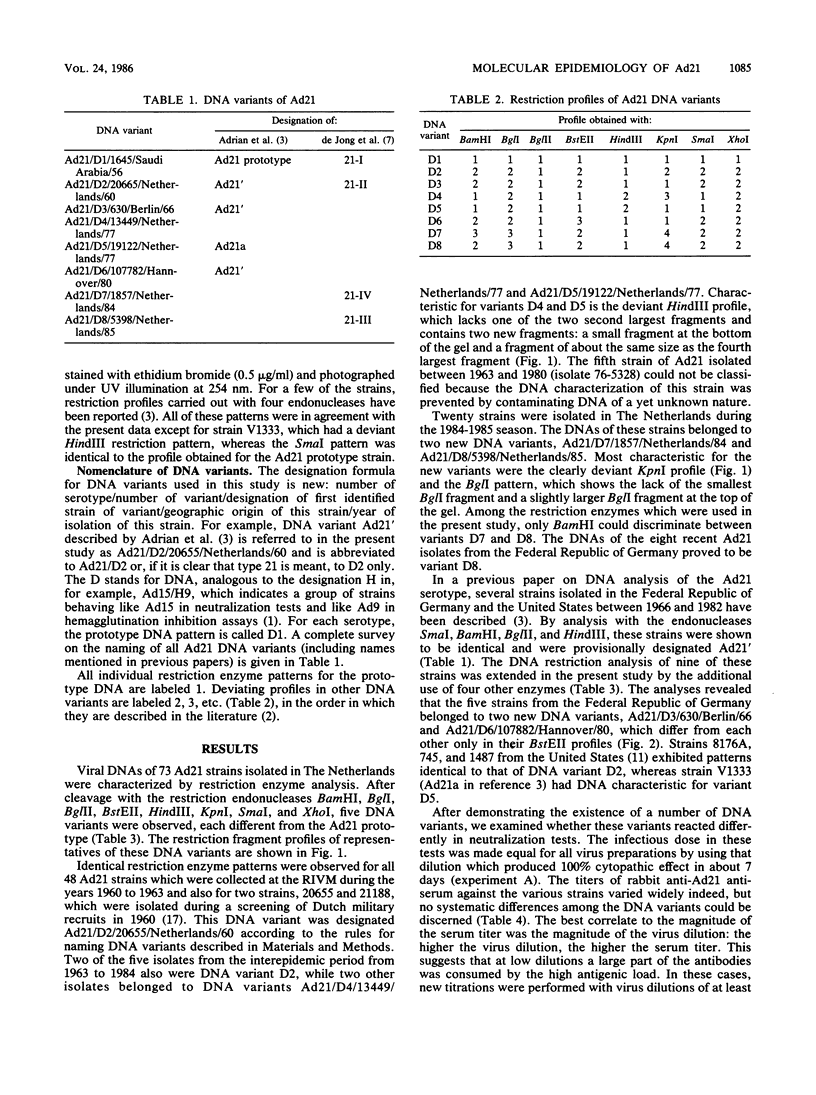
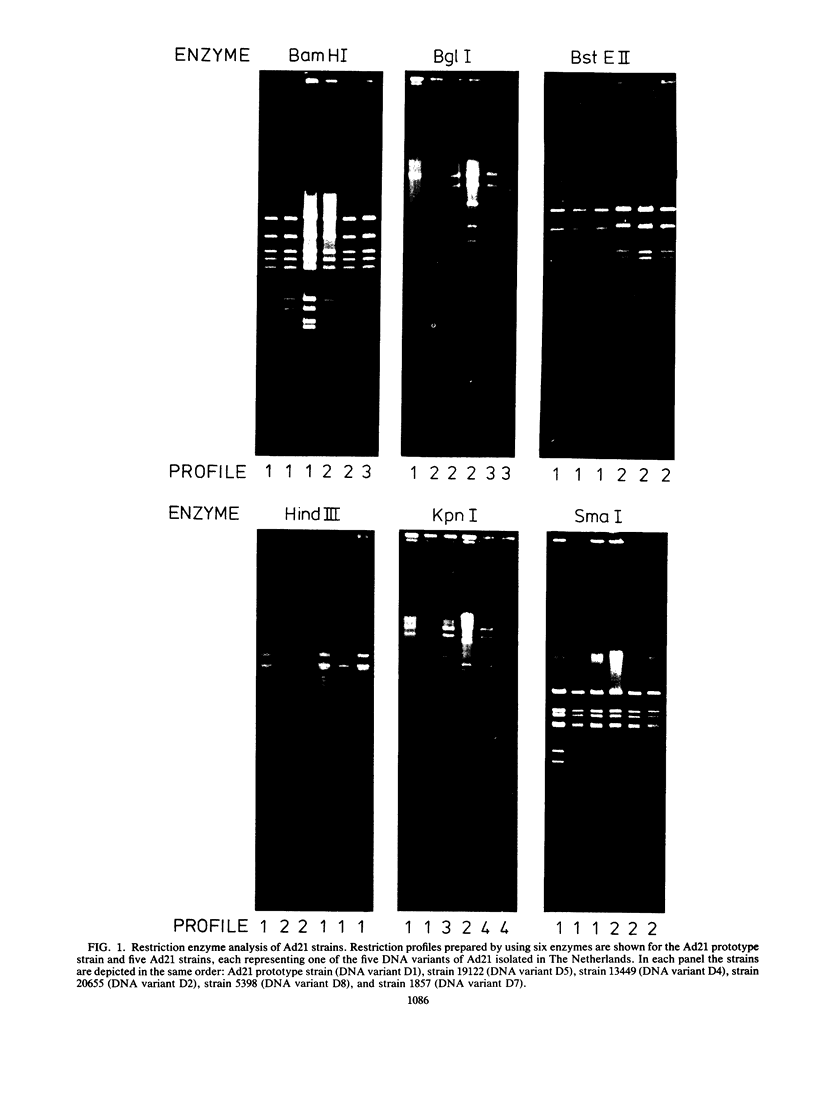
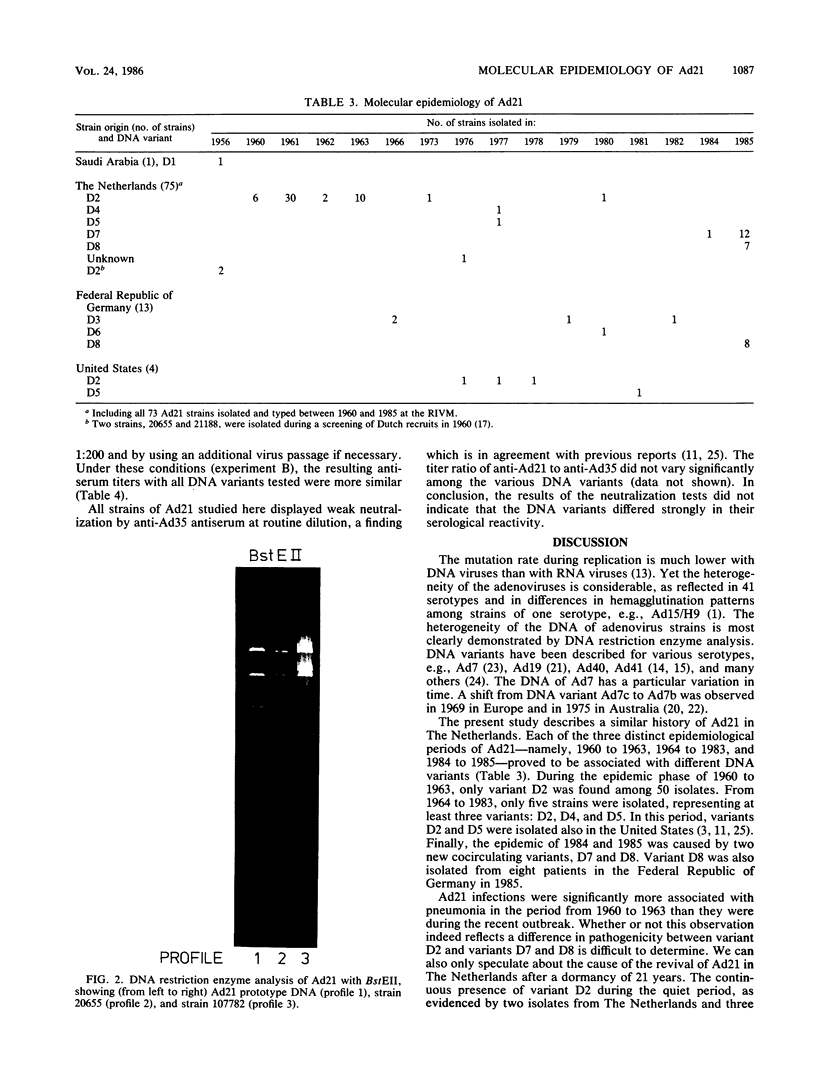
After a period of high prevalence in the early 1960s, adenovirus serotype 21 (Ad21) was identified in The Netherlands only very sporadically for more than 20 years. From December 1984 to July 1985, Ad21 was isolated relatively often from hospitalized children living in different parts of The Netherlands. The patients in question suffered from respiratory, gastrointestinal, meningeal, or ocular disorders. An increase in the incidence of Ad21 infections was also observed in the Federal Republic of Germany during this period. The DNAs of 93 isolates of Ad21 were subjected to restriction enzyme analysis with eight endonucleases. All 50 strains isolated in The Netherlands between 1960 and 1963 proved to be DNA variant Ad21/D2/20655/Netherlands/60. This variant has already been described (T. Adrian, R. Wigand, and J. C. Hierholzer, Arch. Virol. 84:79-89, 1985) as typical for the Ad21 strains circulating since 1960. Analysis of the DNAs of the 28 Ad21 strains isolated in The Netherlands or in the Federal Republic of Germany in 1984 and 1985 showed them to belong to two new, closely related DNA variants designated Ad21/D7/1857/Netherlands/84 and Ad21/D8/5398/Netherlands/85. The BglI and KpnI restriction profiles were characteristic for these recent DNA variants.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian T., Bastian B., Benoist W., Hierholzer J. C., Wigand R. Characterization of adenovirus 15/H9 intermediate strains. Intervirology. 1985;23(1):15–22. doi: 10.1159/000149562. [DOI] [PubMed] [Google Scholar]
- Adrian T., Best B., Wigand R. A proposal for naming adenovirus genome types, exemplified by adenovirus type 6. J Gen Virol. 1985 Dec;66(Pt 12):2685–2691. doi: 10.1099/0022-1317-66-12-2685. [DOI] [PubMed] [Google Scholar]
- Adrian T., Wigand R., Hierholzer J. C. Immunological and biochemical characterization of human adenoviruses from subgenus B. II. DNA restriction analysis. Arch Virol. 1985;84(1-2):79–89. doi: 10.1007/BF01310555. [DOI] [PubMed] [Google Scholar]
- Buitenwerf J., Louwerens J. J., De Jong J. C. A simple and rapid method for typing adenoviruses 40 and 41 without cultivation. J Virol Methods. 1985 Jan;10(1):39–44. doi: 10.1016/0166-0934(85)90086-2. [DOI] [PubMed] [Google Scholar]
- Fatz W., Wigand R. Die Durchseuchung mit Adenovirus 1 bis 39 im Kleinkindesalter und bei Erwachsenen. Immun Infekt. 1985 May;13(3):108–112. [PubMed] [Google Scholar]
- Green M., Mackey J. K., Wold W. S., Rigden P. Thirty-one human adenovirus serotypes (Ad1-Ad31) form five groups (A-E) based upon DNA genome homologies. Virology. 1979 Mar;93(2):481–492. doi: 10.1016/0042-6822(79)90251-4. [DOI] [PubMed] [Google Scholar]
- Hierholzer J. C., Torrence A. E., Wright P. F. Generalized viral illness caused by an intermediate strain of adenovirus (21/H21 + 35). J Infect Dis. 1980 Mar;141(3):281–288. doi: 10.1093/infdis/141.3.281. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Kidd A. H., Berkowitz F. E., Blaskovic P. J., Schoub B. D. Genome variants of human adenovirus 40 (subgroup F). J Med Virol. 1984;14(3):235–246. doi: 10.1002/jmv.1890140307. [DOI] [PubMed] [Google Scholar]
- Kidd A. H. Genome variants of adenovirus 41 (subgroup G) from children with diarrhoea in South Africa. J Med Virol. 1984;14(1):49–59. doi: 10.1002/jmv.1890140108. [DOI] [PubMed] [Google Scholar]
- Wadell G., Cooney M. K., da Costa Linhares A., de Silva L., Kennett M. L., Kono R., Gui-Fang R., Lindman K., Nascimento J. P., Schoub B. D. Molecular epidemiology of adenoviruses: global distribution of adenovirus 7 genome types. J Clin Microbiol. 1985 Mar;21(3):403–408. doi: 10.1128/jcm.21.3.403-408.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadell G. Molecular epidemiology of human adenoviruses. Curr Top Microbiol Immunol. 1984;110:191–220. doi: 10.1007/978-3-642-46494-2_7. [DOI] [PubMed] [Google Scholar]
- Wadell G., Varsanyi T. M. Demonstration of three different subtypes of adenovirus type 7 by DNA restriction site mapping. Infect Immun. 1978 Jul;21(1):238–246. doi: 10.1128/iai.21.1.238-246.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadell G., de Jong J. C. Restriction endonucleases in identification of a genome type of adenovirus 19 associated with keratoconjunctivitis. Infect Immun. 1980 Feb;27(2):292–296. doi: 10.1128/iai.27.2.292-296.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadell G., de Jong J. C., Wolontis S. Molecular epidemiology of adenoviruses: alternating appearance of two different genome types of adenovirus 7 during epidemic outbreaks in Europe from 1958 to 1980. Infect Immun. 1981 Nov;34(2):368–372. doi: 10.1128/iai.34.2.368-372.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigand R., Sehn N., Hierholzer J. C., de Jong J. C., Adrian T. Immunological and biochemical characterization of human adenoviruses from subgenus B. I. Antigenic relationships. Arch Virol. 1985;84(1-2):63–78. doi: 10.1007/BF01310554. [DOI] [PubMed] [Google Scholar]
- de Jong J. C., Wigand R., Kidd A. H., Wadell G., Kapsenberg J. G., Muzerie C. J., Wermenbol A. G., Firtzlaff R. G. Candidate adenoviruses 40 and 41: fastidious adenoviruses from human infant stool. J Med Virol. 1983;11(3):215–231. doi: 10.1002/jmv.1890110305. [DOI] [PubMed] [Google Scholar]
- de Jong J. C., Wigand R., Wadell G., Keller D., Muzerie C. J., Wermenbol A. G., Schaap G. J. Adenovirus 37: identification and characterization of a medically important new adenovirus type of subgroup D. J Med Virol. 1981;7(2):105–118. doi: 10.1002/jmv.1890070204. [DOI] [PubMed] [Google Scholar]
- de Jong J. C., van der Avoort H. G., Wigand R., Adrian T. Revival of adenovirus 21 in Netherlands and West Germany. Lancet. 1985 Oct 5;2(8458):776–776. doi: 10.1016/s0140-6736(85)90649-x. [DOI] [PubMed] [Google Scholar]
- van Loon A. E., Rozijn T. H., de Jong J. C., Sussenbach J. S. Physicochemical properties of the DNAs of the fastidious adenovirus species 40 and 41. Virology. 1985 Jan 15;140(1):197–200. doi: 10.1016/0042-6822(85)90461-1. [DOI] [PubMed] [Google Scholar]
- van der Veen J., Oei K. G., Abarbanel M. F. Patterns of infections with adenovirus types 4, 7 and 21 in military recruits during a 9-year survey. J Hyg (Lond) 1969 Jun;67(2):255–268. doi: 10.1017/s0022172400041668. [DOI] [PMC free article] [PubMed] [Google Scholar]