Abstract
The binding of complement factor H (fH) to meningococci was recently found to be specific for human fH. Therefore, passive protective antibody activity measured in animal models of meningococcal bacteremia may overestimate protection in humans, since in the absence of bound fH, complement activation is not downregulated. We developed an ex vivo model of meningococcal bacteremia using nonimmune human blood to measure the passive protective activity of stored sera from 36 adults who had been immunized with an investigational meningococcal multicomponent recombinant protein vaccine. Before immunization, human complement-mediated serum bactericidal activity (SBA) titers of ≥1:4 against group B strains H44/76, NZ98/254, and S3032 were present in 19, 11, and 8% of subjects, respectively; these proportions increased to 97, 22, and 36%, respectively, 1 month after dose 3 (P < 0.01 for H44/76 and S3032). Against the two SBA-resistant strains, NZ98/254 and S3032, passive protective titers of ≥1:4 were present in 11 and 42% of sera before immunization, respectively, and these proportions increased to 61 and 94% after immunization (P < 0.001 for each strain). Most of the sera with SBA titers of <1:4 and passive protective activity showed a level of killing in the whole-blood assay (>1 to 2 log10 decreases in CFU/ml during a 90-min incubation) similar to that of sera with SBA titers of ≥1:4. In conclusion, passive protective activity was 2.6- to 2.8-fold more frequent than SBA after immunization. The ability of SBA-negative sera to kill Neisseria meningitidis in human blood where fH is bound to the bacteria provides further evidence that SBA titers of ≥1:4 measured with human complement may underestimate meningococcal immunity.
Complement-mediated serum bactericidal activity (SBA) confers protection against meningococcal disease (reviewed in reference 14), and an SBA titer of ≥1:4 measured with human complement is a widely accepted surrogate of vaccine efficacy (5). While a positive bactericidal titer is specific for protection against meningococcal disease, seroepidemiologic data suggest that some persons with SBA titers of <1:4 also may be protected. For example, in the study performed by Goldschneider et al. that established that military recruits with SBA titers of ≥1:4 were protected from developing meningococcal disease, the majority of recruits with SBA titers of <1:4 who became colonized by the epidemic strain did not develop disease (13). Further, in a recent study in the United Kingdom, the incidence of meningococcal group B disease was reported to decrease more than 10-fold between the ages of 6 months and 4 years without a corresponding age-related increase in the prevalence of SBA titers of ≥1:4 (36). Thus, while SBA titers of ≥1:4 appear to be specific for protective meningococcal immunity, SBA at titers of <1:4 and/or opsonic activity may also be sufficient to confer protection (26, 38).
New meningococcal vaccines are being developed for the prevention of disease caused by group B strains, for which there is currently no licensed vaccine in the United States or Europe. Efficacy is being inferred from immunogenicity data (1, 2, 4), because the incidence of group B disease is too low for randomized studies to determine the efficacy of these new vaccines. If a candidate antigen does not elicit SBA, it may not be included in a new vaccine, because of the difficulty of establishing that the antigen contributes to protective immunity. More-sensitive assays that predict protective meningococcal immunity, therefore, are needed to assess the vaccine potential of such antigens.
We recently described an ex vivo model of meningococcal bacteremia that used human blood (38) anticoagulated with lepirudin, a specific thrombin inhibitor, which does not activate complement (30). Neisseria meningitidis strains survived or increased in CFU/ml when incubated for 2 to 4 h in blood from healthy adults who apparently had no naturally acquired immunity. In the present study, we have adapted this ex vivo model to measure the ability of heat-inactivated test sera from adults immunized with an investigational meningococcal multicomponent recombinant protein vaccine to confer passive protection (PP) against bacteremia. The PP assay, which is based on the interactions of human antibodies with physiologic concentrations of human complement and human polymorphonuclear leukocytes (PMNs), appears to be more sensitive for measuring the killing of N. meningitidis than a standard complement-mediated bactericidal assay using human complement or an opsonic assay with human complement and human PMNs.
MATERIALS AND METHODS
Serum samples.
Stored serum samples were available from 36 subjects, 19 to 40 years old, who were immunized with an investigational meningococcal multicomponent recombinant protein vaccine as part of two previously performed phase I clinical trials. These studies were conducted at three sites: Lincoln, NE (principal investigator, James C. Kisicki), Austin, TX (principal investigator, David Carter), and Tacoma, WA (principal investigator, Royce Morrison). The sponsor was Chiron Corporation (now Novartis Vaccines and Diagnostics, Inc., Cambridge, MA). The vaccine contained three recombinant proteins (three components; thus, an “r3C” vaccine) (12). Two of the components were fusion proteins: one was a fusion of genome-derived neisserial antigen 2091 (GNA 2091) and a factor H binding protein (fHbp) from the antigenic variant 1 group (22), and the other consisted of GNA 2132 (25, 40) and GNA 1030. The third component of the vaccine was recombinant NadA (6, 7).
Subjects were given three intramuscular doses, separated by 1-month intervals. Each dose contained 150 μg of protein (50 μg of each component), which was adsorbed with aluminum hydroxide (total of 1.5 mg per injection). Stored serum samples obtained immediately before immunization and at 1 month after the third dose were provided by Novartis Vaccines. The selection of the sera was based on the availability of sufficient volumes for our assays. The clinical research protocols and consent forms for the studies were approved by independent institutional review boards at each of the clinical sites, and permission for the investigations of the stored sera reported here was granted by the institutional review board of Children's Hospital & Research Center, Oakland, CA.
Neisseria meningitidis strains.
We used three group B strains. H44/76-SL (B:15;P1.7,16; sequence type 32 [ST-32]) was from an epidemic in Norway (3, 11) and is referred to as H44/76. NZ98/254 (B:4;P1.7-2,4; ST-41/44 complex) was from a recent epidemic in New Zealand (8), and S3032 (B:19,7;P1.12,16; ST-6875) was from a patient hospitalized in the United States (10). None of these strains had the gene for NadA, which was one of the components of the vaccine. Strains H44/76 and NZ98/254 each had fHbp in the variant 1 group. The fHbp expressed by H44/76 was relatively abundant (41) and matched the amino acid sequence of the fHbp antigen in the vaccine, while that of NZ98/254 was relatively sparse and had a small number of amino acid differences from the sequence of the recombinant protein vaccine (39, 41). The fHbp of strain S3032 was a naturally occurring chimera of fHbp from the variant 2 and 3 groups (GenBank accession number EU921901) and thus was heterologous to the fHbp in the vaccine. The third major antigen in the vaccine, GNA 2132, was expressed by all three strains. Strains H44/76 and NZ98/254 expressed a GNA 2132 containing a homologous 63-amino acid-peptide that was also present in the recombinant vaccine antigen. While this segment was absent in GNA 2132 from strain S3032 (40), it is not known whether this segment is surface exposed or contains epitopes that are targets of SBA antibodies.
Serum bactericidal assay.
The SBA assay was performed as described elsewhere (26, 41) using early-log-phase, broth-grown N. meningitidis and dilutions of test sera that had been heated at 56°C for 30 min to inactivate internal complement. The complement source was serum from a healthy adult with normal total hemolytic complement activity and no detectable serum bactericidal antibodies against any of the test strains. A positive SBA titer was defined by a >50% decrease in CFU/ml after a 60-min incubation in the reaction mixture compared with the CFU/ml in negative control wells at time zero. Note that bacteria incubated with the negative-control serum and complement typically showed a 150% increase in CFU/ml during the 60 min of incubation.
Opsonophagocytic killing assay.
The opsonophagocytic activity (OPA) assay was performed as described elsewhere (26), except that we used C6-sufficient serum as the complement source instead of C6-depleted serum. The complement donor was the same as the donor for the SBA assay. The OPA procedure was essentially the same as the SBA method except that the OPA reaction also contained human PMNs (ratio, 40 PMNs per CFU of bacteria). A positive OPA titer was defined by a 50% decrease in CFU/ml after a 60-min incubation in the reaction mixture compared with that of the negative controls at time zero.
Ex vivo PP.
The PP assay was adapted from the method previously described (38), which was performed in tubes, to the use of a 96-well microtiter plate format. In brief, fresh blood from a healthy adult was obtained using a syringe containing recombinant hirudin (lepirudin; final concentration, 27.8 μg/ml) as the anticoagulant. The blood donor was the same person whose serum was used for complement to measure SBA and OPA. To each well of the microtiter plate, 65 μl of blood, 25 μl of heat-inactivated test sera, 10 μl of phosphate-buffered saline buffer containing 15% heated complement and 1% bovine serum albumin (Equitech), and approximately 4,000 CFU were added. The test strains were NZ98/254 and S3032, described above. The positive antibody controls were a relevant anti-PorA monoclonal antibody (anti-P1.4 or anti-P1.12; NIBSC) and human serum samples with high, medium, or low OPA and SBA against the respective test strains. Negative controls consisted of heat-inactivated serum from the complement donor and buffer alone. The microtiter plates were incubated for 90 min at 37°C under 5% CO2 with agitation on an MS 3 digital minishaker (IKA, Wilmington, NC) at 500 oscillations per min. Samples were removed from the wells, and quantitative cultures were performed on chocolate agar plates, which were incubated at 37°C under 5% CO2. The following day, the CFU/ml was ascertained, and the results were calculated as the log10 change in CFU/ml at 90 min compared to that at time zero with negative-control test sera. Based on the reproducibility of replicate assays performed on different days (see Results), we defined a positive PP activity as a serum giving a ≥0.5 log10 decrease in CFU/ml from that at time zero (i.e., a ∼70% decrease in CFU/ml). All assays were performed in duplicate, and the results reported were from independent experiments performed on at least three occasions.
Statistical analyses.
Statistical calculations were performed using GraphPad Prism 5 for Mac OSX, version 5.0a. The respective geometric means of the bactericidal titers were computed by exponentiating (base10). For log transformation, titers below the 1:4 lower limit of detection were assigned a value of half of the lower limit (i.e., 1:2). The proportion of sera with serum bactericidal titers of ≥1:4 (considered a protective titer when measured with human complement [5, 13]) was computed along with the respective 95% confidence intervals. The latter were calculated according to the method of Wilson (24) using a website calculator (http://faculty.vassar.edu/lowry/prop1.html). The differences found in the proportion of subjects in the respective groups were compared by Fisher's exact test (two-tailed). Bactericidal activity in the whole-blood assay was defined by a decrease of ≥0.5 log10 in CFU/ml after a 1.5-h incubation compared with the respective CFU/ml at time zero (see Results).
RESULTS
Complement-mediated serum bactericidal responses.
Before vaccination, the percentages of subjects with SBA titers of ≥1:4 ranged from 8 to 19% depending on the test strain (Table 1). After vaccination, the respective proportion of subjects with titers of ≥1:4 increased to 97% against strain H44/76 (P < 0.0001), which was chosen as a test strain because of its known susceptibility to SBA by antibodies elicited by the vaccine, and 22% (P > 0.3) and 36% (P < 0.01), respectively, against strains NZ98/254 and S3032, which were chosen as test strains because of their known resistance to SBA by antibodies elicited by the vaccine. The reciprocal geometric mean titers (GMT) of antibodies after vaccination were 1:64, 1:3.8, and 1:3.8 against strains H44/76, NZ98/254 and S3032, respectively, significantly higher than the respective reciprocal GMT before vaccination (P < 0.001 by paired t test) (Table 1).
TABLE 1.
Serum bactericidal responses after three doses of an investigational meningococcal multicomponent recombinant protein vaccinea
| Strain | 1/GMT (95% CI)b
|
% of subjects with SBA titers of ≥1:4 (95% CI)
|
||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| H44/76 | 2.9 (2.2-3.8) A | 64 (38-110) B | 19 (8-36) C | 97 (85-100) D |
| NZ98/254 | 2.5 (2.0-3.0) A | 3.8 (2.4-5.8) B | 11 (3-26) E | 22 (10-39) F |
| S3032 | 2.2 (1.9-2.4) A | 3.8 (2.8-5.3) B | 8 (2-22) G | 36 (21-54) H |
The vaccine contained two fusion proteins, GNA 2091-fHbp variant 1 and GNA 2132-GNA 1030, and a third component, recombinant NadA (12). Sera from 36 subjects were tested, except for strain H44/76, for which sera from 35 subjects were tested because of insufficient volume of 1 postimmunization sample.
CI, confidence interval; Pre and Post, prevaccination and postvaccination, respectively. For A versus B, P < 0.001 by a paired t test. P values by the Fisher exact test are as follows: <0.001 for C versus D, >0.3 for E versus F, and <0.01 for G versus H.
Complement-mediated opsonophagocytic killing.
Serum OPA responses against the two SBA-resistant strains were measured. The proportions of subjects with serum OPA titers of ≥1:4 before immunization were 11% for both strains, increasing after immunization to 36% against strain NZ98/254 and 42% against S3032 (for each strain, P < 0.03 by Fisher's exact test).
PP activity.
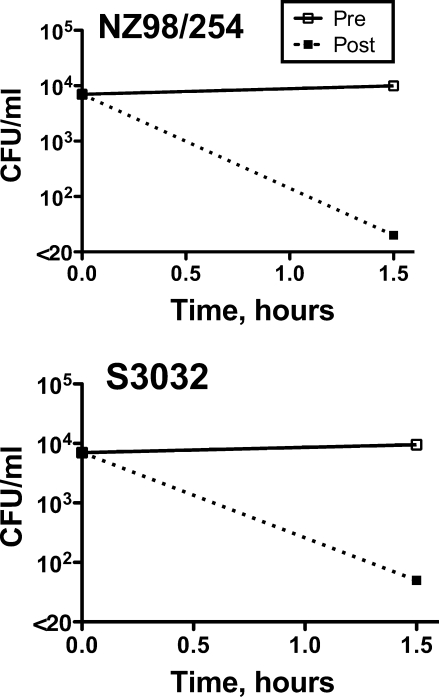
When a 1:4 dilution of preimmunization serum from a representative subject was added to anticoagulated blood from the nonimmune donor along with N. meningitidis from strain NZ98/254 or S3032, the bacteria survived or increased slightly in CFU/ml during the 90 min of incubation (Fig. 1). In contrast, the addition of a 1:4 dilution of the respective postvaccination serum resulted in a >2 log10 decrease in the CFU/ml for both strains. The killing in the whole-blood PP assay occurred despite that fact that the SBA titer of this subject was <1:4 against both strains.
FIG. 1.
Serum PP activity in a human ex vivo model of meningococcal bacteremia. N. meningitidis strains NZ98/254 and S3032 were incubated with human blood from the donor whose serum was used as the complement source for measuring serum SBA and OPA, together with a 1:4 dilution of heat-inactivated pre- or postimmunization serum from a subject whose SBA titers were <1:4 against both strains. With the preimmunization serum (solid lines), the CFU/ml of both strains were unchanged during the 90-min incubation period, whereas in the presence of the postimmunization serum (dotted line), there were >2 log10 decreases in the respective CFU/ml from those at time zero.
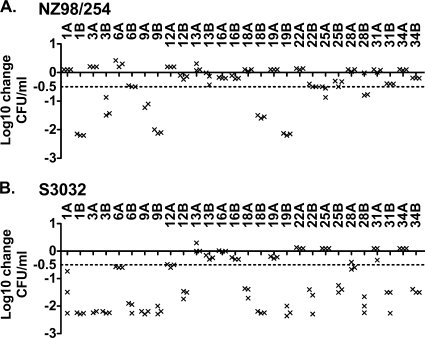
The PP results were reproducible, as evidenced by the results of three independent assays performed over 3 months on paired pre- and postimmunization serum samples from each of the subjects. The data points for each of the assays of sera from the first 14 subjects are shown in Fig. 2, with each data point representing the result of an individual assay. The only exception to good reproducibility was serum sample 1A (preimmune) against strain S3032, with −0.73, −1.49, and −2.26 log10 changes in CFU/ml in the three assays.
FIG. 2.
Reproducibility of measurements of PP activity in sera from individual subjects. (A) Strain NZ98/254; (B) strain S3032. PP was measured in three independent assays, performed over 3 months on test sera diluted 1:4. Data from the first 14 subjects are shown, represented by three data points (X) per subject for each serum sample. Serum samples are labeled with the subject number followed by the letter A for preimmunization sera and B for postimmunization sera.
We defined a positive serum PP activity as a sample giving a ≥0.5 log10 decrease in CFU/ml from that at time zero. A total of 72 sera were assayed for PP activity (36 pre- and 36 postimmunization sera). Against strain NZ98/254, 26 of these samples were positive in assay 1. Of these, 24 of 26 (92%) and 23 of 24 (96%) tested were positive by assays 2 and 3, respectively (Table 2). Forty-six samples were negative for PP against strain NZ98/254 in assay 1. Of these, one was positive by assay 2 (2%), and two (4%), including the sample positive in assay 2, were positive by assay 3. For the second strain, S3032, the respective data for the three assays showed a level of reproducibility similar to that observed with strain NZ98/254 (Table 2).
TABLE 2.
Reproducibility of passive protective activity as measured in three independent assaysa
| Assay 1 result (n) | No. positive/total no. (%) at retesting
|
|
|---|---|---|
| Assay 2 | Assay 3 | |
| Strain NZ98/254 | ||
| Positive (26) | 24/26 (92) | 23/24b (96) |
| Negative (46) | 1/46 (2) | 2/46c (4) |
| Strain S3032 | ||
| Positive (51) | 50/51 (98) | 47/50d (94) |
| Negative (21) | 0/21 (0) | 0/21 (0) |
A positive serum PP activity was defined as a sample giving a ≥0.5 log10 decrease in CFU/ml compared with the CFU/ml of negative-control sera measured at time zero (see the text). Data were combined for 36 preimmunization and 36 postimmunization sera (n = 72).
Two samples, each positive in assays 1 and 2, were unavailable for assay 3 because of insufficient quantities.
One of the two samples positive in assay 3 was also positive in assay 2.
One sample, which was positive in assays 1 and 2, was not available for assay 3 because of insufficient quantity.
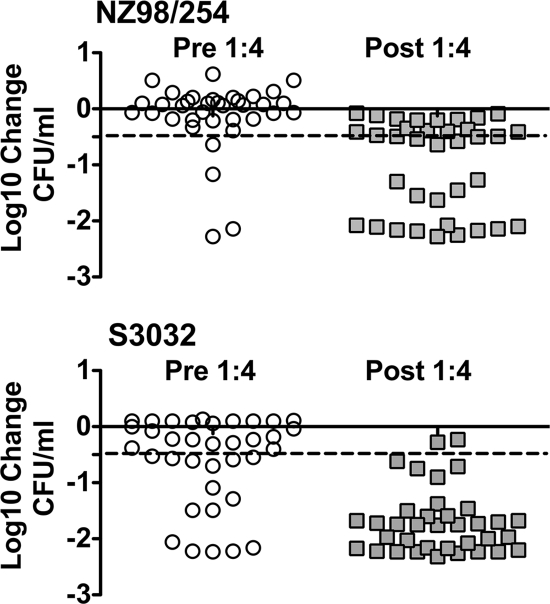
Figure 3 shows the PP results for individual pre- and postimmunization sera from the 36 adults immunized with three doses of the meningococcal recombinant protein vaccine. Each symbol represents the mean from at least two assays. Against strain NZ98/254, 11% of the preimmunization sera were positive for PP, and this proportion increased to 61% after immunization (P < 0.001). For strain S3032, the proportions of sera with PP activity were 42% and 94%, respectively (P < 0.001).
FIG. 3.
PP activity against meningococcal bacteremia in an ex vivo model using human blood. The results are shown for individual pre- and postimmunization sera from the 36 adults immunized with three doses of the meningococcal recombinant protein vaccine. Each symbol represents the mean from two to three assays. The dashed lines represent a −0.5 log10 change, the threshold considered positive in the assay (see Table 2). Against strain NZ98/254, 11% of the preimmunization sera were positive for PP, and this proportion increased to 61% after immunization (P < 0.001). For strain S3032, the proportions of sera with PP activity before and after immunization were 42% and 94%, respectively (P < 0.001).
For strain NZ98/254, the mean log10 change in CFU/ml for the 36 preimmunization sera was −0.12 log10 before immunization and −1.0 log10 after three doses of vaccine (P < 0.001). The respective median values before and after immunization were 0.065 and −0.52 log10 change in CFU/ml (P < 0.0001 by the Mann-Whitney test). For strain S3032, the mean log10 change in CFU/ml for the 36 preimmunization sera was −0.59 before immunization and −1.69 after immunization (P < 0.001). The respective median values before and after immunization were −0.30 and −1.75 log10 change in CFU/ml (P < 0.0001 by the Mann-Whitney test).
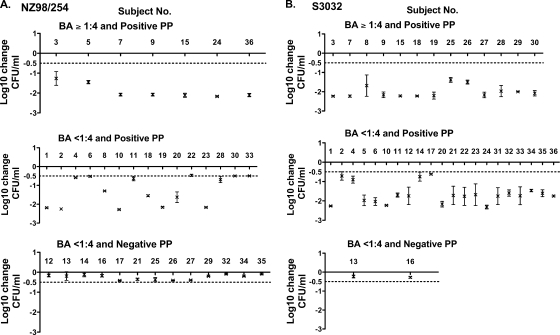
PP activity in relation to SBA.
We stratified the individual postimmunization PP results in relation to the respective SBA titers. With one exception, the sera could be stratified into three categories: SBA titers of ≥1:4 and positive PP activity, SBA titers of <1:4 and positive PP activity, and SBA titers of <1:4 and negative PP activity. (The exception was one serum sample with an SBA titer of 1:7 and a PP of −0.4 log10 change in CFU/ml against strain NZ98/254.) Figure 4 shows the results of the remaining individual sera stratified by each of the three categories. All of the sera with SBA titers of ≥1:4 had strong PP activity in the whole-blood assay (>1 to 2 log10 decreases in CFU/ml [Fig. 4, upper panels]). However, a large proportion of the sera with SBA titers of <1:4 and positive PP activity (Fig. 4, center panels) showed strong killing in the whole-blood assay, similar to that of the sera with SBA titers of ≥1:4.
FIG. 4.
Serum PP activity in relation to complement-mediated serum bactericidal titers. Each point represents the mean ± standard deviation for three assays performed on different days on 1:4 dilutions of postimmunization sera from individual subjects. (A) Strain NZ98/254; (B) strain S3032. Positive PP (dashed lines) was defined by a ≥0.5 log10 decrease in CFU/ml at 90 min from the CFU/ml of the negative control at time zero. The log10 changes in CFU/ml in the whole-blood assay for many of the sera with SBA titers of <1:4 and positive PP (center panels) were similar in magnitude to those observed for sera with bactericidal titers of ≥1:4 and positive PP (upper panels). Note that there was one subject with a postimmunization bactericidal titer of 1:7 and negative PP (−0.4 log10 decrease) against strain NZ98/254. Since no other sera exhibited positive SBA and negative PP, a fourth panel with the single data point is not shown.
Prevalence of SBA, OPA, and PP.
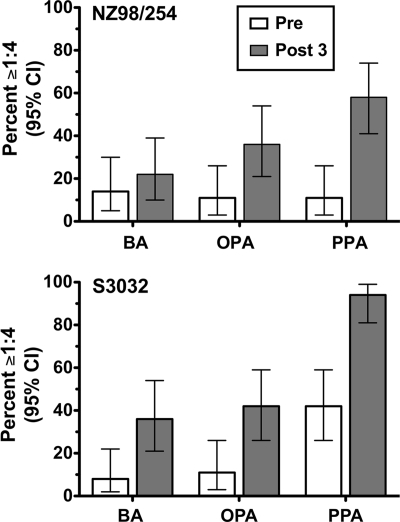
Figure 5 compares the respective percentages of subjects with positive serum titers of ≥1:4 before and after immunization for each of the three antibody functional assays. Depending on the strain, the proportions of subjects with serum PP after vaccination were 2.6- to 2.8-fold higher than those with SBA (61 versus 22%, respectively, for NZ98/254 and 94 versus 36%, respectively, for S3032). The corresponding proportions of postimmunization sera with OPA were 36 and 42% for strains NZ98/254 and S3032, respectively.
FIG. 5.
Summary of the proportions of immunized subjects with titers of ≥1:4 in SBA, OPA, or PP assays. The bars represent the proportions of preimmunization (open bars) or postimmunization (filled bars) sera that were positive when tested at a 1:4 dilution in each assay. Error bars, 95% confidence intervals (CI). A positive serum SBA or OPA titer was defined as a 50% decrease, after a 60-min incubation of bacteria, from the CFU/ml at time zero. Positive PP activity was defined as a ≥0.5 log10 decrease, after a 90-min incubation, from the CFU/ml at time zero. Depending on the strain, postimmunization PP activity was 2.6- to 2.8-fold more frequent then an SBA titer of ≥1:4.
DISCUSSION
Complement-mediated SBA is a well-accepted serologic marker of protection against meningococcal disease (5). The recombinant protein vaccine given to the subjects in the present study contained five antigens, of which three, NadA, fHbp, and GNA 2132, were found in previous studies to be important targets of serum bactericidal antibodies (12). The remaining two antigens, GNA 1030 and GNA 2091, elicited antibodies that bound to the surface of N. meningitidis and/or conferred protection in animal models but individually had minimal or no bactericidal activity (12). The vaccine that is currently in phase III clinical studies contains the three recombinant proteins (five antigens) combined with an outer membrane vesicle vaccine prepared from strain NZ98/254. The present studies were limited to sera from adults given the multicomponent recombinant protein vaccine without the outer membrane vesicle vaccine.
Subjects given this vaccine showed strong SBA responses when measured with human complement against strain H44/76, which is a naturally high expresser of fHbp with an amino acid sequence that matches that of the vaccine antigen. As expected, the SBA responses against the other two strains, NZ98/254 and S3032, which were selected because they were known to be resistant to SBA by antibodies elicited by the recombinant protein vaccine, were much lower. Here we present data showing that the vaccine-induced antibodies of some subjects with SBA titers of <1:4 killed the two SBA-resistant strains in OPA and/or PP assays.
In previous studies, a whole-blood assay that measured both serum and opsonophagocytic bactericidal activities against N. meningitidis also was reported to give positive results for many persons whose SBA titers were <1:4 (18-20, 38), including children or adults immunized with outer membrane vesicle vaccines (9, 23). These assays were performed over many months on fresh whole-blood samples obtained from individuals, an approach that is not likely to be realistic for the measurement of antibody responses to vaccines in large clinical trials (38). What is novel about the present approach is the use of fresh blood from a nonimmune donor to assay the PP activity of stored sera from subjects enrolled in a vaccine trial. Thus, pre- and postimmunization sera from individuals could be assayed in parallel. We also used blood that was anticoagulated with lepirudin, a specific thrombin inhibitor not known to activate complement, whereas the previous studies used citrate or heparin, which are known to affect complement activation (29). In future studies, the PP activities of sera from persons given different vaccines can be compared directly in single assays.
The antigenic targets of the antibodies responsible for the OPA or PP were not defined. However, none of the test strains had the gene for NadA, and one of the strains, S3032, expressed fHbp from an antigenic group different from the variant 1 protein contained in the vaccine. This strain also was resistant to SBA, OPA, and PP activity by mouse antiserum to recombinant fHbp in the variant 1 group (titer, <1:10) but was susceptible to the SBA of a mouse antiserum to GNA 2132 (titer, ∼1:200 with human complement). Thus, it is likely that the functional antibody activities observed against strain S3032 in the sera from the vaccinees in the present study reflected, at least in part, antibodies elicited by GNA 2132. This conclusion is consistent with our previous results from studies of mouse and/or human anti-GNA 2132 antibodies (26, 40).
Recently two laboratories reported that factor H (fH), a downregulating molecule in the complement pathway, bound to N. meningitidis, which enabled the organism to evade innate immunity (21, 28). In a subsequent study, we found that the binding of fH to N. meningitidis was specific for human fH (16). Further, when N. meningitidis was incubated with infant rat or rabbit serum, there was unregulated complement activation and C3 deposition on the bacterial surface. The addition of human fH to the reaction mixture inhibited rat or rabbit C3 activation and lowered the SBA titers, measured with rabbit complement, of human vaccinee sera more than 10-fold (16). Thus, human species-specific binding of fH by N. meningitidis may explain why serum bactericidal titers measured with rabbit complement are higher than titers measured with human complement (27, 43), where human fH was bound to the bacteria.
The infant rat model has been used to measure the ability of antibodies to confer PP against meningococcal bacteremia (15, 17, 31-35, 37, 39, 40). Although nonbactericidal antibodies to fHbp and/or GNA 2132 conferred protection in this model (40, 42), we must now consider that the observed in vivo protection occurred in the presence of unregulated complement activation. Therefore, it may not be possible to extrapolate the PP results from the animal model to protection of humans from meningococcal disease, where fH is bound to the bacterial surface and complement activation is downregulated, rendering the organism resistant to bacteriolysis.
Based on titers of <1:4 in the SBA or OPA assay, only a minority of the adults immunized with the investigational recombinant protein vaccine in this study was predicted to be protected against disease caused by N. meningitidis strain NZ98/254 or S3032. However, the results of the serum PP assays indicated that a majority of the test sera, when added to nonimmune human blood, could kill these strains. Thus, a much higher proportion of the vaccinated subjects may have been protected than that predicted by the SBA or OPA results. The greater sensitivity of the PP assay may reflect the higher complement concentrations in the ex vivo model of bacteremia using whole human blood than that in the SBA or OPA assay (20%), but whether the PP results overestimate protection is unknown. Thus, the actual extent to which SBA or OPA responses underestimate the meningococcal immunity induced by vaccination, and the ability of the serum PP assay to predict protection, will need to be validated by additional studies.
Acknowledgments
We are grateful for the excellent technical assistance provided by Ray Chen, Ryan Palapaz, and Tracy Wong.
Footnotes
Published ahead of print on 1 April 2009.
REFERENCES
- 1.Andrews, N., R. Borrow, and E. Miller. 2003. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from post-licensure surveillance in England. Clin. Diagn. Lab. Immunol. 10780-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balmer, P., and R. Borrow. 2004. Serologic correlates of protection for evaluating the response to meningococcal vaccines. Expert Rev. Vaccines 377-87. [DOI] [PubMed] [Google Scholar]
- 3.Bjune, G., J. K. Gronnesby, E. A. Hoiby, O. Closs, and H. Nokleby. 1991. Results of an efficacy trial with an outer membrane vesicle vaccine against systemic serogroup B meningococcal disease in Norway. NIPH Ann. 14125-130. [PubMed] [Google Scholar]
- 4.Borrow, R., P. Balmer, and E. Miller. 2005. Meningococcal surrogates of protection—serum bactericidal antibody activity. Vaccine 232222-2227. [DOI] [PubMed] [Google Scholar]
- 5.Borrow, R., G. M. Carlone, N. Rosenstein, M. Blake, I. Feavers, D. Martin, W. Zollinger, J. Robbins, I. Aaberge, D. M. Granoff, E. Miller, B. Plikaytis, L. van Alphen, J. Poolman, R. Rappuoli, L. Danzig, J. Hackell, B. Danve, M. Caulfield, S. Lambert, and D. Stephens. 2006. Neisseria meningitidis group B correlates of protection and assay standardization—international meeting report Emory University, Atlanta, Georgia, United States, 16-17 March 2005. Vaccine 245093-5107. [DOI] [PubMed] [Google Scholar]
- 6.Capecchi, B., J. Adu-Bobie, F. Di Marcello, L. Ciucchi, V. Masignani, A. Taddei, R. Rappuoli, M. Pizza, and B. Arico. 2005. Neisseria meningitidis NadA is a new invasin which promotes bacterial adhesion to and penetration into human epithelial cells. Mol. Microbiol. 55687-698. [DOI] [PubMed] [Google Scholar]
- 7.Comanducci, M., S. Bambini, B. Brunelli, J. Adu-Bobie, B. Arico, B. Capecchi, M. M. Giuliani, V. Masignani, L. Santini, S. Savino, D. M. Granoff, D. A. Caugant, M. Pizza, R. Rappuoli, and M. Mora. 2002. NadA, a novel vaccine candidate of Neisseria meningitidis. J. Exp. Med. 1951445-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dyet, K. H., and D. R. Martin. 2006. Clonal analysis of the serogroup B meningococci causing New Zealand's epidemic. Epidemiol. Infect. 134377-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Findlow, J., S. Taylor, A. Aase, R. Horton, R. Heyderman, J. Southern, N. Andrews, R. Barchha, E. Harrison, A. Lowe, E. Boxer, C. Heaton, P. Balmer, E. Kaczmarski, P. Oster, A. Gorringe, R. Borrow, and E. Miller. 2006. Comparison and correlation of Neisseria meningitidis serogroup B immunologic assay results and human antibody responses following three doses of the Norwegian meningococcal outer membrane vesicle vaccine MenBvac. Infect. Immun. 744557-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frasch, C. E., W. D. Zollinger, and J. T. Poolman. 1985. Serotype antigens of Neisseria meningitidis and a proposed scheme for designation of serotypes. Rev. Infect. Dis. 7504-510. [DOI] [PubMed] [Google Scholar]
- 11.Fredriksen, J. H., E. Rosenqvist, E. Wedege, K. Bryn, G. Bjune, L. O. Froholm, A. K. Lindbak, B. Mogster, E. Namork, U. Rye, et al. 1991. Production, characterization and control of MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 1467-79. [PubMed] [Google Scholar]
- 12.Giuliani, M. M., J. Adu-Bobie, M. Comanducci, B. Arico, S. Savino, L. Santini, B. Brunelli, S. Bambini, A. Biolchi, B. Capecchi, E. Cartocci, L. Ciucchi, F. Di Marcello, F. Ferlicca, B. Galli, E. Luzzi, V. Masignani, D. Serruto, D. Veggi, M. Contorni, M. Morandi, A. Bartalesi, V. Cinotti, D. Mannucci, F. Titta, E. Ovidi, J. A. Welsch, D. Granoff, R. Rappuoli, and M. Pizza. 2006. A universal vaccine for serogroup B meningococcus. Proc. Natl. Acad. Sci. USA 10310834-10839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 1291307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granoff, D. M., L. Harrison, and R. Borrow. 2008. Meningococcal vaccines, p. 399-434. In S. A. Plotkin, P. Offit, and W. A. Orenstein (ed.), Vaccines, 5th ed. Saunders Elsevier, Philadelphia, PA.
- 15.Granoff, D. M., A. Morgan, and J. A. Welsch. 2005. Persistence of group C anticapsular antibodies two to three years after immunization with an investigational quadrivalent Neisseria meningitidis-diphtheria toxoid conjugate vaccine. Pediatr. Infect. Dis. J. 24132-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granoff, D. M., J. A. Welsch, and S. Ram. 2009. Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera. Infect. Immun. 77764-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou, V. C., O. Koeberling, J. A. Welsch, and D. M. Granoff. 2005. Protective antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed genome-derived neisserial antigen 1870. J. Infect. Dis. 192580-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ison, C. A., N. Anwar, M. J. Cole, R. Galassini, R. S. Heyderman, N. J. Klein, J. West, A. J. Pollard, S. Morley, M. Levin, and the Meningococcal Research Group. 1999. Assessment of immune response to meningococcal disease: comparison of a whole-blood assay and the serum bactericidal assay. Microb. Pathog. 27207-214. [DOI] [PubMed] [Google Scholar]
- 19.Ison, C. A., N. Anwar, M. J. Cole, A. J. Pollard, S. L. Morley, K. Fidler, C. Sandiford, J. Banks, S. J. Kroll, and M. Levin. 2003. Age dependence of in vitro survival of meningococci in whole blood during childhood. Pediatr. Infect. Dis. J. 22868-873. [DOI] [PubMed] [Google Scholar]
- 20.Ison, C. A., R. S. Heyderman, N. J. Klein, M. Peakman, and M. Levin. 1995. Whole blood model of meningococcal bacteraemia—a method for exploring host-bacterial interactions. Microb. Pathog. 1897-107. [DOI] [PubMed] [Google Scholar]
- 21.Madico, G., J. A. Welsch, L. A. Lewis, A. McNaughton, D. H. Perlman, C. E. Costello, J. Ngampasutadol, U. Vogel, D. M. Granoff, and S. Ram. 2006. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J. Immunol. 177501-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masignani, V., M. Comanducci, M. M. Giuliani, S. Bambini, J. Adu-Bobie, B. Arico, B. Brunelli, A. Pieri, L. Santini, S. Savino, D. Serruto, D. Litt, S. Kroll, J. A. Welsch, D. M. Granoff, R. Rappuoli, and M. Pizza. 2003. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J. Exp. Med. 197789-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morley, S. L., M. J. Cole, C. A. Ison, M. A. Camaraza, F. Sotolongo, N. Anwar, I. Cuevas, M. Carbonero, H. C. Campa, G. Sierra, and M. Levin. 2001. Immunogenicity of a serogroup B meningococcal vaccine against multiple Neisseria meningitidis strains in infants. Pediatr. Infect. Dis. J. 201054-1061. [DOI] [PubMed] [Google Scholar]
- 24.Newcombe, R. G. 1998. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat. Med. 17857-872. [DOI] [PubMed] [Google Scholar]
- 25.Pizza, M., V. Scarlato, V. Masignani, M. M. Giuliani, B. Arico, M. Comanducci, G. T. Jennings, L. Baldi, E. Bartolini, B. Capecchi, C. L. Galeotti, E. Luzzi, R. Manetti, E. Marchetti, M. Mora, S. Nuti, G. Ratti, L. Santini, S. Savino, M. Scarselli, E. Storni, P. Zuo, M. Broeker, E. Hundt, B. Knapp, E. Blair, T. Mason, H. Tettelin, D. W. Hood, A. C. Jeffries, N. J. Saunders, D. M. Granoff, J. C. Venter, E. R. Moxon, G. Grandi, and R. Rappuoli. 2000. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 2871816-1820. [DOI] [PubMed] [Google Scholar]
- 26.Plested, J. S., and D. M. Granoff. 2008. Vaccine-induced opsonophagocytic immunity to Neisseria meningitidis group B. Clin. Vaccine Immunol. 15799-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santos, G. F., R. R. Deck, J. Donnelly, W. Blackwelder, and D. M. Granoff. 2001. Importance of complement source in measuring meningococcal bactericidal titers. Clin. Diagn. Lab. Immunol. 8616-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider, M. C., R. M. Exley, S. Ram, R. B. Sim, and C. M. Tang. 2007. Interactions between Neisseria meningitidis and the complement system. Trends Microbiol. 15233-240. [DOI] [PubMed] [Google Scholar]
- 29.Sprong, T., P. Brandtzaeg, M. Fung, A. M. Pharo, E. A. Hoiby, T. E. Michaelsen, A. Aase, J. W. van der Meer, M. van Deuren, and T. E. Mollnes. 2003. Inhibition of C5a-induced inflammation with preserved C5b-9-mediated bactericidal activity in a human whole blood model of meningococcal sepsis. Blood 1023702-3710. [DOI] [PubMed] [Google Scholar]
- 30.Sprong, T., A. S. Moller, A. Bjerre, E. Wedege, P. Kierulf, J. W. van der Meer, P. Brandtzaeg, M. van Deuren, and T. E. Mollnes. 2004. Complement activation and complement-dependent inflammation by Neisseria meningitidis are independent of lipopolysaccharide. Infect. Immun. 723344-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toropainen, M., H. Kayhty, L. Saarinen, E. Rosenqvist, E. A. Hoiby, E. Wedege, T. Michaelsen, and P. H. Makela. 1999. The infant rat model adapted to evaluate human sera for protective immunity to group B meningococci. Vaccine 172677-2689. [DOI] [PubMed] [Google Scholar]
- 32.Toropainen, M., L. Saarinen, P. van der Ley, B. Kuipers, and H. Kayhty. 2001. Murine monoclonal antibodies to PorA of Neisseria meningitidis show reduced protective activity in vivo against B:15:P1.7,16 subtype variants in an infant rat infection model. Microb. Pathog. 30139-148. [DOI] [PubMed] [Google Scholar]
- 33.Toropainen, M., L. Saarinen, G. Vidarsson, and H. Kayhty. 2006. Protection by meningococcal outer membrane protein PorA-specific antibodies and a serogroup B capsular polysaccharide-specific antibody in complement-sufficient and C6-deficient infant rats. Infect. Immun. 742803-2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toropainen, M., L. Saarinen, E. Wedege, K. Bolstad, P. H. Makela, and H. Kayhty. 2005. Passive protection in the infant rat protection assay by sera taken before and after vaccination of teenagers with serogroup B meningococcal outer membrane vesicle vaccines. Vaccine 234821-4833. [DOI] [PubMed] [Google Scholar]
- 35.Toropainen, M., L. Saarinen, E. Wedege, K. Bolstad, T. E. Michaelsen, A. Aase, and H. Kayhty. 2005. Protection by natural human immunoglobulin M antibody to meningococcal serogroup B capsular polysaccharide in the infant rat protection assay is independent of complement-mediated bacterial lysis. Infect. Immun. 734694-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trotter, C., J. Findlow, P. Balmer, A. Holland, R. Barchha, N. Hamer, N. Andrews, E. Miller, and R. Borrow. 2007. Seroprevalence of bactericidal and anti-outer membrane vesicle antibodies to Neisseria meningitidis group B in England. Clin. Vaccine Immunol. 14863-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vu, D. M., J. A. Welsch, P. Zuno-Mitchell, J. V. Dela Cruz, and D. M. Granoff. 2006. Antibody persistence 3 years after immunization of adolescents with quadrivalent meningococcal conjugate vaccine. J. Infect. Dis. 193821-828. [DOI] [PubMed] [Google Scholar]
- 38.Welsch, J. A., and D. Granoff. 2007. Immunity to Neisseria meningitidis group B in adults despite lack of serum bactericidal activity. Clin. Vaccine Immunol. 141596-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welsch, J. A., and D. Granoff. 2004. Naturally acquired passive protective activity against Neisseria meningitidis group C in the absence of serum bactericidal activity. Infect. Immun. 725903-5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Welsch, J. A., G. R. Moe, R. Rossi, J. Adu-Bobie, R. Rappuoli, and D. M. Granoff. 2003. Antibody to genome-derived neisserial antigen 2132, a Neisseria meningitidis candidate vaccine, confers protection against bacteremia in the absence of complement-mediated bactericidal activity. J. Infect. Dis. 1881730-1740. [DOI] [PubMed] [Google Scholar]
- 41.Welsch, J. A., S. Ram, O. Koeberling, and D. M. Granoff. 2008. Complement-dependent synergistic bactericidal activity of antibodies against factor H-binding protein, a sparsely distributed meningococcal vaccine antigen. J. Infect. Dis. 1971053-1061. [DOI] [PubMed] [Google Scholar]
- 42.Welsch, J. A., R. Rossi, M. Comanducci, and D. M. Granoff. 2004. Protective activity of monoclonal antibodies to genome-derived neisserial antigen 1870, a Neisseria meningitidis candidate vaccine. J. Immunol. 1725606-5615. [DOI] [PubMed] [Google Scholar]
- 43.Zollinger, W. D., and R. E. Mandrell. 1983. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect. Immun. 40257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]