Abstract
Respiratory syncytial virus (RSV) infects airway epithelial cells, causing bronchiolitis and pneumonia. Inflammation is mediated by various cytokines secreted from RSV-infected airway epithelial cells, and it promotes the pathogenesis of RSV-related diseases. Fosfomycin (FOF) is approved as a treatment for various bacterial infectious diseases, including respiratory infectious diseases, in Japan. FOF is suggested to exhibit immunomodulatory effects on lipopolysaccharide-stimulated monocytes and T lymphocytes, in addition to its antimicrobial activity. We investigated the effect of FOF on the cytokine production of an airway epithelial cell line, A549, infected with RSV. RSV-induced cytokines, such as regulated on activation, normal T-cell expressed and secreted (RANTES), interleukin-8 (IL-8), and IL-6, in infected A549 cells. We found that FOF decreased the levels of RSV-induced RANTES and IL-8 but not the level of RSV-induced IL-6. The RANTES promoter was activated by RSV infection. Site-directed mutagenesis analysis of the RANTES promoter showed that NF-κB-binding motifs had a critical role in RSV-induced RANTES promoter activity. A luciferase reporter gene assay and a DNA-binding assay indicated that FOF suppressed the NF-κB activity induced by RSV infection. These results demonstrate that FOF treatment suppresses the RSV-induced transcription of the chemokines RANTES and IL-8 in airway epithelial cells.
Respiratory syncytial virus (RSV) is one of the most important infectious agents causing acute lower respiratory tract illness in infants and young children, and RSV infection sometimes results in life-threatening acute bronchiolitis (28, 29). Necrosis of the airway epithelium is associated with the infiltration of monocytes and T lymphocytes, mainly in the peribronchial and perivascular regions in patients with bronchiolitis and between the interalveolar walls in patients with pneumonia, which leads to alveolar filling (26). Recruitment of these immunocompetent cells to the site of RSV infection is regulated by the cytokines/chemokines secreted from infected epithelial cells. Elevated levels of proinflammatory cytokines (e.g., interleukin-1β [IL-1β] and IL-6) and chemokines (e.g., IL-8 and regulated on activation, normal T-cell expressed and secreted [RANTES]) have been observed in nasal swab samples from infants with RSV infection (23). RANTES produced in response to RSV infection plays an important role in the pathogenesis of RSV-induced lung inflammation (5). RANTES is well known to be an eosinophil chemotactic factor involved in the pathogenesis of asthma (34). Furthermore, RANTES levels were found to increase significantly in infants experiencing wheezing after an RSV infection (7). Therefore, RANTES induced by RSV infection is thought to be highly associated with wheezing and childhood asthma (1, 9). Respiratory epithelial cells are a major source of RANTES in patients with lung inflammation (9, 30).
Several antimicrobial agents, such as the 14-membered-ring macrolides and fluoroquinolones, affect the immunological response of the host. The abilities of these antibiotics to inhibit the secretion of proinflammatory cytokines are thought to be mediated by inhibition of NF-κB (3, 13). Furthermore, pretreatment with erythromycin or clarithromycin has been reported to inhibit rhinovirus infection by suppressing the expression of virus receptors and reducing the rhinovirus-induced inflammatory cytokine response (14, 31). Fosfomycin (FOF) is a structurally unique antibiotic that is chemically unrelated to any other known antimicrobial agent (16). Apart from these antibacterial activities, FOF also possesses a novel immunomodulatory activity, which has been observed both in vitro and in vivo (10, 21, 22). FOF suppressed IL-1β, IL-2, IL-8, and tumor necrosis factor alpha (TNF-α) secretion in vitro from human monocytes and/or lipopolyssacharide (LPS)-stimulated T lymphocytes. Yoneshima et al. demonstrated that FOF suppresses NF-κB activation induced by TNF-α in monocyte and T-lymphocyte cell lines (36). FOF also strongly suppressed the mixed lymphocyte reaction and IL-2 production in vivo. However, the effect of FOF on the immunomodulation during virus infection has not been studied. FOF is approved as a treatment for various bacterial infectious diseases, including respiratory infectious diseases, in Japan. Therefore, we investigated the effect and the mechanism of action of FOF on RSV-induced inflammatory cytokine upregulation in respiratory epithelial cells, which are the primary and main targets of RSV infection.
MATERIALS AND METHODS
Epithelial cell culture and viral infection.
The A549 human lung adenocarcinoma epithelial cell line (ATCC, Manassas, VA) was maintained in RPMI 1640 medium with 10% fetal bovine serum. The human RSV Long and A2 strains (ATCC), which belong to subgroup A, were grown in the HEp-2 human laryngeal carcinoma cell line. For infection, A549 cells at ∼80% confluence were adsorbed at an RSV multiplicity of infection (MOI) of 1 for 60 min at 37°C. After adsorption, the viral solutions were removed and the cells were rinsed twice with phosphate-buffered saline and incubated with growth medium. The virus titers in the supernatant were determined by a plaque-forming assay with HEp-2 cells. Expression of RSV mRNA was confirmed by reverse transcription-PCR (RT-PCR).
FOF treatment.
Cells were treated with medium containing FOF (0, 10, 100, and 1,000 μg/ml) at 37°C. FOF was provided by Meiji Seika Kaisha, Ltd. (Tokyo, Japan).
Cell viability.
After 24 h of FOF treatment, cell viability was assessed with Cell Counting Kit-8 (Dojindo, Kumamoto, Japan), according to the manufacturer's protocol. Cell Counting Kit-8 solution, which contains the tetrazolium salt WST-8 [2-(2-methyl-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt], was added to the culture medium, and the plates were incubated at 37°C for 4 h. The absorbance at 450 nm was determined with a multiwell plate reader.
Semiquantitative RT-PCR.
Total cellular RNA was prepared from cells with an RNeasy kit (Qiagen, Hilden, Germany), according to the manufacturer's protocol. RT-PCR was performed with a One-Step RT-PCR kit (Qiagen). The quantitative nature of the PCR was validated by the linearity of the determination curve obtained with various concentrations of RNA. The sequences of the primers (Sigma-Genosys, Ishikari, Japan) were described previously (24). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as a control.
Measurement of cytokine production.
A549 cells were infected with RSV in the presence or absence of FOF for 24 h. The culture supernatants were stored at −80°C until they were assayed. The RANTES, IL-8, and IL-6 levels were measured with a DuoSet enzyme-linked immunosorbent assay (ELISA) development kit (R&D Systems, Minneapolis, MN), according to the manufacturer's instructions.
Luciferase reporter gene assay.
The luciferase reporter gene assay was carried out as described previously (35). Plasmids containing fragments of the RANTES promoter (−884 bp to +1 bp) and its mutants were kindly provided by S. Matsukura, Showa University School of Medicine (Tokyo, Japan) (12). The reporter plasmids, pISRE and pNF-κB, were purchased from Stratagene (La Jolla, CA). pISRE and pNF-κB harbor the enhancer elements of the interferon-stimulated response element [ISRE; (TAGTTTCACTTTCCC)5] and NF-κB [(TGGGGACTTTCCGC)5], respectively. These plasmids contain a firefly luciferase reporter gene and were cotransfected into cells with a reference plasmid, pRL-TK (Promega, Madison WI), by using the SuperFect transfect reagent (Qiagen). Twenty-four hours after transfection, cells were either infected or not infected with RSV in the presence or absence of FOF. After the cells were lysed, the luciferase activity was measured with the dual-luciferase reporter assay system (Promega) and a Fluoroskan Ascent FL apparatus (Labsystem, San Diego, CA).
ELDIA.
The DNA-binding activities of transcription factors in cells were determined by an enzyme-linked DNA-protein interaction assay (ELDIA), essentially by a previously described method (35). Nuclear extracts were prepared with the NE-PER nuclear and cytoplasmic extraction reagents (Pierce, Rockford, IL). The DNA binding of NF-κB p50 was determined with a Mercury TransFactor NF-κB p50 kit (BD Bioscience, Palo Alto, CA). The specificities of binding to a DNA motif were confirmed by inhibition experiments with unlabeled double-stranded competitor oligonucleotides at a concentration of 200 ng/well. The following oligonucleotides were used as competitors: NF-κB p50 wild type (WT; GCCATGGGGGGATCCCGGGC) and its mutant (GCCATGGGCCGATCCCGGGC, where the underlining indicates the mutated nucleotides).
Statistical analysis.
Determination of statistical significance was carried out by Student's t test. Data groups were considered significantly different when the P value was <0.05.
RESULTS
Effect of FOF on viral replication.
We first examined the effect of FOF on A549 cells infected with RSV. FOF (10 to 1,000 μg/ml) did not show any cytotoxicity on A549 cells (Fig. 1A). In A549 cells infected with RSV, FOF treatment did not alter the cytopathic effect, the release of infectious RSV particles, or the transcription of the viral genome (Fig. 1B and C). These results suggest that FOF does not influence cell viability or RSV replication.
FIG. 1.
FOF is not cytotoxic to A549 cells and does not influence RSV replication. (A) A549 cells were incubated with FOF (10 to 1,000 μg/ml) for 24 h, and their viabilities were measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Cell viability under cultivation without FOF was used as a control (100%; white bar). Each bar represents the mean ± standard deviation for three samples. (B) Microscopic observation of A549 cells infected or not infected with RSV (Long strain at an MOI of 1) for 24 h in the presence of FOF (0 to 1,000 μg/ml). (C) Effects of FOF on viral titers in culture supernatants and expression of RSV glycoprotein mRNA (RSV/G) in RSV-infected A549 cells. Each bar represents the mean ± standard deviation for three samples.
FOF reduces the induction of chemokines.
We examined the effects of various schedules of FOF treatment (Fig. 2, left panel). RSV infection increased the level of RANTES production (Fig. 2, bar 1). When FOF was applied before the incubation period (1 h), during the RSV infection period (1 h), and after infection (23 h), the level of RANTES was significantly reduced (Fig. 2, bar 4). Treatment with FOF only during the preincubation period (1 h) did not alter the level of RANTES (Fig. 2, bar 2). In the absence of FOF during the postinfection period, the level of RANTES tended to decrease, although the inhibitory effects were not significant compared to those observed in the absence of FOF (Fig. 2; see bars 3 and 5 versus bar 1). Addition of FOF only after infection (Fig. 2, bar 7) significantly inhibited the level of RANTES production, which was reduced to the same level as that seen when FOF was present during the preincubation and the absorption periods or during the absorption period and after infection (Fig. 2, bars 4 and 6). Taken together, FOF appears to be effective in suppressing the RANTES induced by RSV, even after infection.
FIG. 2.
Effects of FOF treatment schedule on RANTES production by RSV-infected A549 cells. A549 cells were infected with the RSV Long strain at an MOI of 1 in either the presence of FOF (1,000 μg/ml) or the absence of FOF before infection (1 h), at the RSV adsorption step (1 h), and after infection (23 h). (Left panel) The gray regions of each bar indicate the period of FOF treatment; (right panel) the concentration of RANTES in the culture supernatant was determined by ELISA. Each bar represents the mean ± standard deviation for three samples. **, P < 0.01 compared with the results of incubation without FOF (bar 1, open bar).
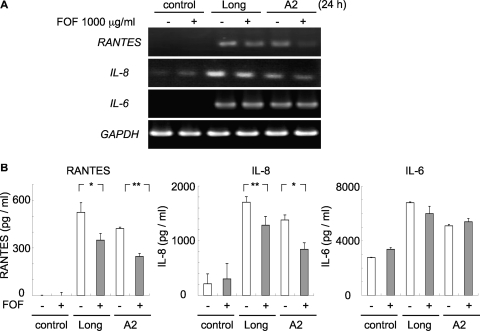
We evaluated whether FOF affected the expression levels of cytokines/chemokines induced by infection with the RSV Long and A2 strains (Fig. 3). RSV-infected cells were treated with FOF (1,000 μg/ml) during the postinfection period. RSV infection resulted in increases in RANTES, IL-8, and IL-6 at the mRNA (Fig. 3A) and the protein (Fig. 3B) levels. The increased levels of expression of RANTES and IL-8 in response to RSV infection was significantly inhibited by FOF treatment (Fig. 3). However, IL-6 expression was not influenced by FOF treatment.
FIG. 3.
FOF suppresses RANTES and IL-8 production but not IL-6 production in RSV-infected A549 cells. RSV-infected A549 cells were treated with FOF (1,000 μg/ml) for 23 h postinfection. (A) The levels of expression of RANTES, IL-8, and IL-6 mRNA were determined by RT-PCR. Analysis of GAPDH mRNA was performed as a control. (B) The concentrations of RANTES, IL-8, and IL-6 in the culture supernatant were determined by ELISA. Each bar represents the mean ± standard deviation of three samples. *, P < 0.05; **, P < 0.01.
Mechanism of RANTES suppression by FOF.
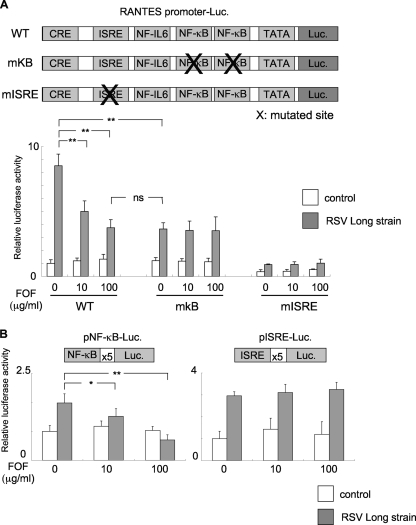
Since FOF effectively reduced the level of RANTES production by RSV-infected A549 cells, we determined the effect of FOF on the transcriptional activity of the RANTES gene. RANTES transcription is controlled by multiple cis enhancer elements, such as ISRE, NF-κB, the cis-acting replication element (CRE), and NF-IL-6, which bind to the interferon regulatory factors, NF-κB, jun/CREB/ATF, and C/EBP, respectively (5). We examined the role of the binding sites for NF-κB and ISRE during induction of the RANTES promoter in response to RSV infection, because NF-κB and ISRE were previously found to be critical for RSV-induced RANTES production (5, 32). We performed a luciferase reporter assay with plasmids containing the WT RANTES promoter or mutant forms of the RANTES promoter which were either mutated at two binding sites for NF-κB (mkB) or at an ISRE (mISRE) (12). Cells were transfected with each plasmid and infected with RSV, and the luciferase activity was measured. As shown in Fig. 4A, RSV infection upregulated the luciferase activity of the WT RANTES promoter. FOF treatments reduced the level of RSV-induced luciferase activity in a dose-dependent manner. The mkB RANTES promoter showed reduced levels of RSV-induced luciferase activity in the absence of FOF, with the mkB RANTES promoter being expressed at a level similar to that of the WT RANTES promoter upon FOF (100 μg/ml) treatment. The luciferase activity of the mkB RANTES promoter was not further reduced by FOF treatment. The mISRE RANTES promoter almost abolished both basal and RSV-inducible luciferase activity.
FIG. 4.
Luciferase reporter gene analyses of promoter and transcription factors contributing to the suppression of RANTES production in RSV-infected cells treated with FOF. (A) Mutations in the NF-κB-binding sites of the RANTES promoter reduce the level of RSV-induced RANTES transcription at a level similar reduced by FOF treatment. The luciferase (Luc.) activities of WT and mutant (mkB and mISRE) RANTES promoters were determined with or without RSV infection and with or without FOF treatment. Twenty-four hours after the transfection of each plasmid to A549 cells, FOF (0 to 100 μg/ml) was added to the A549 cell culture before infection (1 h) and after infection (24 h) with RSV. (B) NF-κB activation by RSV is suppressed by FOF treatment. The luciferase activities of the plasmids containing tandem repeats of the NF-κB-binding site (pNF-κB-Luc) and ISRE (pISRE-Luc) were determined with or without RSV infection and with or without FOF treatment. White bars, no RSV infection; gray bars, infection with RSV. The results are expressed as the level of fold induction (± standard deviation) relative to the value obtained from uninfected cells transfected with the WT RANTES promoter without FOF treatment (A) and from uninfected cells transfected with each reporter plasmid (B). Each bar represents the mean ± standard deviation for three samples. **, P < 0.01; ns, not significant.
We also carried out a reporter gene assay using plasmids harboring the luciferase reporter under the control of tandem repeats of binding motifs of either NF-κB or ISRE (Fig. 4B). The luciferase activities of both plasmids were upregulated by RSV infection (Fig. 4B, FOF at 0 μg/ml). FOF suppressed RSV-induced NF-κB activity in a dose-dependent manner (Fig. 4B, left panel). In contrast, ISRE activities were not affected by FOF treatment (Fig. 4B, right panel).
To confirm that FOF alters the NF-κB activity induced by RSV infection, the ability of NF-κB to bind to DNA was determined by ELDIA with nuclear extracts prepared from RSV-infected cells with or without FOF treatment. As shown in Fig. 5, RSV infection markedly increased the level of binding of NF-κB p50 to its specific binding consensus motif (Fig. 5A). NF-κB p50 binding was inhibited by the addition of an NF-κB-binding motif-containing oligonucleotide (data not shown). The nuclear extract prepared from FOF-treated, RSV-infected cells contained less activated NF-κB than that prepared from nontreated RSV-infected cells. This effect of FOF occurred in a dose-dependent manner (Fig. 5B). The data from the luciferase assay and ELDIA indicate that the RANTES promoter activity is suppressed by FOF in RSV-infected cells due to the suppression of NF-κB activity and is not mediated by an ISRE-dependent mechanism.
FIG. 5.
FOF suppresses the ability of NF-κB to bind to an oligonucleotide in A549 cells infected with RSV. The ability of NF-κB to bind to its binding consensus motif was determined by an ELDIA. (A) A549 cells infected with RSV for 3, 6, or 9 h. (B) A549 cells either infected or not infected with RSV for 24 h were treated with FOF (0 to 100 μg/ml). FOF was added before infection (1 h), during the RSV adsorption step (1 h), and after infection (5 h). The cells were harvested at the indicated times, and the nuclear fractions were prepared. The nuclear extracts were assessed by ELDIA, which used the authentic NF-κB p50-binding motif as a coated oligonucleotide. The specificity of binding of NF-κB was confirmed by the inhibition of NF-κB binding to a mutated oligonucleotide DNA in the presence of a soluble oligonucleotide DNA as a competitor (data not shown). Each bar represents the mean ± standard deviation for three samples. *, P < 0.05; **, P < 0.01.
DISCUSSION
In the present study, we have shown that FOF suppressed RSV-induced chemokine production, specifically, the production of RANTES and IL-8, in respiratory epithelial cells. The ability of FOF to suppress RSV-induced RANTES production depended on the suppression of NF-κB activity. Our results are the first to indicate the suppression of a virus-induced chemokine by FOF in epithelial cells, which are the primary target of viral infection.
We and other researchers showed that NF-κB activity is important in RSV-induced RANTES promoter regulation (Fig. 4 and 5) (5, 32). The production of IL-8 that was induced by RSV infection was suppressed by FOF treatment (Fig. 3). The IL-8 promoter also has an NF-κB-binding site, and the production of IL-8 that is induced by RSV infection involves the activation of NF-κB (6). We inferred from these results that FOF treatment suppresses IL-8 production by inhibiting NF-κB activation. Although the IL-6 promoter also has an NF-κB-binding site (20), the production of IL-6 was not affected by the FOF treatment (Fig. 3). It was reported that FOF treatment enhances IL-6 production in LPS-stimulated human monocytes (22). The production of IL-6 may thus mainly be controlled by transcription factors other than NF-κB.
Some researchers reported on the modulatory effect of FOF on cytokine production by examining LPS-stimulated monocytes and/or T cells (10, 21, 22). They demonstrated that FOF suppressed IL-1β, IL-2, and IL-8 but not IL-6 or IL-10. Yoneshima et al. reported that FOF suppressed NF-κB activation in TNF-α-stimulated human monocyte and T-cell lines (36). We found no reports on the effect of FOF on virus-induced cytokine production or on epithelial cells, which are the main targets of RSV infection. Because airway epithelial cells are the primary targets of RSV replication, the immune response that develops within the lungs of infected individuals dictates the subsequent immune response. Among the chemokines and proinflammatory cytokines, RANTES and IL-8 are strongly expressed in RSV-infected epithelial cells (25, 27) and are key factors in the pathophysiology of RSV infection (8). The RANTES expressed in airway epithelial cells serves as a chemotactic and activation factor for eosinophils, basophils, monocytes, and neutrophils (17); promotes the adhesion and infiltration of these leukocytes, especially the eosinophils; and induces allergic reactions in individuals with asthma who have been exposed to an allergen (15). The examination of bronchoalveolar lavage fluid from children with RSV bronchiolitis showed markedly elevated levels of IL-8, which significantly correlated with the neutrophil numbers (18). IL-8 primarily targets the neutrophil and promotes neutrophil migration to the site of inflammation. It is suggested that neutrophils induced by IL-8 inflammation play an important role during airway RSV infection (37). RANTES and IL-8 induced by RSV infection activate the release of leukotrienes and histamine in primed mast cells and basophils (2, 4). The release of these chemical mediators in the respiratory tract induces RSV-related bronchiolitis and asthma (19). John et al. demonstrated that the RSV-induced release of leukotrienes is suppressed by the reduction of RANTES activity (15). Furthermore, FOF has the capacity to suppress histamine release from anti-immunoglobulin E-stimulated basophils and leukotriene release from neutrophils (10, 11). The suppression of RANTES and IL-8 in RSV-infected respiratory epithelial cells by FOF treatment may result in relief from the RSV-related symptoms. We also found that FOF suppressed the production of RANTES and IL-8, even if it was added postinfection (Fig. 2). These results suggest that FOF suppresses the RSV-induced allergic property that is triggered by the inflammatory immune reaction of both epithelial and leukocyte cells.
Fourteen-membered-ring macrolides, such as erythromycin and clarithromycin, have been reported to reduce the titers of rhinovirus and influenza virus and the cytokine response induced by these viruses (14, 31, 33). Our results indicate that FOF modulates chemokine production via the suppression of NF-κB activation independently of the replication of RSV (Fig. 1B). Thus, FOF has the ability to suppress cytokine production via the NF-κB induced not only by RSV infection but also by other infectious agents.
The inflammation mediated by chemokines promotes the pathogenesis of RSV-induced infectious diseases. Our results have shown that the levels of RANTES and IL-8 induced by RSV in epithelial cells are decreased by FOF via the suppression of NF-κB activation. FOF should improve the clinical symptoms induced by RSV not only by preventing secondary bacterial infections but also by imparting an immunomodulatory effect.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science and a grant from Meiji Seika Kaisha, Ltd.
Footnotes
Published ahead of print on 15 April 2009.
REFERENCES
- 1.Alam, R., J. York, M. Boyars, S. Stafford, J. A. Grant, J. Lee, P. Forsythe, T. Sim, and N. Ida. 1996. Increased MCP-1, RANTES, and MIP-1alpha in bronchoalveolar lavage fluid of allergic asthmatic patients. Am. J. Respir. Crit. Care Med. 1531398-1404. [DOI] [PubMed] [Google Scholar]
- 2.Bacon, K. B., L. Flores-Romo, J. P. Aubry, T. N. Wells, and C. A. Power. 1994. Interleukin-8 and RANTES induce the adhesion of the human basophilic cell line KU-812 to human endothelial cell monolayers. Immunology 82473-481. [PMC free article] [PubMed] [Google Scholar]
- 3.Bailly, S., Y. Mahe, B. Ferrua, M. Fay, T. Tursz, H. Wakasugi, and M. A. Gougerot-Pocidalo. 1990. Quinolone-induced differential modification of IL-1 alpha and IL-1 beta production by LPS-stimulated human monocytes. Cell. Immunol. 128277-288. [DOI] [PubMed] [Google Scholar]
- 4.Bischoff, S. C., M. Krieger, T. Brunner, A. Rot, V. von Tscharner, M. Baggiolini, and C. A. Dahinden. 1993. RANTES and related chemokines activate human basophil granulocytes through different G protein-coupled receptors. Eur. J. Immunol. 23761-767. [DOI] [PubMed] [Google Scholar]
- 5.Casola, A., R. P. Garofalo, H. Haeberle, T. F. Elliott, R. Lin, M. Jamaluddin, and A. R. Brasier. 2001. Multiple cis regulatory elements control RANTES promoter activity in alveolar epithelial cells infected with respiratory syncytial virus. J. Virol. 756428-6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casola, A., R. P. Garofalo, M. Jamaluddin, S. Vlahopoulos, and A. R. Brasier. 2000. Requirement of a novel upstream response element in respiratory syncytial virus-induced IL-8 gene expression. J. Immunol. 1645944-5951. [DOI] [PubMed] [Google Scholar]
- 7.Chung, H. L., and S. G. Kim. 2002. RANTES may be predictive of later recurrent wheezing after respiratory syncytial virus bronchiolitis in infants. Ann. Allergy Asthma Immunol. 88463-467. [DOI] [PubMed] [Google Scholar]
- 8.Culley, F. J., A. M. Pennycook, J. S. Tregoning, J. S. Dodd, G. Walzl, T. N. Wells, T. Hussell, and P. J. Openshaw. 2006. Role of CCL5 (RANTES) in viral lung disease. J. Virol. 808151-8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalo, J. A., C. M. Lloyd, D. Wen, J. P. Albar, T. N. Wells, A. Proudfoot, A. C. Martinez, M. Dorf, T. Bjerke, A. J. Coyle, and J. C. Gutierrez-Ramos. 1998. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J. Exp. Med. 188157-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honda, J., Y. Okubo, M. Kusaba, M. Kumagai, N. Saruwatari, and K. Oizumi. 1998. Fosfomycin (FOM: 1R-2S-epoxypropylphosphonic acid) suppress the production of IL-8 from monocytes via the suppression of neutrophil function. Immunopharmacology 39149-155. [DOI] [PubMed] [Google Scholar]
- 11.Ida, S., Y. Shindoh, and T. Takishima. 1987. Effect of antibiotics on immediate hypersensitivity reactions in vitro: suppression of IgE-mediated histamine release from peripheral blood basophils by fosfomycin. Microbiol. Immunol. 31975-984. [DOI] [PubMed] [Google Scholar]
- 12.Ieki, K., S. Matsukura, F. Kokubu, T. Kimura, H. Kuga, M. Kawaguchi, M. Odaka, S. Suzuki, S. Watanabe, H. Takeuchi, R. P. Schleimer, and M. Adachi. 2004. Double-stranded RNA activates RANTES gene transcription through co-operation of nuclear factor-kappaB and interferon regulatory factors in human airway epithelial cells. Clin. Exp. Allergy 34745-752. [DOI] [PubMed] [Google Scholar]
- 13.Iino, Y., M. Toriyama, K. Kudo, Y. Natori, and A. Yuo. 1992. Erythromycin inhibition of lipopolysaccharide-stimulated tumor necrosis factor alpha production by human monocytes in vitro. Ann. Otol. Rhinol. Laryngol. Suppl. 15716-20. [DOI] [PubMed] [Google Scholar]
- 14.Jang, Y. J., H. J. Kwon, and B. J. Lee. 2006. Effect of clarithromycin on rhinovirus-16 infection in A549 cells. Eur. Respir. J. 2712-19. [DOI] [PubMed] [Google Scholar]
- 15.John, A. E., A. A. Berlin, and N. W. Lukacs. 2003. Respiratory syncytial virus-induced CCL5/RANTES contributes to exacerbation of allergic airway inflammation. Eur. J. Immunol. 331677-1685. [DOI] [PubMed] [Google Scholar]
- 16.Kahan, F. M., J. S. Kahan, P. J. Cassidy, and H. Kropp. 1974. The mechanism of action of fosfomycin (phosphonomycin). Ann. N. Y. Acad. Sci. 235364-386. [DOI] [PubMed] [Google Scholar]
- 17.Kalina, W. V., and L. J. Gershwin. 2004. Progress in defining the role of RSV in allergy and asthma: from clinical observations to animal models. Clin. Dev. Immunol. 11113-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim, C. K., S. W. Kim, Y. K. Kim, H. Kang, J. Yu, Y. Yoo, and Y. Y. Koh. 2005. Bronchoalveolar lavage eosinophil cationic protein and interleukin-8 levels in acute asthma and acute bronchiolitis. Clin. Exp. Allergy 35591-597. [DOI] [PubMed] [Google Scholar]
- 19.Krishnan, S., M. Halonen, and R. C. Welliver. 2004. Innate immune responses in respiratory syncytial virus infections. Viral Immunol. 17220-233. [DOI] [PubMed] [Google Scholar]
- 20.Kuderer, N. M., H. G. San-Juan-Vergara, X. Kong, R. Esch, R. F. Lockey, and S. S. Mohapatra. 2003. Mite and cockroach proteases activate p44/p42 MAP kinases in human lung epithelial cells. Clin. Mol. Allergy 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morikawa, K., M. Nonaka, I. Torii, and S. Morikawa. 2003. Modulatory effect of fosfomycin on acute inflammation in the rat air pouch model. Int. J. Antimicrob. Agents 21334-339. [DOI] [PubMed] [Google Scholar]
- 22.Morikawa, K., H. Watabe, M. Araake, and S. Morikawa. 1996. Modulatory effect of antibiotics on cytokine production by human monocytes in vitro. Antimicrob. Agents Chemother. 401366-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noah, T. L., S. S. Ivins, P. Murphy, I. Kazachkova, B. Moats-Staats, and F. W. Henderson. 2002. Chemokines and inflammation in the nasal passages of infants with respiratory syncytial virus bronchiolitis. Clin. Immunol. 10486-95. [DOI] [PubMed] [Google Scholar]
- 24.Okabayashi, T., H. Kariwa, S. Yokota, S. Iki, T. Indoh, N. Yokosawa, I. Takashima, H. Tsutsumi, and N. Fujii. 2006. Cytokine regulation in SARS coronavirus infection compared to other respiratory virus infections. J. Med. Virol. 78417-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olszewska-Pazdrak, B., A. Casola, T. Saito, R. Alam, S. E. Crowe, F. Mei, P. L. Ogra, and R. P. Garofalo. 1998. Cell-specific expression of RANTES, MCP-1, and MIP-1α by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. J. Virol. 724756-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel, J. A., M. Kunimoto, T. C. Sim, R. Garofalo, T. Eliott, S. Baron, O. Ruuskanen, T. Chonmaitree, P. L. Ogra, and F. Schmalstieg. 1995. Interleukin-1 alpha mediates the enhanced expression of intercellular adhesion molecule-1 in pulmonary epithelial cells infected with respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 13602-609. [DOI] [PubMed] [Google Scholar]
- 27.Saito, T., R. W. Deskin, A. Casola, H. Haeberle, B. Olszewska, P. B. Ernst, R. Alam, P. L. Ogra, and R. Garofalo. 1997. Respiratory syncytial virus induces selective production of the chemokine RANTES by upper airway epithelial cells. J. Infect. Dis. 175497-504. [DOI] [PubMed] [Google Scholar]
- 28.Sigurs, N., R. Bjarnason, F. Sigurbergsson, and B. Kjellman. 2000. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am. J. Respir. Crit. Care Med. 1611501-1507. [DOI] [PubMed] [Google Scholar]
- 29.Stein, R. T., D. Sherrill, W. J. Morgan, C. J. Holberg, M. Halonen, L. M. Taussig, A. L. Wright, and F. D. Martinez. 1999. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 354541-545. [DOI] [PubMed] [Google Scholar]
- 30.Stellato, C., L. A. Beck, G. A. Gorgone, D. Proud, T. J. Schall, S. J. Ono, L. M. Lichtenstein, and R. P. Schleimer. 1995. Expression of the chemokine RANTES by a human bronchial epithelial cell line. Modulation by cytokines and glucocorticoids. J. Immunol. 155410-418. [PubMed] [Google Scholar]
- 31.Suzuki, T., M. Yamaya, K. Sekizawa, M. Hosoda, N. Yamada, S. Ishizuka, A. Yoshino, H. Yasuda, H. Takahashi, H. Nishimura, and H. Sasaki. 2002. Erythromycin inhibits rhinovirus infection in cultured human tracheal epithelial cells. Am. J. Respir. Crit. Care Med. 1651113-1118. [DOI] [PubMed] [Google Scholar]
- 32.Thomas, L. H., J. S. Friedland, M. Sharland, and S. Becker. 1998. Respiratory syncytial virus-induced RANTES production from human bronchial epithelial cells is dependent on nuclear factor-kappa B nuclear binding and is inhibited by adenovirus-mediated expression of inhibitor of kappa B alpha. J. Immunol. 1611007-1016. [PubMed] [Google Scholar]
- 33.Tsurita, M., M. Kurokawa, M. Imakita, Y. Fukuda, Y. Watanabe, and K. Shiraki. 2001. Early augmentation of interleukin (IL)-12 level in the airway of mice administered orally with clarithromycin or intranasally with IL-12 results in alleviation of influenza infection. J. Pharmacol. Exp. Ther. 298362-368. [PubMed] [Google Scholar]
- 34.Venge, J., M. Lampinen, L. Hakansson, S. Rak, and P. Venge. 1996. Identification of IL-5 and RANTES as the major eosinophil chemoattractants in the asthmatic lung. J. Allergy Clin. Immunol. 971110-1115. [DOI] [PubMed] [Google Scholar]
- 35.Yokota, S., T. Okabayashi, N. Yokosawa, and N. Fujii. 2004. Growth arrest of epithelial cells during measles virus infection is caused by upregulation of interferon regulatory factor 1. J. Virol. 784591-4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoneshima, Y., T. Ichiyama, H. Ayukawa, T. Matsubara, and S. Furukawa. 2003. Fosfomycin inhibits NF-kappaB activation in U-937 and Jurkat cells. Int. J. Antimicrob. Agents 21589-592. [DOI] [PubMed] [Google Scholar]
- 37.Yoon, J. S., H. H. Kim, Y. Lee, and J. S. Lee. 2007. Cytokine induction by respiratory syncytial virus and adenovirus in bronchial epithelial cells. Pediatr. Pulmonol. 42277-282. [DOI] [PubMed] [Google Scholar]