Abstract
P4, a 28-amino-acid peptide, is a eukaryotic cellular activator that enhances specific in vitro opsonophagocytic killing of multiple bacterial pathogens. In a previous study, we successfully recreated this phenomenon in mice in vivo by using a two-dose regimen of P4 and pathogen-specific antibodies, which significantly reduced moribundity in mice. For the present study, we hypothesized that the inclusion of a low-dose antibiotic would make it possible to treat the infected mice with a single dose containing a mixture of P4 and a pathogen-specific antibody. A single dose consisting of P4, intravenous immunoglobulin (IVIG), and ceftriaxone effectively reduced moribundity compared to that of untreated controls (n = 10) by 75% (P < 0.05) and rescued all (10 of 10) infected animals (P < 0.05). If rescued animals were reinfected with Streptococcus pneumoniae and treated with a single dose containing P4, IVIG, and ceftriaxone, they could be rerescued. This observation of the repeated successful use of P4 combination therapy demonstrates a low risk of tolerance development. Additionally, we examined the polymorphonuclear leukocytes (PMN) derived from infected mice and observed that P4 enhanced in vitro opsonophagocytic killing (by >80% over the control level; P < 0.05). This finding supports our hypothesis that PMN are activated by P4 during opsonophagocytosis and the recovery of mice from pneumococcal infection. P4 peptide-based combination therapy may offer an alternative and rapid immunotherapy to treat fulminant pneumococcal infection.
Infectious diseases are a global public health problem that is compounded by the emergence of multidrug-resistant pathogens; treating infections caused by such organisms poses a challenge to human and animal health care (9). New approaches to address this growing public health concern are needed. Research in the field of host-microbe interaction and immunity has formed the basis for the development of immune therapies. As early as 1891, patients with life-threatening bacterial infections were treated with immune sera derived from rabbits or horses, with remarkable reductions in both morbidity and mortality (2). Several reports have described the successful treatment of bacterial infections in animals and humans by using immune sera (2, 3, 8). Despite the success, a variety of factors have impeded the clinical use of immune sera. The incidence of serum sickness raised serious concerns over the safety of immune sera in passive immunization. Interestingly, the early 1900s also witnessed an explosive development in the field of antibiotics. Thus, the introduction of sulfonamides in 1937 made passive immune therapy a less attractive therapeutic option with questionable safety.
Serum or antibody therapy, now known as passive immunization, has come full circle, with recent advances in antibody harvesting and monoclonal antibody production increasing interest in passive immunization (6). The emergence of multidrug-resistant bacterial pathogens, viral infections that sever the cellular arms of the immune system, and autoimmune diseases have prompted researchers and clinicians to revisit antibody-based passive immune therapy. At present, passive immune therapy is largely confined to treating cancer and autoimmune diseases (5, 6, 19), although antibodies are used passively to treat cytomegalovirus or Clostridium difficile infections in critically ill or immunocompromised patients.
Previously, we developed a combination immunotherapy employing P4, a 28-amino-acid peptide, combined with specific polyclonal antibody and successfully treated mice infected with a lethal strain of Streptococcus pneumoniae (12). In this study, we have taken up the question of whether P4 combination therapy can be delivered in a single dose, which would reduce the time needed to treat a patient. In addition, we explored whether the combination therapy consisting of the P4 peptide, pathogen-specific antibodies, and ceftriaxone can be given for subsequent infections without the development of immune tolerance. We observed that P4 combination therapy offers an alternative and rapid immunotherapy for treating an otherwise fatal pneumococcal (Pnc) infection.
MATERIALS AND METHODS
Bacterium, peptide, antibodies, and antibiotic used in this study.
S. pneumoniae serotype 3 (WU2) was used for mouse infections as described previously (12). P4, a 28-amino-acid peptide, was synthesized, purified, and prepared for combination therapy as described previously (11). Gamma globulin (intravenous immunoglobulin [IVIG]; Gamunex, Telecris, NC) was used as a source of Pnc serotype-specific polysaccharide antibodies (7, 10, 13). Ceftriaxone (catalog no. C5793; Sigma-Aldrich, St. Louis, MO), an expanded-spectrum cephalosporin, was dissolved in phosphate-buffered saline (0.01 M), and working dilutions in phosphate-buffered saline were made for mouse inoculations.
Mice.
Female Swiss Webster mice (Charles River Laboratories, Wilmington, MA) 6 to 10 weeks of age were used in this study. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) and conducted according to the institutional ethical guidelines for animal experiments and safety guidelines.
Intranasal infection.
Intranasal infections of mice with a Pnc isolate were carried out by adopting the methodology described previously (1). Briefly, a mouse was injected intraperitoneally (i.p.) with 20 μl of 100-mg/ml ketamine hydrochloride (Ketaset; Wyeth). Once the mouse was lethargic, 40 μl of the bacterial suspension (∼2.1 × 107 cells/mouse) was dispensed drop by drop close to the nose, allowing the mouse to inhale the bacteria. Scruffiness combined with a hunched posture or lethargy indicated moribundity in a mouse. Most mice (80%) were moribund at 48 h postexposure. Moribund mice were divided into various control and treatment groups (n = 10/group) as shown in Table 1. Control groups included untreated animals or animals that received P4 alone, IVIG alone, or ceftriaxone (at 0.3, 3.0, 300, or 3,000 μg/mouse) alone. Treatment groups included mice that received a single inoculum containing P4 and IVIG with or without ceftriaxone at one of three different doses (0.3, 3.0, or 300 μg/mouse).
TABLE 1.
Study design
| Treatment | Groupa | Useb of:
|
|||||
|---|---|---|---|---|---|---|---|
| P4 | Gamma globulin | Ceftriaxone
|
|||||
| 3,000 μg | 300 μg | 3.0 μg | 0.3 μg | ||||
| Combination therapy | 1 | − | − | − | − | − | − |
| 2 | + | − | − | − | − | − | |
| 3 | − | + | − | − | − | − | |
| 4 | − | − | + | − | − | − | |
| 5 | − | − | − | + | − | − | |
| 6 | − | − | − | − | + | − | |
| 7 | − | − | − | − | − | + | |
| 8 | + | + | − | − | − | − | |
| 9 | + | + | − | + | − | − | |
| 10 | + | + | − | − | + | − | |
| 11 | + | + | − | − | − | + | |
| Repeat therapy | 10 | + | + | − | − | + | − |
n = 10/group. All groups received S. pneumoniae serotype 3 (WU2) intranasally at ∼2.1 × 107 cells/40 μl/mouse.
P4 at 50 μg in a 100-μl volume and 100 μl of gamma globulin were administered i.v.; ceftriaxone was administered i.p. at the indicated doses in a 100-μl volume. +, included in the therapeutic mixture; −, not included in the therapeutic mixture.
Combination therapy.
Mice were restrained using a Tailveiner restrainer (model no. TV-150; Braintree Scientific, Braintree, MA). IVIG and/or P4 was administered intravenously (i.v.) using a 25-gauge needle and a 1-ml syringe. IVIG (100-μl volume/mouse) was administered first, followed 20 min later by P4 (50 μg in a 100-μl volume/mouse). Ceftriaxone was administered (i.p. in a 100-μl volume/mouse) 30 min after P4 administration. Animals were monitored daily for 7 days or (in the repeat infection study) 36 days posttreatment for clinical signs of disease progression. For repeat therapy, mice rescued with P4 combination therapy were reinfected on day 28 posttreatment and treated again with P4 combination therapy. Even though 25 to 30% of mice in the control group survived the first challenge, they later either succumbed to infection or were terminally ill and hence were humanely euthanized. Hence, we had no control mice from the first challenge for the repeat therapy.
Test for bacteremia.
Blood samples from P4-treated and untreated mice were collected (12), and 100-μl aliquots of the heparinized blood samples were spread onto plates of blood agar (blood agar base plus 5% sheep blood plus gentamicin [2.5 mg/liter]). The plates were incubated for 18 to 24 h at 37°C in 5% CO2, and bacteria were counted.
P4-enhanced opsonophagocytosis.
We used the in vitro opsonophagocytic killing assay (OPKA) as described previously by Romero-Steiner et al. (15) with polymorphonuclear leukocytes (PMNs) isolated from mice (4). Peripheral blood samples were collected from mice 1 and 2 h postinfection (with Pnc WU2) or from uninfected controls as described previously (7), and the buffy coat fractions were separated (4) and used as the source of effector cells. Gamma globulin (Gamunex, Telecris, NC) was used as the serotype-specific antibody source. S. pneumoniae serotype 3 (WU2) was propagated, stored, and used in this assay as described previously (10, 14). A 100-μg/ml P4 peptide solution was added to the OPKA mixture at the preopsonization stage, and the control wells received diethyl pyrocarbonate water.
ELISA for anti-P4 IgG.
Blood samples from mice treated with P4 therapy were collected 14 days postinfection, and the serum fractions were separated by adopting the methodology described previously (12). An enzyme-linked immunosorbent assay (ELISA) was performed to detect and quantify antiprotein immunoglobulin G (IgG) as described previously, with minor modifications (16, 17). Briefly, ELISA plates were coated with the P4 peptide at a 5-μg/ml concentration. Plates were incubated at 4°C overnight and used to detect and quantify anti-P4 IgG in mouse sera. Horseradish peroxidase-labeled anti-mouse IgG (Sigma, St. Louis, MO) was used as the reporter antibody. SureBlue 3,3′,5,5′-tetramethylbenzidine (KPL, Gaithersburg, MD) was used as the substrate, and 1 N HCl was used as the stop solution. Samples were assayed in triplicate, and suitable positive and negative controls were included.
Statistics.
The in vivo combination therapy experiments were repeated three to five times, and repeat therapy was performed once. The numbers of moribund animals up to 7 and 36 days after the combination and repeat therapies, respectively, were recorded, and the data were analyzed for significant differences among various groups by using the paired two-sample t test for means in Microsoft Excel 2003.
RESULTS
Combination therapy.
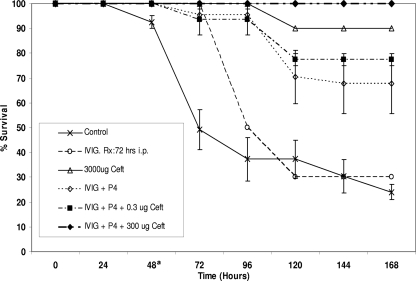
Untreated control mice (n = 10) had a 30% survival rate at 168 h. Mice treated with ceftriaxone alone at 3,000 μg had a 90% survival rate (Fig. 1). On the other hand, ceftriaxone at lower doses (300, 3.0, and 0.3 μg) or P4 alone offered poor protection, with survival rates comparable to that of untreated controls (data not shown). Treating moribund mice with a P4 concentration of 50 μg with IVIG administered in a single dose led to a survival rate of 70% (Fig. 1). Combining a low dose (300 μg) of ceftriaxone with this IVIG-P4 therapeutic mixture increased the mouse survival rate to 100%, significantly better than that of untreated controls (P < 0.05) (Fig. 1).
FIG. 1.
Survival of mice in various treatment arms and of control mice following exposure to S. pneumoniae serotype 3 (WU2). A single-dose i.v. injection of a mixture of P4 and gamma globulin (IVIG) with i.p. injection of ceftriaxone (Ceft) provided highly significant protection (100%; P < 0.05) compared to that of untreated controls. a, treatment administered.
Repeat therapy.
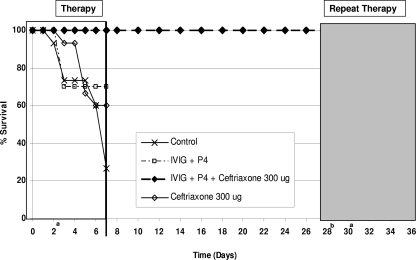
Mice (n = 10) rescued from fatal S. pneumoniae WU2 infection with combination (P4, IVIG, and ceftriaxone) therapy were reinfected with S. pneumoniae WU2 after 28 days and retreated with the combination therapy when they appeared to be moribund. P4-mediated combination therapy rescued all the infected animals (Fig. 2).
FIG. 2.
Mice previously rescued (♦) with P4 combination therapy were reinfected with S. pneumoniae serotype 3(WU2) on day 28. A single dose of P4, gamma globulin (IVIG), and ceftriaxone was administered 2 days later. All animals (100%) were protected. a, treatment; b, reinfection.
Test for bacteremia.
Blood samples from P4-treated and untreated mice were drawn and tested for bacteremia. Blood samples from the untreated control mice contained loads of bacteria too numerous to count. Samples from treated animals had no bacteremia (data not shown).
P4-enhanced opsonophagocytosis.
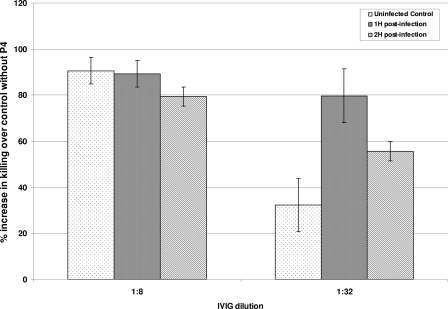
An OPKA with PMNs from either infected or uninfected mice was performed. The addition of P4 significantly increased (by >80%; P < 0.05) the in vitro opsonophagocytic killing of Pnc (WU2) over the control level in the presence of serotype-specific IgG (Fig. 3).
FIG. 3.
An in vitro OPKA with peripheral blood PMNs isolated from mice 1 and 2 h postinfection or from uninfected mice was performed. Gamma globulin (IVIG) was used as the source for serotype-specific antibodies. The addition of P4 increased the opsonophagocytic killing of S. pneumoniae serotype 3 (WU2) by ≥80% over that by PMNs from control mice not receiving P4 (P < 0.05).
ELISA for anti-P4 IgG.
Serum samples from P4-treated mice were tested for anti-P4 IgG by using a mouse IgG-specific ELISA. All the samples were negative for anti-P4 IgG (data not shown).
DISCUSSION
The results of the present study demonstrate the potential value of P4 therapy in treating bacterial infection. Antibiotics are the first line of therapeutic intervention for critically ill patients. But the successful treatment of these patients is complicated, and patients may die even with optimal antibiotic therapy (18). Immune therapy or passive immunization in these severe cases may provide additional therapeutic benefit. The administration of pathogen-specific antibodies may supplement the host immune system. P4 combination therapy takes this therapeutic alternative one step further. While the antibiotic component in the P4 combination therapy lowers the bacterial load in the host, pathogen-specific antibodies from the IVIG and the host form immune complexes and initiate the opsonization cascade. Because of its cellular activation characteristics, P4 upregulates the phagocytes, which may lead to an enhanced response to the opsonized bacterial pathogen and successful killing and/or elimination of the pathogen.
Previously, we have shown that the P4 peptide activates phagocytes, resulting in an enhancement of in vitro opsonophagocytic killing/uptake of various bacterial pathogens, including S. pneumoniae, Streptococcus pyogenes, Neisseria meningitidis, and Staphylococcus aureus (12). This effect requires pathogen-specific antibodies, complement, phagocytes, and P4. In our previous in vivo study, without antibiotics, we found that the combination of P4 and IVIG must be administered in two doses, with P4 given at 100 μg/mouse, for the complete elimination of symptoms and the recovery of mice at 192 h posttreatment. While P4 therapy may prove useful in enhancing the immune response in potentially lethal streptococcal infections and rescuing individuals, as we found in mice, it is desirable to have a single-dose regimen with a shorter recovery time. In this context, combining an expanded-spectrum cephalosporin, ceftriaxone, at a low dose (300 μg/mouse) with the P4 peptide and IVIG was beneficial. This combination rescued all animals (10 of 10) with a single dose of the P4 therapeutic mixture (Fig. 1), and we were able to reduce the P4 concentration in the mixture from 100 to 50 μg/mouse. This synergistic benefit was also observed when we examined the P4 combination therapy and observed reduced bacterial loads in the treated animals, as we failed to recover the bacterium from blood or nasal wash samples from the treated mice (data not shown).
Interestingly, P4-mediated combination therapy can also be repeated to treat recurrent bacterial infections, as mice treated with the combination therapy were not refractive to repeat P4 therapy. We anticipated no development of tolerance to repeat P4 therapy, as the P4 peptide is poorly immunogenic in its monomeric form (data not shown).
Based on the findings of our previous studies (11, 12), we hypothesized that in vivo, P4 activates the PMNs, leading to enhanced opsonophagocytosis and the recovery of the animals from the Pnc infection. The result that P4 enhanced in vitro opsonophagocytic killing of bacteria (by ≥80% over the control level; P < 0.05) by PMNs isolated from infected mice supports our hypothesis.
The success or failure of a host response to a bacterial infection is determined by the quantity and quality of the immune response. Although in some instances severe infections result in an exaggerated immune response that damages the host tissues or organs (e.g., in acute respiratory distress syndrome), exogenous support, such as that from P4, that reduces the pathogen load may change the equation to the advantage of the host.
The rise in the frequencies of multidrug-resistant bacterial and viral pathogens has rekindled the interest in passive immunization, or immune therapy. While researchers focus their resources to develop antibodies with desired biological activities, P4-mediated immune therapy offers promise for treating critical illness due to a range of bacterial pathogens.
Acknowledgments
We thank Cynthia Whitney, chief, Respiratory Diseases Branch, DBD, CDC, Atlanta, and Nadine Rouphael, Emory University Hospitals, for critical review of the manuscript.
Footnotes
Published ahead of print on 22 April 2009.
REFERENCES
- 1.Briles, D. E., S. K. Hollingshead, J. C. Paton, E. W. Ades, L. Novak, F. W. van Ginkel, and W. H. Benjamin, Jr. 2003. Immunizations with pneumococcal surface protein A and pneumolysin are protective against pneumonia in a murine model of pulmonary infection with Streptococcus pneumoniae. J. Infect. Dis. 188339-348. [DOI] [PubMed] [Google Scholar]
- 2.Chudwin, D. S. 1989. Prophylaxis and treatment of pneumococcal bacteremia by immune globulin intravenous in a mouse model. Clin. Immunol. Immunopathol. 5062-71. [DOI] [PubMed] [Google Scholar]
- 3.De Hennezel, L., F. Ramisse, P. Binder, G. Marchal, and J.-M. Alonso. 2001. Effective combination therapy for invasive pneumococcal pneumonia with ampicillin and intravenous immunoglobulins in a mouse model. Antimicrob. Agents Chemother. 45316-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devi, R. S., B. Shoba, A. R. Jayakumar, R. Muthusamy, and A. Namasivayam. 1995. Peripheral immune response in albino rats following cerebroventricular and intraperitoneal antigen challenge. Indian J. Physiol. Pharmacol. 39354-360. [PubMed] [Google Scholar]
- 5.Foster, J. K., G. Verdile, K. A. Bates, and R. N. Martins. 2008. Immunization in Alzheimer's disease: naïve hope or realistic clinical potential? Mol. Psychiatry 14239-251. [DOI] [PubMed] [Google Scholar]
- 6.Ledford, H. 2008. Monoclonal antibodies come of age. Nature 455437. [DOI] [PubMed] [Google Scholar]
- 7.Mikolajczyk, M. G., N. F. Concepcion, T. Wang, D. Frazier, B. Golding, C. E. Frasch, and D. E. Scott. 2004. Characterization of antibodies to capsular polysaccharide antigens of Haemophilus influenzae type b and Streptococcus pneumoniae in human immune globulin intravenous preparations. Clin. Diagn. Lab. Immunol. 111158-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pennington, J. E. 1988. Introduction to the symposium: intravenous immunoglobulin therapy in serious infections. J. Hosp. Infect. 121-2. [PubMed] [Google Scholar]
- 9.Pepper, J. W. 2008. Defeating pathogen drug resistance: guidance from evolutionary theory. Evolution 623185-3191. [DOI] [PubMed] [Google Scholar]
- 10.Rajam, G., G. M. Carlone, and S. Romero-Steiner. 2007. Functional antibodies to the O-acetylated pneumococcal serotype 15B capsular polysaccharide have low cross-reactivities with serotype 15C. Clin. Vaccine Immunol. 141223-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajam, G., D. J. Phillips, E. White, J. Anderton, C. W. Hooper, J. S. Sampson, G. M. Carlone, E. W. Ades, and S. Romero-Steiner. 2008. A functional epitope of the pneumococcal surface adhesin A activates nasopharyngeal cells and increases bacterial internalization. Microb. Pathog. 44186-196. [DOI] [PubMed] [Google Scholar]
- 12.Rajam, G., J. Skinner, N. Melnick, J. Martinez, G. M. Carlone, J. S. Sampson, and E. W. Ades. 2009. A 28-aa pneumococcal surface adhesin A-derived peptide, P4, augments passive immunotherapy and rescues mice from fatal pneumococcal infection. J. Infect. Dis. 1991233-1238. [DOI] [PubMed] [Google Scholar]
- 13.Romero-Steiner, S., C. Frasch, N. Concepcion, D. Goldblatt, H. Kayhty, M. Vakevainen, C. Laferriere, D. Wauters, M. H. Nahm, M. F. Schinsky, B. D. Plikaytis, and G. M. Carlone. 2003. Multilaboratory evaluation of a viability assay for measurement of opsonophagocytic antibodies specific to the capsular polysaccharides of Streptococcus pneumoniae. Clin. Diagn. Lab. Immunol. 101019-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romero-Steiner, S., C. E. Frasch, G. Carlone, R. A. Fleck, D. Goldblatt, and M. H. Nahm. 2006. Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin. Vaccine Immunol. 13165-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero-Steiner, S., D. Libutti, L. B. Pais, J. Dykes, P. Anderson, J. C. Whitin, H. L. Keyserling, and G. M. Carlone. 1997. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin. Diagn. Lab. Immunol. 4415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott, J. A. G., Z. Mlacha, J. Nyiro, S. Njenga, P. Lewa, J. Obiero, H. Otieno, J. S. Sampson, and G. M. Carlone. 2005. Diagnosis of invasive pneumococcal disease among children in Kenya with enzyme-linked immunosorbent assay for immunoglobulin G antibodies to pneumococcal surface adhesin A. Clin. Diagn. Lab. Immunol. 121195-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott, J. A. G., J. Obiero, A. J. Hall, and K. Marsh. 2002. Validation of immunoglobulin G enzyme-linked immunosorbent assay for antibodies to pneumococcal surface adhesin A in the diagnosis of pneumococcal pneumonia among adults in Kenya. J. Infect. Dis. 186220-226. [DOI] [PubMed] [Google Scholar]
- 18.Witte, W., C. Cuny, I. Klare, U. Nübel, B. Strommenger, and G. Werner. 2008. Emergence and spread of antibiotic-resistant gram-positive bacterial pathogens. Int. J. Med. Microbiol. 298365-377. [DOI] [PubMed] [Google Scholar]
- 19.Yap, P. L., and P. E. Williams. 1988. The treatment of human immunodeficiency virus infected patients with intravenous immunoglobulin. J. Hosp. Infect. 1235-46. [DOI] [PubMed] [Google Scholar]