Abstract
The induction of innate immune responses by toll-like receptor (TLR) agonists is the subject of intense investigation. In large part, this reflects the potential of such compounds to be effective vaccine adjuvants. For that reason, we analyzed the activation of innate cells in swine by TLR7 and TLR8 agonists. These agonists activated porcine NK cells by increasing gamma interferon (IFN-γ) expression and perforin storage. The activation of porcine NK cells was mediated by accessory cells, since their depletion resulted in reduced cytotoxicity toward target cells. Accessory cells were stimulated to produce interleukin 12 (IL-12), IL-15, IL-18, and IFN-α after treatment with TLR7 or TLR8 agonists. Neutralization of these cytokines reduced but did not completely inhibit the induction of NK cell cytotoxicity. Direct stimulation of NK cells with TLR7 or TLR8 agonists resulted in minimal cytotoxicity but levels of IFN-γ equivalent to those detected in the presence of accessory cells. Porcine NK cells express both TLR7 and TLR8 mRNAs, and treatment with these TLR agonists induced higher mRNA expression levels of TRAIL and IL-15Rα, which may contribute to the activity of NK cells. These data indicate that TLR7 and TLR8 agonists indirectly or directly activate porcine NK cells but that optimum levels of activation require cytokine secretion by accessory cells activated by these compounds. Interestingly, NK cells activated by TLR7 or TLR8 agonists were cytotoxic against foot-and-mouth disease virus (FMDV)-infected cells in vitro, indicating that these TLR agonists may be beneficial as adjuvants to stimulate the innate immunity against FMDV.
Toll-like receptors (TLRs) are pathogen-associated molecular pattern recognition receptors responsible for signaling intrusion by pathogens. These receptors are expressed by cells mediating innate responses. Pathogens are recognized directly by the binding of pathogen-associated molecules. For example, TLR3 recognizes double-stranded RNA during virus replication; TLR5 binds bacterial flagellin; TLR9 detects nonmethylated, CG-rich prokaryotic DNA, i.e., CpG; and TLR7 and TLR8 recognize single-stranded RNA (44), although recognition by TLR8 may be species specific, as demonstrated recently by Forsbach et al. (11). The consequence of engaging TLRs is the induction of signals that lead to the expression of proinflammatory cytokines, antimicrobial and antiviral effector molecules, and costimulatory molecules on macrophages (Mφ) and dendritic cells (DCs) (25, 39). Overall, such events affect the activation and functional status of innate immune cells, such as natural killer (NK) cells and DCs, and further influence the organization of adaptive immune responses.
NK cells perform a critical role in innate immunity, leading to protection against various pathogens well before the adaptive immune responses develop. NK cells are lymphocyte-derived cells that engage nonspecific target recognition mechanisms to eliminate malignant or virus-infected cells (10). However, it has recently been shown that some receptors on NK cells engage viral gene products. For example, Ly49H recognizes m157 of murine cytomegalovirus in mice, while NKp44 and NKp46 bind influenza virus hemagglutinin (4, 27). Moreover, NK cells express both inhibitory and activating receptors, which directly influence the outcome of NK cell activation. Besides the expression of such receptors (22), NK cells have other mechanisms that enhance their function as natural spontaneous effector cells (24). Such mechanisms include the expression of TLRs, which possibly allow NK cells to respond to the presence of pathogens by direct activation via these receptors.
NK cells express TLR9 in mice (26) and TLR1 to TLR10 in humans (12, 17, 23). However, not much information is currently available on the expression of these receptors on immune cells of domestic livestock species, such as porcine or bovine species. Direct stimulation of human NK cells through TLR2, TLR3, TLR7, and TLR8 leads to the upregulation of gamma interferon (IFN-γ) secretion, although in some instances this response requires the presence of interleukin 12 (IL-12) (6, 12, 17). Furthermore, activation via TLR5 is reported to stimulate NK cell proliferation but not IFN-γ production (45). Additionally, stimulation via TLR2 or TLR7 induces chemokines such as CCL3, CCL4, and CCL5 (36). Although TLR9 is expressed in NK cells, it does not induce IFN-γ production directly unless the NK cells are presented with antibody-coated target cells or are cultured on plates with an immobilized antibody against immunoglobulin G (35). Therefore, TLR expression in NK cells may be involved in the differential regulation of these vital cells of the innate response. However, not much is known about the direct effect of TLR stimulation on the expression of NK cell effector molecules, such as perforin, granzymes, and cytokines.
Using this class of molecules, it is now possible to formulate vaccine adjuvants that prime cell-mediated immunity. Engaging TLR receptors with specific synthetic agonists introduces a new way of inducing early innate responses as well as increasing the potency of adaptive immunity. The importance of such an approach is exemplified by several clinical studies currently under way. TLR9 and TLR4 agonist are being tested as vaccine adjuvants, and a TLR7 agonist is being tested in the treatment of genital warts caused by herpes simplex virus (28). In addition, TLR4 is being tested for the treatment of allergies, endotoxemia, and liver disease, TLR7 for cancer treatment, and TLR9 as a treatment for melanoma (reviewed by Ulevitch [46]).
Foot-and-mouth disease virus (FMDV) infects cloven-hoofed animals, leading to devastating economic consequences (16). This is a highly contagious viral infection that causes a very acute disease. Clinical symptoms are detected within 1 or 2 days of exposure and resolve within a week to 10 days. Viral clearance may be mediated in part by antibodies, but under controlled experimental conditions, viremia is gone by day 3 or 4 after infection when anti-FMDV immunoglobulin M is barely detectable (2, 13). These results strongly suggest that other antiviral mechanisms contribute to the elimination of the virus in vivo. Innate responses, including the activation of DCs and NK cells, are likely involved.
Therapeutic approaches involving TLR stimulation via synthetic agonists can augment innate responses, indicating that such therapeutics have the potential to induce early protection against foot-and-mouth disease (29, 30). Therefore, we have studied the effects of TLR agonists on porcine NK cells in vitro. We report that TLR7 and TLR8 agonists and a combined TLR7/8 agonist activate porcine CD2+ CD8+ CD3− NK cells through accessory-cell-mediated mechanisms, such as the secretion of cytokines, including IFN-α, IL-12, IL-15, and IL-18. In addition, porcine NK cells are partially activated by the direct interaction of TLR7 and TLR8 agonists through these receptors expressed by NK cells. Activated cells show enhanced secretion of IFN-γ and storage of perforin granules and can effectively lyse tumor or FMDV-infected targets. These results are discussed in the context of rational approaches to antiviral measures against FMDV.
MATERIALS AND METHODS
Animals.
Yorkshire pigs were purchased from Animal Biotech Inc., Danboro, PA, at the age of 3 to 4 months and were used in experiments after 1 week of acclimatization. These animal studies were reviewed and approved by the Institutional Animal Care and Use Committee at Plum Island Animal Disease Center.
TLR agonist compounds.
Agonists for TLR7, TLR8, and a mixed compound activating both, TLR7/8, have been described previously (14) and were kindly provided by Richard Miller of 3M Pharmaceuticals (Minneapolis, MN). These compounds were originally designed as human TLR agonists, but recently Dumitru et al. have reported their ability to stimulate both human and murine NK cells (8).
Preparation of PBMC.
Blood was drawn from pigs into heparin-containing tubes and diluted with phosphate-buffered saline, and peripheral blood mononuclear cells (PBMC) were separated on a Lymphoprep gradient as described by the manufacturer (Axis-Shield, PoC AS, Oslo, Norway). Cells were finally suspended in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM HEPES, 2 mM l-glutamine, and antibiotic-antimycotic (Invitrogen, Carlsbad, CA).
NK cell enrichment.
To obtain the fraction of PBMC that contains porcine NK cells, CD2+ CD8+ CD3− cells were enriched by negative selection using antibodies bound to magnetic beads, with either the magnetic-activated cell-sorting (MACS) (Miltenyi Biotech, Germany) or the Dynabead (Dynal Biotech ASA, Oslo, Norway) system, according to the manufacturers' instructions. Mouse antibodies against porcine CD3 (clone PPT3), CD4 (clone 74-12-4), CD21 (B cells; clone BB6-11C9.6), and CD172 (clone 74-22-15) were purchased from Southern Biotech (Birmingham, AL). Antibodies used for cell enrichment were sodium azide free. Adherent monocytes/Mφ were isolated by incubating PBMC for 2 h at 37°C in flasks and then removing nonadherent cells. Adherent cells were detached with cell dissociation solution (Sigma, St. Louis, MO) at 37°C for 5 to 10 min. Detached cells were washed in RPMI 1640 and used in the assays. CD3+, CD4+, CD21+, and CD172+ cells were positively sorted and rested for 18 to 24 h before any assays were performed on them. This sorting procedure did not cause changes in cell reactivity. Enrichment of cells usually yielded 90 to 95% CD2+ CD8+ CD3− cells, 98% CD3+ cells, 97% CD4+ cells, 90% CD21+ cells, and 96% CD172+ cells.
NK cell lytic activity assay.
A flow cytometry-based assay was developed to determine the lytic activity of NK cells. K562 tumor cells (a human erythroleukemia cell line) expressing green fluorescent protein (GFP) (K562-GFP cells) were a gift from Mike Olin (University of Minnesota, School of Veterinary Medicine, St. Paul). Target cells (K562-GFP) were added to effector cells (PBMC or CD2+ CD8+ CD3− cells) at effector-to-target cell (E:T) ratios of 50:1, 25:1, and 12:1, followed by the addition of 5 μl 7-aminoactinomycin D (7AAD; BD Bioscience, San Diego CA), a dead/live discriminating dye. Cells were mixed, spun for 1 min at 500 × g, and incubated at 37°C under 5% CO2 for 4 h. Data were acquired with a FACSCalibur flow cytometer and were processed with CellQuest Pro software (BD Biosciences, San Diego, CA). A gate was created on K562-GFP cells to display the double-positive (GFP plus 7AAD) cells that represented the killed target cells. The level of killing was determined by the calculation R1/(R1 + R2) × 100 = % lysis, where R1 stands for double-positive cells and R2 stands for GFP-positive cells.
Cytotoxicity against FMDV-infected targets was assessed with SK6 or IBRS2 cells (porcine kidney fibroblast cell lines). Briefly, SK6 cells were infected with the LL-KGE virus at a multiplicity of infection of 10. The LL-KGE virus is an FMDV O1 Campos strain capsid inserted into a strain A12 backbone with a positive charge mutation, as well as another mutation in the RGD sequence to KGE to allow for binding of the heparin sulfate receptor, allowing infection of a wide variety of cells. Additionally, this virus has no leader protease, which permits in vitro attenuation. IBRS2 cells are persistently infected with classical swine fever virus. Cells were stained with carboxyfluorescein succinimidyl ester (CFSE) at 5 μmol/ml for 15 min at room temperature and were washed extensively with RPMI 1640 containing 10% fetal bovine serum. Labeled cells were used in the NK assay as described above.
Stimulation of porcine NK cells.
PBMC (1 × 106/well) or CD2+ CD8+ CD3− cells (1 × 105/well) were stimulated with a TLR7 agonist (3M-001), a TLR8 agonist (3M-002), a TLR7/8 agonist (3M-011), or the control compound (3M-006) at 1 mM for 18 to 24 h and were then used in the NK assay or assessed for IFN-γ secretion or perforin expression. The 3M compounds were active over a range of concentrations (1 mM, 2 mM, and 3 mM) with similar levels of NK cytotoxicity, so for these studies we chose the lowest concentration. In some experiments, NK cells were stimulated with human IL-2 or porcine IL-15, purchased from Hemagen Diagnostics, Inc. (Columbia, MD), or Invitrogen (Carlsbad, CA) and used at 20 ng/ml or 25 ng/ml, respectively.
Intracellular staining for IFN-γ and perforin.
Cells, either stimulated or left unstimulated, were placed in 96-well microtiter plates at 1 × 106 (PBMC) or 1 × 105 (NK cells) per well and were incubated for 5 h in the presence of 3.0 μg/ml brefeldin A (eBioscience, San Diego, CA). A mixture of phorbol myristate acetate and ionomycin (Sigma, St. Louis, MO) at 100 ng/ml and 10 ng/ml, respectively, was added to cells that were treated as positive controls. Next, cells were surface stained with mouse anti-porcine CD2 (clone MSA4; Accurate Chemical, Westbury, NY) and biotin-labeled mouse anti-porcine CD8 (clone 76-2-11; Southern Biotech, Birmingham, AL), followed by fluorescein isothiocyanate- or streptavidin-peridinin chlorophyll protein-labeled secondary antibodies, respectively (BD Biosciences, San Diego, CA). IFN-γ or perforin was labeled intracellularly with phycoerythrin-conjugated anti-porcine IFN-γ (clone P2G10; 1 μg/ml) or an anti-human perforin antibody that cross-reacts with porcine perforin (clone δG9; 20 μl per 1 × 106 cells), respectively (BD Biosciences, San Diego, CA). Data were acquired with a FACSCalibur flow cytometer and analyzed by CellQuest Pro (BD Biosciences, San Diego, CA).
Cytokine detection.
An enzyme-linked immunosorbent assay (ELISA) was used to determine the levels of IFN-α, IL-12, IL-15, and IL-18 in supernatants from TLR agonist-stimulated cells. Coating and detecting antibodies against porcine IFN-α were purchased from Endogen/Pierce (Rockford, IL); a pIL-12 detection kit was purchased from R&D Systems Inc. (Minneapolis, MN); a pIL-15 detection kit was purchased from Biosource/Invitrogen (Camarillo, CA); and a pIL-18 detection kit was purchased from Bender MedSystems (Vienna, Austria). Plates were coated with antibodies in carbonate buffer or phosphate-buffered saline overnight at 4°C (IFN-α, IL-12, IL-18) or at room temperature (IL-15). ELISA was performed as described by the appropriate manufacturer of the coating and detecting antibodies. Optical densities were acquired on a Bio-Tek (Winooski, VT) ELISA reader, and data were analyzed with a linear regression equation in Microsoft Excel (Microsoft Corp., Bellevue, WA). The following antibodies were used for the neutralization of IL-12, IL-15, and IFN-α. The anti-IL-12 antibody, with a 50% neutralizing dose of 0.01 to 0.04 μg/ml, was purchased from R&D Systems. Anti-human IL-15, which cross-reacts with porcine IL-15 and has a 50% neutralizing dose of 3 to 8 μg/ml, was also purchased from R&D Systems. Anti-porcine IFN-α was purchased from PBL Biomedical Laboratories; 1 U of this antibody neutralizes 1 U of pig IFN-α.
qrt-PCR.
RNA was isolated from nonstimulated CD2+ CD8+ CD3− NK cells or TLR7/8L-stimulated cells, including CD172+ cells, CD21+ cells, and Mφ/monocytes. The TRIzol reagent (Invitrogen, Carlsbad, CA) method was used as described by the manufacturer. Briefly, 700 μl TRIzol reagent was added to 1 × 106 to 2 × 106 cells, and the mixture was left for 5 min at room temperature in order to dissociate the nucleoproteins. RNA was extracted with chloroform, precipitated by isopropyl alcohol, and finally washed in 75% ethanol. RNA was treated with DNase and then transcribed into cDNA using the following reaction mixture: 5× reaction buffer, 10 mM deoxynucleoside triphosphates, 50 ng/μl random primers, 0.1 M dithiothreitol, 40 U RNase inhibitor, and 200 U Moloney murine leukemia virus reverse transcriptase (RT). The cDNA templates were later used in the quantitative real-time RT-PCRs (qrtRT-PCRs) in duplicate. A TaqMan RT-PCR system was used for detection. Primers and probes were designed at the Beltsville Human Nutrition Research Center, Agricultural Research Service, U.S. Department of Agriculture (USDA; Beltsville, MD) (http://www.ars.usda.gov/Services/docs.htm?docid=6065), and sequences are provided in Table 1. PCRs were run in the ABI Prism sequence detector, model 7700 (Applied Biosystems, Foster City, CA). The cycling parameters were as follows: 50°C for 2 min, 95°C for 10 min, and 40 cycles at 95°C for 15 s (denaturation) and 60°C for 1 min (annealing and extension). Ubiquitin (UBC) or peptidylprolyl isomerase A was used as the reference gene to normalize the expression of each gene. Relative expression was calculated by 2−ΔΔCT, and data are expressed as ratios relative to the calibrator (nonstimulated CD2+ CD8+ CD3− cells or CD3+ T cells for baseline expression levels).
TABLE 1.
Primers and probes for TLRs and NK cell-associated genes used in real-time PCR assays
| Gene | Primer or probe designationa | Sequence |
|---|---|---|
| TLR3 | p-TLR3-45F | AAAATCTCCAAGAGCTTCTATTAGCAA |
| p-TLR3-163R | TTGTATTTGATTTGATGACAACTCTAATCTTT | |
| p-TLR3-95T | CGTGAAGAACTTGATTTCCTTGGCAATTCTTC | |
| TLR7 | p-TLR7-3F: | CAGAAGTCCAAGTTTTTCCAGCTT |
| p-TLR7-84R | GGTGAGCCTGTGGATTTGTTG | |
| p-TLR7-62 revT | CCACTCAAGAACAGAACTCCCACAGAGCC | |
| TLR8 | p-TLR8-2821F | GATACCATTGCGGCGATAATATG |
| p-TLR8-2951R | TTTACCTTGGCTAAGCACACATG | |
| p/h-TLR8-2875T | ATGTTGGCTGCCCTGGCTCACC | |
| UBC | p-UBC-47F | GCGCACCCTGTCTGACTACA |
| p-UBC-126R | AGATCTGCATCCCACCTCTGA | |
| p-UBC-79T | AGTCCACCCTGCACCTGGTCCTCC | |
| TNFb (ligand) subfamily member 10 (TRAIL) | p-TNFSF10-277F | TGTGAGAAAGATGATTTTGAGAACCT |
| p-TNFSF10-380R | CTCTCTGTGGACCTTTTTCTCTTTCTA | |
| p-TNFSF10-326T | TCAGAAAAGCAACAAGGCATTCCTCACCT | |
| NCR1 (NKp46) | p-NCR1-1F | CCTTTGACCAGGAGCTTCACA |
| p-NCR1-71R | AGATGTTTTGGCTCCTACAACAAG | |
| p-NCR1-23T | GCTCACTGGGGAAAGACCATGCGT | |
| NCR3 (NKp30) | p-NCR3-197F | TGATCAGGGTCCATCCAGGAT |
| p-NCR3-268R | TGCCCTCCTGAGTACAGATCTCA | |
| p/h-NCR3-219T | CTGTGCTCTCTGGGTGTCCCAGCC | |
| KLRF1 (NKp80) | p-KLRF1-43F | GATTGGACTTAACTTTACCTTCCAGAA |
| p-KLRF1-165R | TCTTTGATGGTAGCACAGCTATTTTC | |
| p-KLRF1-95 revT | AACCATCCACCCAGGTCCATGTCCT | |
| Granzyme A | p-GZMA-279F | GGAGCTCACTCGATAACCAAGAAA |
| p-GZMA-396R | GCTTTAGAAGTTTAAGGTCACCCTCAT | |
| p-GZMA-341T | TCCTTATCCATGCTTTGACCAGGACACAC | |
| Granzyme B | p-GZMB-546F | TCTCCTATGGAAGAAAGGATGGAA |
| p-GZMB-615R | ATCCAGGGCAGGAAACTTGA | |
| p/h-GZMB-572T | CCTCCACGAGCCTGCACCAAAGTCT | |
| Perforin | p-PERF1-241F | TTCGCGGCCCAGAAGAC |
| p-PERF1-311R | CTGTAGAAGCGACACTCCACTGA | |
| p-PERF1-259T | CACCAGGACCAGTACCGCTTCAGCC | |
| KLRK1 (NKG2D) | p-KLRK1-381F | TCTCAAAATTCCAGTCTTCTGAAGATATA |
| p-KLRK1-492R | AGGATCTGTTTGTTGGAATTTGTACTA | |
| p-KLRK1-463 revT | CCCATCCAATGATATGACTTCACCAATTTGA | |
| KLRB1 (CD161) | p-KLRB1-377F | TTTATAAACACTGGAATGACAGTCTAGCT |
| p-KLRB1-470R | TGTATGAGTCTCAATTCCTCATTATCTTG | |
| p-KLRB1-408T | CTGTTCCACAAAAGAATCCAGTCTGCTGC | |
| KLRC1 (NKG2A) | p-KLRC1-390F | GTAATTGTCCAAAGGAATGGTTTACA |
| p-KLRC1-507R | CGAAGTAGAGTAGAATTCCGTGAAGC | |
| p-KLRC1-482 revT | CACAGGCCATCAAACTCTCATTCCATGTC | |
| SH2 domain containing 1B | p-SH2D1B-191F | TGTTAAGGGACAGCGAGTCCAT |
| p-SH2D1B-273R | GAAGATTCGGTATGTGTAGACAAAATTT | |
| p/h-SH2D1B-215T | CAGGAGTCCTGTGCCTCTGTGTCTCGTTTA | |
| KLRA1 (Ly49) | p-KLRA1-185F | TGGCAAGACTGATGAAAAAGAGTT |
| p-KLRA1-257R | GAAACAGAGGATCCCAAGAATCAC | |
| p-KLRA1-211T | CAGTGCTCTGGCATCGCATTGCA |
Probe designations and sequences are given in boldface.
TNF, tumor necrosis factor.
Statistical evaluation.
Where appropriate, a t test was performed to examine the significance of differences. P values of <0.05 were considered significant.
RESULTS
TLR7 and TLR8 agonists activate porcine NK cells.
Generally, the lytic activity of resting NK cells in Yorkshire pigs is low. However, culture of PBMC in the presence of cytokines, such as porcine IL-2 or IL-15, activates porcine NK cells, leading to higher cytotoxicity against K562-GFP cells (Fig. 1A). We were interested in determining the effect of TLR agonists on the cytotoxicity of porcine NK cells. To measure the influence of TLR agonists, we cultured PBMC in the presence of TLR7 ligand (TLR7L), TLR8L, TLR7/8L, or the control compound for 18 h and performed a flow cytometry-based NK cell cytotoxicity assay. As shown in Fig. 1B, the lytic activities of porcine NK cells against K562-GFP cells were 4-, 2.5-, and 4.5-fold higher in PBMC stimulated with TLR7L, TLR8L, and TLR7/8L, respectively, than those of nonstimulated porcine NK cells or cells cultured with the control compound. TLR7L stimulated more lytic activity than TLR8L, but there was only a small difference in stimulatory capacity between TLR7L and TLR7/8L. For all stimulated cells, the difference from the lytic activity of nonstimulated cells was statistically significant (P = 0.033 for TLR7L; P = 0.041 for TLR8L; P = 0.012 for TLR7/8L). The difference between TLR7L and TLR8L stimulation was significant (P = 0.045), but no statistical difference was observed between TLR7L and TLR7/8L. Thus, TLR7L and TLR8L enhanced the cytotoxicity of porcine NK cells when added to whole PBMC.
FIG. 1.
(A) Activation of porcine NK cells by IL-2 or IL-15. PBMC were incubated with porcine IL-2 or IL-15 for 18 h, and an NK cell cytotoxicity assay was performed against K562-GFP cells as described in Materials and Methods. (B) Effect of TLR7L, TLR8L, or TLR7/8L on the cytotoxicity of porcine NK cells. PBMC were cultured in the presence of TLR7L, TLR8L, TLR7/8L, or the control compound, and an NK cell assay was performed after 18 h as described in Materials and Methods. Results are presented as means ± standard deviations (n = 3) for three experiments. P values in relation to nonstimulated (Non-Stim) PBMC are as follows: *, ≤0.033; **, ≤0.041; ***, ≤0.012. #, P ≤ 0.045 in relation to TLR8L-stimulated cells.
TLR7 and TLR8 agonists directly activate porcine NK cells.
It was not clear from the experiments described above whether the TLR agonists activated the porcine NK cells directly or whether the activation was mediated through another cell type. Therefore, we separated the NK cells from PBMC. Due to the lack of a definitive antibody recognizing porcine NK cells, we separated the CD2+ CD8+ CD3− cells, which contain a substantial population of porcine NK cells. Throughout this report we refer to CD2+ CD8+ CD3− cells as NK cells, with the provision, however, that porcine NK cells may be heterogeneous, and thus, the CD2+ CD8+ CD3− cells may not represent all NK cell subsets in swine.
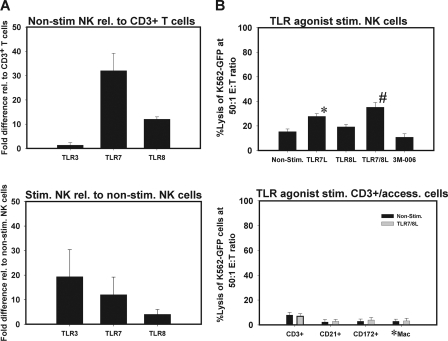
First, we determined the expression of TLR7 and TLR8 on purified NK cells. Since no antibodies reactive with the porcine homologues of these proteins were available, we measured the expression of TLR mRNAs in freshly isolated CD2+ CD8+ CD3− NK cells by using qrt-PCR. With this assay, we confirmed the presence of mRNAs for TLR7 and TLR8 as well as for TLR3 in CD2+ CD8+ CD3− NK cells in relation to levels in CD3+ T cells (Fig. 2A). TLR7 showed higher expression levels than TLR8.
FIG. 2.
Effect of direct stimulation of CD2+ CD8+ CD3− NK cells with TLR7L, TLR8L, or TLR7/8L. (A and C) Expression of TLRs on stimulated and nonstimulated (Non-stim.) CD2+ CD8+ CD3− NK cells, determined by qrt-PCR as described in Materials and Methods. Results are shown as the increase in expression relative (rel.) to that in CD3+ T cells (A) or nonstimulated NK cells (C). (B) The lytic activities of TLR agonist-stimulated or nonstimulated NK cells and accessory cells against K562-GFP cells are compared at an E:T ratio of 50:1. P values for comparison to the control compound are as follows: *, ≤0.43; #, ≤0.28. (D) Cells were sorted into CD2+ CD8+ CD3−, CD3+, CD21+, and CD172+ cells (by the MACS system [Miltenyi Biotech]) and into adherent Mφ/monocytes (*Mac). The cells were cultured with or without TLR7/8L for 18 h and were then used to measure mRNA expression or NK cell cytotoxicity as described in Materials and Methods. The results are means ± standard deviations for separate experiments (n = 3).
Next, CD2+ CD8+ CD3− NK cells were cultured with TLR7L, TLR8L, TLR7/8L, or an inert control compound for 18 h and then tested in NK cell cytotoxicity assays. Figure 2B shows that a degree of NK cell stimulation by TLR agonists was achieved, since the lytic levels against K562-GFP cells were 1.8-, 1.3-, and 2.3-fold higher for TLR7L-, TLR8L-, and TLR7/8L-stimulated cells, respectively, than for nonstimulated or control-treated cells. The difference between TLR7L or TLR7/8L and the control compound was statistically significant (P ≤ 0.043 and P ≤ 0.28, respectively). However, although these were enriched NK cells, their killing capability after direct stimulation was lower than that exhibited by the bulk PBMC cultures stimulated with TLR7L, TLR8L, or TLR7/8L (Fig. 1B). Furthermore, we tested the lytic activity in the CD3+, CD21+, adherent Mφ/monocyte, and CD172+ cell fractions treated with TLR7/8L only. There was no evidence of cytotoxicity in CD21+ cells, adherent Mφ/monocytes, or CD172+ cells, but some residual lytic activity was observed in the CD3+ cell population, presumably containing a fraction of a yet unidentified porcine NK cell subset. This result indicated that TLR7L or TLR8L interacted directly with porcine NK cells, although this stimulus did not highly enhance their cytotoxicity against K562-GFP cells.
Accessory cell involvement in porcine NK cell activation.
The experiment described above showed that removal of accessory cells, i.e., CD172+ cells, CD21+ cells, and adherent Mφ/monocytes, reduced the lytic activity of CD2+ CD8+ CD3− NK cells. DCs and Mφ have been reported to secrete factors that affect the functions of various lymphocyte populations in other animal species (5, 7, 33, 34). We therefore investigated the possible involvement of these cells in porcine NK cell activation following stimulation with TLR agonists. CD2+ CD8+ CD3− NK cells, CD172+ cells, CD21+ cells, and plastic-adherent Mφ/monocytes were enriched separately. The expression of TLR7 and TLR8 mRNAs was first determined by RT-PCR; then a comparison was made between the cytotoxicity of NK cells alone and that of NK cells cultured with CD172+ leukocytes, CD21+ B cells, or adherent cells enriched for Mφ/monocytes following stimulation with TLR agonists. Indeed, all three subsets of accessory cells (CD172+ cells, CD21+ cells, and Mφ/monocytes) do express TLR7 and TLR8 mRNAs, albeit to different degrees and depending on activation status (Fig. 3A).
FIG. 3.
Expression of TLR7 and TLR8 mRNAs in porcine cells by qRT-PCR. (A) Expression of TLR7 and TLR8 in nonstimulated (Non-stim.) CD172+ cells, Mφ/monocytes (Mac.), or CD21+ cells relative to (rel.) that in CD3+ T cells, and expression of TLR7 and TLR8 in TLR7/8L-stimulated (Stim.) CD172+ cells, Mφ/monocytes, or CD21+ cells relative to that in nonstimulated cells of the same type. (B) Influence of accessory cells on NK cell cytotoxicity after stimulation with TLR agonists. Cells were sorted using the MACS or Dynabead system as described in Materials and Methods. Sorted CD2+ CD8+ CD3− NK cells were cocultured either with CD172+ cells (top) or with CD21+ cells or adherent Mφ/monocytes (bottom) in the presence or absence of TLR7L, TLR8L, or TLR7/8L for 18 h. An NK cell cytotoxicity assay against K562-GFP cells was then performed. Data are means ± standard deviations for three experiments (n = 3).
Figure 3B shows that CD2+ CD8+ CD3− NK cells attained higher lytic activity when cocultured with CD172+ cells in the presence of TLR7/8 agonists than CD2+ CD8+ CD3− NK cells alone stimulated with TLR7/8L (Fig. 2B), CD2+ CD8+ CD3− NK cells cocultured with CD172+ cells without stimulation, or NK cell-CD172+ cell cocultures stimulated with either TLR7L or TLR8L separately. Cells were cultured at an NK cell-to-accessory cell ratio of 5:1. Coculture with CD21+ cells or adherent Mφ/monocytes increased the lytic activity of CD2+ CD8+ CD3− NK cells only minimally (Fig. 3B). This suggests that most likely, the accessory cells influenced the lytic activity of porcine NK cells through direct interaction or cytokine secretion. Enriched CD172+, CD21+, or Mφ/monocyte cell populations (isolated from the same animals) had no cytotoxic activity, whether they were stimulated or not. Taken together, these data indicate that it is possible to highly stimulate NK cells with TLR7L or TLR8L when CD172-expressing leukocytes are present.
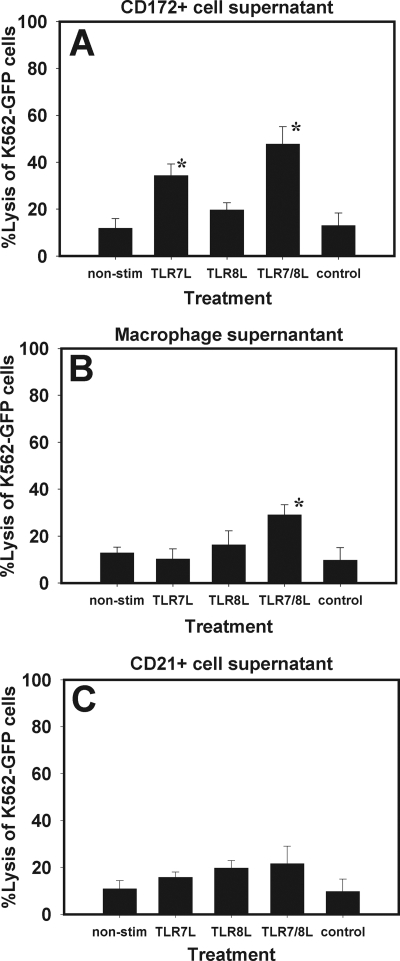
To learn if soluble factors contributed to NK cell activation, we cultured CD172+ cells, CD21+ cells, or adherent Mφ/monocytes with these TLR agonists for 18 h. Supernatants were collected and later added to CD2+ CD8+ CD3− NK cells, which were further cultured for 18 h. Next, NK cell killing assays were performed to assess the activation of porcine NK cells. Figure 4A shows that supernatants from CD172+ cells stimulated with the TLR agonists increased porcine NK cell cytotoxic activity. The effect of TLR7L was greater than that of TLR8L, but supernatants from TLR7/8L-cultured CD172+ cells exerted the most stimulatory effect on porcine NK cells, and this was statistically significant (P < 0.05) compared to supernatants from nonstimulated cells. Interestingly, both supernatants from TLR agonist-stimulated adherent Mφ/monocytes and those from TLR agonist-stimulated CD21+ cells increased porcine NK cell lytic activity slightly, and the effects of TLR8L were greater than those of TLR7L (Fig. 4B and C). However, there was no significant difference between the TLR agonists in either case, with one exception. TLR7/8L stimulated Mφ/monocytes to secrete factors that significantly activated the enriched NK cells (Fig. 4B). This experiment strongly indicated that stimulation of accessory cells induces the secretion of soluble factors that activate porcine NK cell cytotoxicity.
FIG. 4.
Supernatants from TLR agonist-stimulated accessory cells activate porcine NK cells. CD172+ cells, CD21+ cells, and adherent Mφ were separated from PBMC and stimulated with TLR7L, TLR8L, or TLR7/8L for 18 h. Later, CD2+ CD8+ CD3− NK cells were cultured in the presence of the supernatants, and their lytic activity was assessed. Shown is NK cell activation by supernatants from TLR7/8L-stimulated CD172+ cells (A), Mφ/monocytes (B), or CD21+ cells (C). *, P ≤ 0.05 for comparison to supernatants from control compound-treated cells. Data are means ± standard deviations for four experiments (n = 3).
Cytokines produced by CD172+ cells and Mφ contribute to the activation of porcine NK cells.
In order to resolve the character of the soluble factors in supernatants involved in porcine NK cell activation, supernatants from cells stimulated as described in the preceding section were tested for the presence of cytokines that generally have a role in NK cell activation. ELISAs for cytokines such as IL-12p70, IL-15, IL-18, and IFN-α were performed on supernatants from these cultures. IFN-α was detected in supernatants from CD172+ cells, adherent Mφ/monocytes, and PBMC, but only marginal levels could be detected in supernatants from CD21+ cells and nonstimulated cells (Fig. 5A). IL-12 was detected in all supernatants, but only low levels were detected in CD21+ cells. It is worth noting that adherent Mφ/monocytes secreted more IL-12 when stimulated with TLR8L than with TLR7L, in contrast to CD172+ cells (Fig. 5B). IL-18 and IL-15 (Fig. 5C and D) were detected in all supernatants, although supernatants from PBMC contained more of the cytokines, and CD172+ cells secreted more than Mφ/monocytes or CD21+ cells. The cytokine detection level in nonstimulated cells was below the sensitivity of the assays used. This was true when an enzyme-linked immunospot assay was used, as well (see Fig. S1 in the supplemental material).
FIG. 5.
Secretion of cytokines by PBMC following culture in the presence of TLR7L, TLR8L, or TLR7/8L. PBMC or sorted cell populations were incubated with either TLR7L, TLR8L, TLR7/8L, or a control compound for 18 h, and supernatants were collected for cytokine detection by ELISA as described in Materials and Methods. (A) IFN-α; (B) IL-12; (C) IL-18; (D) IL-15. (E) Neutralizing antibodies against IL-12, IL-15, or IFN-α do not completely inhibit the activation of porcine NK cells in PBMC stimulated with TLR7/8L. PBMC were cultured in the presence of TLR7/8L with the addition of antibodies against IL-12, IL-15, IFN-α, or a mixture of the three antibodies. An NK cytotoxicity assay against K562-GFP cells was performed 18 h later as described in Materials and Methods. Data are means ± standard deviations for three experiments (n = 3).
To precisely measure the role of cytokines in the activation of porcine NK cells, we added neutralizing antibodies against IL-12, IL-15, and IFN-α to PBMC cultures stimulated with TLR7/8L (IL-18 was not tested due to the lack of a neutralizing antibody against porcine IL-18). The NK cell killing capability was only reduced, not completely inhibited (Fig. 5E), even at saturating amounts of the neutralizing antibodies (data not shown). Similarly reduced NK cell cytotoxicity was also apparent in cultures where a combination of anti-cytokine antibodies was used. Therefore, although we have shown that these cytokines contribute to NK cell activation, they are not the only source of porcine NK cell activation. Taken together, accessory cells contributed stimuli to the activation of porcine NK cells through the secretion of mediators such as IL-12, IL-15, IL-18, and IFN-α.
TLR7 and TLR8 agonists induce cytokine production in CD2+ CD8+ CD3− cells.
The data presented to this point (Fig. 1 to 5) show that the response of NK cells to the TLR7/8 agonist is more often significant than the responses to TLR7L and TLR8L, which often are not statistically different from the controls. Therefore, for the balance of this study, we report only the analysis of responses to the TLR7/8 agonist.
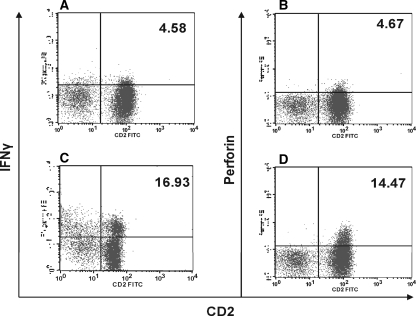
We further analyzed porcine NK cells for the production of IFN-γ and intracellular storage of perforin following activation with these TLR agonists. CD2+ CD8+ CD3− NK cells were incubated with the TLR7/8 agonist 3M-011 for 18 h and were tested in an intracellular staining assay. The presence of both IFN-γ and perforin could be demonstrated in resting CD2+ CD8+ CD3− NK cells. Whereas baseline IFN-γ levels differed considerably from animal to animal, perforin stayed within the range of 4 to 7% (Fig. 6A and B). Following stimulation with TLR agonists, both perforin storage and IFN-γ production increased at least threefold (Fig. 6C and D). The increases in cytokine and perforin storage did not wholly correlate with the increase in the cytotoxicity of sorted NK cells following stimulation with these agonists, since a minimal increase in cytotoxicity was observed. Although TLR7/8 agonists appeared to induce cytokine production substantially, they did not significantly enhance NK cell cytotoxicity, suggesting that the direct engagement of TLR7 and TLR8 on NK cells by their agonists is directed toward the upregulation of cytokines such as IFN-γ.
FIG. 6.
IFN-γ and perforin induction by TLR7/8L in CD2+ CD8+ CD3− NK cells. Sorted cells were cultured in the presence or absence of TLR7/8L for 18 h and were later stained intracellularly for IFN-γ or perforin as detailed in Materials and Methods. Cells were gated on CD2+ cells. FITC, fluorescein isothiocyanate. (A and B) IFN-γ and perforin, respectively, in nonstimulated cells; (C and D) IFN-γ and perforin, respectively, in TLR7/8L-stimulated cells. Data are representative of five separate experiments. Numbers in upper right corners of histograms are percentages of IFN-γ- or perforin-producing cells.
The TLR7/8 agonist enhances the ability of porcine NK cells to lyse FMDV-infected cells.
We were interested in examining the ability of activated porcine NK cells to lyse porcine target cells infected with FMDV. We tested nonstimulated and TLR7/8 agonist-stimulated PBMC in a killing assay using IBRS2 cells and SK6 cells infected with the LL-KGE virus. Neither nonstimulated nor TLR7/8 agonist-stimulated porcine NK cells could lyse noninfected SK6 cells (Fig. 7A), while stimulated NK cells showed much higher lytic capability toward infected cells than nonstimulated NK cells. Moreover, these activated porcine NK cells could kill IBRS2 cells, which are endogenously infected with classical swine fever virus (Fig. 7B). Taken together, stimulation of porcine NK cells with TLR7/8 agonists renders them capable of lysing virus-infected cells in vitro.
FIG. 7.
TLR7/8L enhances the cytotoxicity of NK cells against FMDV-infected cells. Sorted CD2+ CD8+ CD3− cells or bulk PBMC were cultured in the presence of TLR7/8L for 18 h and were later used in an NK cell killing assay against SK6 cells previously infected (Inf) with LL-KGE virus (A) or IBRS2 cells (B). Data are representative of three individual experiments (n = 3).
Quantitative mRNA expression.
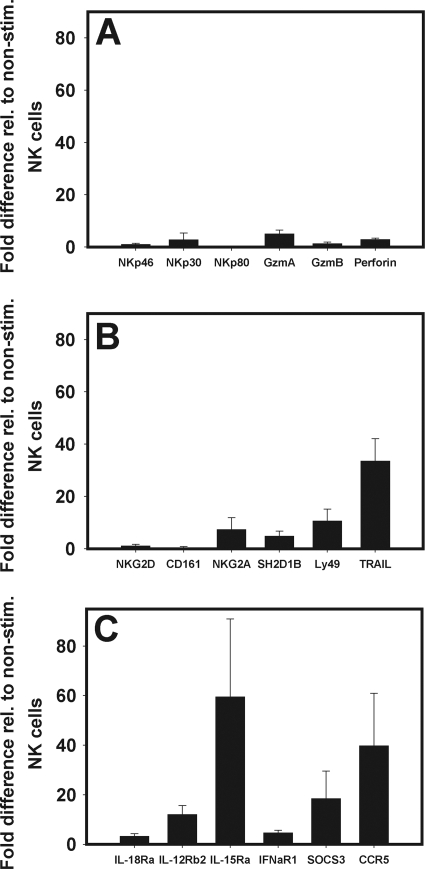
To learn about the possible molecular mechanisms that could be involved in TLR-mediated activation of porcine NK cells, we performed qrtRT-PCR on RNA isolated from purified CD2+ CD8+ CD3− NK cells previously cultured in the presence of TLR7/8L. The mRNAs of most activating receptors, i.e., NKp46, NKp80 (Fig. 8A), NKG2D, and CD161 (Fig. 8B), were not upregulated, whereas a minimal increase in expression relative to that in nonstimulated NK cells was observed for NKp30 (at least threefold) (Fig. 8A). Only Ly49 was considerably upregulated (10-fold) (Fig. 8B). Interestingly, direct stimulation with TLR agonists induced upregulation of mRNAs for two inhibitory receptor genes, NKG2A and SH2D1B, six- and fourfold, respectively (Fig. 8B). Among the mRNAs for effector molecule genes, granzyme A was upregulated to the greatest extent (4.7-fold), followed by perforin (2.7-fold) and granzyme B (1.2-fold) (Fig. 8A). TRAIL, which plays an important role in apoptosis induction, was highly upregulated (33-fold) (Fig. 8B). Analysis of the expression of cytokine receptor mRNA showed that direct stimulation of NK cells with TLR agonists appeared to affect the regulation of all cytokine receptors assessed. The greatest effects were observed on IL-15Rα and IL-12Rβ2 (Fig. 8C). Additionally, CD2+ CD8+ CD3− NK cells expressed mRNAs for the SOCS3 and CCR5 genes, which in this instance were upregulated following stimulation with the TLR7/8 agonist. The increase in SOCS3 expression could be due to the increased production of cytokines by these cells, while CCR5 in the natural setting could increase expression in preparation for migration toward the chemokine gradient, e.g., in infected tissues.
FIG. 8.
Quantitative mRNA expression of NK cell-associated genes. RNA was isolated from CD2+ CD8+ CD3− NK cells stimulated with TLR7/8 agonists for qrt-PCR. (A and B) Activating and inhibiting receptor genes; (C) cytokine receptor, SOCS, and CCR5 genes. Expression is shown relative to that in nonstimulated CD2+ CD8+ CD3− NK cells. Data are representative of four determinations (n = 3). Gzm, granzyme.
DISCUSSION
Cell-mediated immunity to pathogens can be improved by applying adjuvants, which, among other functions, nonspecifically activate inflammatory responses. Some of the most effective of these function by initially stimulating DCs. TLRs are expressed by multiple types of DCs, and therefore, TLR agonists have been studied for adjuvant capacity.
We addressed the impact of TLR agonist stimulation on the functional activity of porcine NK cells, and we show that TLR7/8L can activate porcine NK cells to produce IFN-γ and perforin and can enhance cytotoxicity against tumor cells and FMDV-infected cells in vitro. The results also show that the activation of NK cells is most effectively achieved through the activation of DCs and, to a lesser extent, Mφ/monocytes. B cells are not significantly responsive. Moreover, the results suggest at least two main pathways of TLR activation of porcine NK cells. First, CD2+ CD8+ CD3− NK cells express mRNAs for both TLR7 and TLR8. Exposure of these highly enriched NK cells to TLR7L or TLR8L is characterized by IFN-γ production. However, these cells exhibited minimal cytotoxicity when directly cultured in the presence of the TLR agonists. Second, activation can be mediated by soluble factors produced by CD172+ cells, Mφ/monocytes, and B cells stimulated with the TLR7/8 agonists. Innate cytokines secreted by these cell populations include IL-12, IL-18, IL-15, and IFN-α. Although this study does not provide direct evidence for cell-cell interaction in activating NK cells, it could be an alternative pathway for CD172+ cells to enhance NK cell cytotoxicity.
Considering the evidence collected in this study, we propose possible mechanistic pathways of porcine NK cell activation following stimulation with TLR7/8 agonists (Fig. 9). The agonists likely act through three cell types: (i) DCs, (ii) Mφ/monocytes, and (iii) B cells. These may, at variable levels, produce cytokines such as IL-12, IL-15, IL-18, or IFN-α, which in turn stimulate NK cells. In addition, stimulation with TLR agonists may induce the expression of ligands on DCs that interact with receptors on NK cells, enabling the latter to become activated in a cell-cell-dependent manner. This was shown for human DCs that were induced to express major histocompatibility complex class I chain-related gene A (MICA) and MICB, which, in turn, activated NK cells through binding to NKG2D (19, 20). Moreover, maximal IFN-γ production by NK cells required direct contact of NK cells with DCs as well as stimulation by cytokines secreted by DCs after stimulation with TLR agonists (21). Additionally, activation of porcine NK cells occurs via direct interaction with TLR7/8 receptors expressed by NK cells. The consequence of such NK cell stimulation is increased IFN-γ production, increased perforin storage, and a small increase in cytotoxicity.
FIG. 9.
Possible pathways of porcine NK cell activation by TLR7/8L. (Arrow 1) Addition of TLR7/8L to a culture of porcine PBMC may induce cytokine production in DCs, Mφ/monocytes, or B cells. (Arrow 2) Cytokines such as IL-12, IL-15, IL-18, or IFN-α may then act on NK cells to enhance their cytolytic activity as well as their IFN-γ production. (Arrow 3) Once DCs are activated by TLR7/8L, they may express ligands such as MICA or MICB and thereby interact directly with NK cells through NKG2D to promote their cytotoxic and secretory functions. (Arrow 4) TLR7/8L themselves may directly activate the NK cells through direct engagement of their appropriate receptors expressed on the NK cells, thus driving them to increased NK cell activity.
It is not surprising that several types of cells are targets of TLR agonist induction of cytokines, resulting in NK cell activation. Protamine-condensed mRNA has been found to have activating properties on DCs, monocytes, B cells, NK cells, and granulocytes when added to whole PBMC (37). In those studies, the results suggested that the effect was mediated through TLR7 and TLR8. This demonstrates that several cell types in PMBCs express TLR7 or TLR8 and may react to stimulation. However, Zhang et al. (49) recently reported that TLR7 is expressed only by antigen-presenting cells (B cells and Mφ), and not by T cells, in swine. The reasons for this discrepancy are not known, and those authors did not explain how the T cells examined were obtained and purified. Recently, Adams et al. (1) examined skin biopsy specimens for in situ immunomodulatory effects of imiquimod (a TLR7 agonist) and found that the cell infiltrate was mostly populated with monocytes, Mφ, myeloid DCs, NK cells, T cells, and a small proportion of plasmacytoid DCs (pDCs). The goal of that study was to show the applicability of TLR7 as an adjuvant in immunization against melanoma. Here we show that TLR7 and TLR8 mRNAs are expressed on CD172+, CD21+, and Mφ/monocyte cell populations of swine and that all these cell populations are stimulated to produce cytokines that activate porcine NK cells by agonists of these receptors.
It is encouraging that the stimulation of NK cells by TLR7/8 agonists renders these cells highly cytotoxic against FMDV-infected cells, suggesting that these compounds may be beneficial as adjuvants for vaccines against FMDV. The advantage of using TLR7/8 agonists as adjuvants to enhance innate responses resides in the fact that following stimulation of DCs, the function of antigen uptake is preserved, in contrast to stimulation through other TLRs, such as TLR3 or TLR4. Activation through those receptors leads to maturation of DCs and may induce programs that disable them from particulate antigen uptake (47). Therefore, TLR7/8 stimulation may afford rapid generation of innate responses while allowing antigen-presenting cells to further participate in the development of an adaptive immune response.
To our knowledge, there are no data on the expression profiles of TLR7 and TLR8 on porcine NK cells. Ideally, demonstration of protein expression would have been more informative; however, no reagents are available yet for swine. Here we show that TLR7 and TLR8 mRNAs are present in CD2+ CD8+ CD3− NK cells as well as in CD172+ cells, Mφ/monocytes, and B cells. Therefore, the observed stimulation of sorted NK cells is likely mediated through direct interaction with TLR agonists. However, in a mixed-cell environment, stimulation of NK cells could result from a concerted effort of the stimuli by both direct and indirect interactions with the TLR agonist. In such a situation, it is difficult to discern whether TLR7/8 agonist-induced NK cell activation was secondary to accessory cell activation. A similar observation has been made in the case of CpG stimulation of NK cells in mice, where the pDCs expressed TLR9, and the authors conclude that there was an amplification of signals in such an environment (40).
Treatment with cytokines that normally enhance NK cell activity entails upregulation of genes involved in the function of NK cells (3, 31, 48). Therefore, it was reasonable to expect that TLR7/8 agonist treatment, which stimulated the production of such cytokines, would induce the expression of activating receptors on these cells. However, we did not find significant increases in mRNA expression for NKp46, NKp80, NKG2D, or CD161, but we observed a very high upregulation of TRAIL mRNA. Since we did not measure the protein expression, caution should be exercised in interpreting these data, because the function of certain receptors or effector molecules may be upregulated without the necessity of an mRNA increase. This has been reported for perforin following the treatment of NK cells with IL-18 (18). In a recent study by Schlaepfer and Speck (38), the level of NKG2D protein expression on human NK cells did not increase after treatment with TLR7/8 agonists, and the expression levels of other inhibitory and activating receptors were similarly unaltered. However, the expression of TRAIL was upregulated, a pattern similar to the mRNA expression profile obtained in our study. Given the function of TRAIL, it is anticipated that it could have played a major role in enhancing porcine NK cell cytotoxicity.
The soluble factors mostly involved in NK cell activation following the treatment of PBMC with TLR7 or TLR8 are likely the cytokines IL-12, IL-15, IL-18, and IFN-α. We detected these cytokines in the supernatants of PBMC, CD172+ cells, CD21+ cells, or Mφ/monocytes incubated with the TLR7/8 agonists. We have previously shown that these cytokines enhanced porcine NK cell cytotoxicity and IFN-γ production when applied directly to NK cell cultures (43). Although the cytokines may have contributed to the activation of porcine NK cells, their precise role is not clear, because blocking with neutralizing antibodies against IL-12, IL-15, or IFN-α did not completely eliminate the cytotoxicity of NK cells. A lack of complete inhibition of NK cell cytotoxicity was also reported in similar studies (38).
Porcine pDCs can produce IFN-α (42), but no detailed information is available as to whether they produce cytokines such as IL-15 or IL-18. We have previously reported that both the pDCs and the monocyte-derived DCs in the circulation express CD1 and CD172 in addition to class II major histocompatibility complex (31a and C. K. Nfon, F. N. Toda, M. Kenney, J. Pacheco-Tobin, and W. T. Golde, submitted for publication). Therefore, we used CD172 expression for the isolation of leukocytes containing these DC populations. These cells produced IL-12, IL-15, IL-18, and IFN-α in response to TLR7/8 agonists. Neutralizing antibodies to these cytokines only partially inhibited cytokine-dependent NK cell activation. This indicates that the activation of porcine NK cells by TLR7/8 agonists may be a multifactor signaling process, involving direct cell-cell interaction with cells such as DCs and/or Mφ/monocytes, cytokine mediated stimulation, and direct stimulation through TLRs expressed by the NK cells.
DC-NK cell interaction has been shown for murine NK cells (9), while direct stimulation with TLR agonists has been demonstrated for human NK cells through TLR7, although some controversy between reports exists (15). Direct activation of porcine NK cells by cytokines has been reported by Pintaric et al. (32). However, in our case we allow for the possibility that TLR7/8 may not have directly activated porcine NK cells and that the observed increase in cytotoxicity following the incubation of purified NK cells with TLR7/8 agonists could have been due to the stimulation of residual DCs. Since we lack a single exclusive marker for porcine NK cells, we could achieve only 90 to 95% pure CD2+ CD8+ CD3− cells, which are enriched for, but are not exclusively, NK cells.
Human peripheral blood pDCs and monocyte DCs have been reported to express perforin and granzyme B following culture in the presence of the TLR7 agonist imiquimod. Moreover, these DCs had the ability to kill K562 cells in vitro (41). We did not observe any killing of K562 by CD172+ cells cultured in the presence of the TLR7/8 agonist. This may indicate a species difference in reactivity to TLR stimulation or a divergence of innate responses in swine using bystander activation of lymphocytes to achieve tumor cell and infected-cell killing, particularly by NK cells.
In summary, the data presented in this study clearly show that TLR7/8 agonists activate porcine NK cells. Both direct and indirect stimulatory pathways appear to be involved in generating an innate immune response of porcine NK cells following stimulation with these compounds. CD172+ cells and Mφ/monocytes are directly involved in this activation process through cytokine production. Moreover, we show the expression of TLR7 or TLR8 mRNA by both porcine NK cells and accessory cells. TLR agonist-activated NK cells are activated to produce IFN-γ and increase intracellular perforin levels, and they are capable of killing FMDV-infected cells. However, it remains to be determined whether these TLR agonists can enhance NK cell activity in vivo and thereby afford protection against viral infection. Interventional vaccine formulations incorporating these molecules, targeting DCs and NK cells, could constitute a vital strategy for enhancing innate immunity and adaptive responses to FMDV in swine.
Supplementary Material
Acknowledgments
This work was supported by CRIS 1940-32000-052-00D (to W.T.G.) and 1235-51000-051-00 (to H.D.) from the Agricultural Research Service, USDA, and by an interagency agreement (60-1940-8-037) between the Department of Homeland Security, Science and Technology Directorate, and the USDA (W.T.G.). C. K. Nfon was the recipient of a Plum Island Animal Disease Center Research Participation Program fellowship, administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy (DOE) and the USDA.
We thank Richard Miller of 3M Pharmaceuticals, Inc. (Minneapolis, MN), for the generous gift of the TLR7 and TLR8 agonist compounds. We thank Mary Kenney for technical assistance and the animal care staff at the Plum Island Animal Disease Center for professional support and assistance. Finally, we thank D. Mark Estes, University of Texas Medical Branch at Galveston, for consultation and discussion of this work.
No competing financial interests exist. 3M Pharmaceuticals provided TLR7 and TLR8 agonists for analysis of the response of porcine cells under a Material Transfer Agreement between the company and the USDA. The USDA and its scientists hold no proprietary interest in these compounds.
Footnotes
Published ahead of print on 15 April 2009.
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1.Adams, S., D. W. O'Neill, D. Nonaka, E. Hardin, L. Chiriboga, K. Siu, C. M. Cruz, A. Angiulli, F. Angiulli, E. Ritter, R. M. Holman, R. L. Shapiro, R. S. Berman, N. Berner, Y. Shao, O. Manches, L. Pan, R. R. Venhaus, E. W. Hoffman, A. Jungbluth, S. Gnjatic, L. Old, A. C. Pavlick, and N. Bhardwaj. 2008. Immunization of malignant melanoma patients with full-length N.Y.-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. J. Immunol. 181776-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bautista, E. M., G. S. Ferman, and W. T. Golde. 2003. Induction of lymphopenia and inhibition of T cell function during acute infection of swine with foot and mouth disease virus (FMDV). Vet. Immunol. Immunopathol. 9261-73. [DOI] [PubMed] [Google Scholar]
- 3.Boyiadzis, M., S. Memon, J. Carson, K. Allen, M. J. Szczepanski, B. A. Vance, R. Dean, M. R. Bishop, R. E. Gress, and F. T. Hakim. 2008. Up-regulation of NK cell activating receptors following allogeneic hematopoietic stem cell transplantation under a lymphodepleting reduced intensity regimen is associated with elevated IL-15 levels. Biol. Blood Marrow Transplant. 14290-300. [DOI] [PubMed] [Google Scholar]
- 4.Brown, M. G., A. O. Dokun, J. W. Heusel, H. R. Smith, D. L. Beckman, E. A. Blattenberger, C. E. Dubbelde, L. R. Stone, A. A. Scalzo, and W. M. Yokoyama. 2001. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science 292934-937. [DOI] [PubMed] [Google Scholar]
- 5.Cantell, K., and J. Pirhonen. 1996. IFN-γ enhances production of IFN-α in human macrophages but not in monocytes. J. Interferon Cytokine Res. 16461-463. [DOI] [PubMed] [Google Scholar]
- 6.Chalifour, A., P. Jeannin, J. F. Gauchat, A. Blaecke, M. Malissard, T. N′Guyen, N. Thieblemont, and Y. Delneste. 2004. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers alpha-defensin production. Blood 1041778-1783. [DOI] [PubMed] [Google Scholar]
- 7.Doherty, T. M., R. A. Seder, and A. Sher. 1996. Induction and regulation of IL-15 expression in murine macrophages. J. Immunol. 156735-741. [PubMed] [Google Scholar]
- 8.Dumitru, C. D., M. A. Antonysamy, K. S. Gorski, D. D. Johnson, L. G. Reddy, J. L. Lutterman, M. M. Piri, J. Proksch, S. M. McGurran, E. A. Egging, F. R. Cochran, K. E. Lipson, M. A. Tomai, and G. W. Gullikson. 2009. NK1.1+ cells mediate the antitumor effects of a dual Toll-like receptor 7/8 agonist in the disseminated B16-F10 melanoma model. Cancer Immunol. Immunother. 58575-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez, N. C., A. Lozier, C. Flament, P. Ricciardi-Castagnoli, D. Bellet, M. Suter, M. Perricaudet, T. Tursz, E. Maraskovsky, and L. Zitvogel. 1999. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat. Med. 5405-411. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald, P., and C. Lopez. 1986. Natural killer cells active against viral, bacterial, protozoan and fungal infections, p. 107-111. In E. Lotzova and R. B. Herberman (ed.), Immunobiology of natural killer cells, vol. 2. CRC Press, Boca Raton, FL. [Google Scholar]
- 11.Forsbach, A., J. G. Nemorin, C. Montino, C. Muller, U. Samulowitz, A. P. Vicari, M. Jurk, G. K. Mutwiri, A. M. Krieg, G. B. Lipford, and J. Vollmer. 2008. Identification of RNA sequence motifs stimulating sequence-specific TLR8-dependent immune responses. J. Immunol. 1803729-3738. [DOI] [PubMed] [Google Scholar]
- 12.Girart, M. V., M. B. Fuertes, C. I. Domaica, L. E. Rossi, and N. W. Zwirner. 2007. Engagement of TLR3, TLR7, and NKG2D regulate IFN-γ secretion but not NKG2D-mediated cytotoxicity by human NK cells stimulated with suboptimal doses of IL-12. J. Immunol. 1793472-3479. [DOI] [PubMed] [Google Scholar]
- 13.Golde, W. T., J. M. Pacheco, H. Duque, T. Doel, B. Penfold, G. S. Ferman, D. R. Gregg, and L. L. Rodriguez. 2005. Vaccination against foot-and-mouth disease virus confers complete clinical protection in 7 days and partial protection in 4 days: use in emergency outbreak response. Vaccine 235775-5782. [DOI] [PubMed] [Google Scholar]
- 14.Gorden, K. B., K. S. Gorski, S. J. Gibson, R. M. Kedl, W. C. Kieper, X. Qiu, M. A. Tomai, S. S. Alkan, and J. P. Vasilakos. 2005. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J. Immunol. 1741259-1268. [DOI] [PubMed] [Google Scholar]
- 15.Gorski, K. S., E. L. Waller, J. Bjornton-Severson, J. A. Hanten, C. L. Riter, W. C. Kieper, K. B. Gorden, J. S. Miller, J. P. Vasilakos, M. A. Tomai, and S. S. Alkan. 2006. Distinct indirect pathways govern human NK-cell activation by TLR-7 and TLR-8 agonists. Int. Immunol. 181115-1126. [DOI] [PubMed] [Google Scholar]
- 16.Grubman, M. J., and B. Baxt. 2004. Foot-and-mouth disease. Clin. Microbiol. Rev. 17465-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart, O. M., V. Athie-Morales, G. M. O'Connor, and C. M. Gardiner. 2005. TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-γ production. J. Immunol. 1751636-1642. [DOI] [PubMed] [Google Scholar]
- 18.Hyodo, Y., K. Matsui, N. Hayashi, H. Tsutsui, S. Kashiwamura, H. Yamauchi, K. Hiroishi, K. Takeda, Y. Tagawa, Y. Iwakura, N. Kayagaki, M. Kurimoto, H. Okamura, T. Hada, H. Yagita, S. Akira, K. Nakanishi, and K. Higashino. 1999. IL-18 up-regulates perforin-mediated NK activity without increasing perforin messenger RNA expression by binding to constitutively expressed IL-18 receptor. J. Immunol. 1621662-1668. [PubMed] [Google Scholar]
- 19.Jinushi, M., T. Takehara, T. Kanto, T. Tatsumi, V. Groh, T. Spies, T. Miyagi, T. Suzuki, Y. Sasaki, and N. Hayashi. 2003. Critical role of MHC class I-related chain A and B expression on IFN-α-stimulated dendritic cells in NK cell activation: impairment in chronic hepatitis C virus infection. J. Immunol. 1701249-1256. [DOI] [PubMed] [Google Scholar]
- 20.Jinushi, M., T. Takehara, T. Tatsumi, T. Kanto, V. Groh, T. Spies, T. Suzuki, T. Miyagi, and N. Hayashi. 2003. Autocrine/paracrine IL-15 that is required for type I IFN-mediated dendritic cell expression of MHC class I-related chain A and B is impaired in hepatitis C virus infection. J. Immunol. 1715423-5429. [DOI] [PubMed] [Google Scholar]
- 21.Kamath, A. T., C. E. Sheasby, and D. F. Tough. 2005. Dendritic cells and NK cells stimulate bystander T cell activation in response to TLR agonists through secretion of IFN-α/β and IFN-γ. J. Immunol. 174767-776. [DOI] [PubMed] [Google Scholar]
- 22.Lanier, L. L. 2005. NK cell recognition. Annu. Rev. Immunol. 23225-274. [DOI] [PubMed] [Google Scholar]
- 23.Lauzon, N. M., F. Mian, R. MacKenzie, and A. A. Ashkar. 2006. The direct effects of Toll-like receptor ligands on human NK cell cytokine production and cytotoxicity. Cell. Immunol. 241102-112. [DOI] [PubMed] [Google Scholar]
- 24.Lee, S. H., T. Miyagi, and C. A. Biron. 2007. Keeping NK cells in highly regulated antiviral warfare. Trends Immunol. 28252-259. [DOI] [PubMed] [Google Scholar]
- 25.Leulier, F., and B. Lemaitre. 2008. Toll-like receptors—taking an evolutionary approach. Nat. Rev. Genet. 9165-178. [DOI] [PubMed] [Google Scholar]
- 26.Liese, J., U. Schleicher, and C. Bogdan. 2007. TLR9 signaling is essential for the innate NK cell response in murine cutaneous leishmaniasis. Eur. J. Immunol. 373424-3434. [DOI] [PubMed] [Google Scholar]
- 27.Mandelboim, O., N. Lieberman, M. Lev, L. Paul, T. I. Arnon, Y. Bushkin, D. M. Davis, J. L. Strominger, J. W. Yewdell, and A. Porgador. 2001. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature 4091055-1060. [DOI] [PubMed] [Google Scholar]
- 28.Mark, K. E., L. Corey, T. C. Meng, A. S. Magaret, M. L. Huang, S. Selke, H. B. Slade, S. K. Tyring, T. Warren, S. L. Sacks, P. Leone, V. A. Bergland, and A. Wald. 2007. Topical resiquimod 0.01% gel decreases herpes simplex virus type 2 genital shedding: a randomized, controlled trial. J. Infect. Dis. 1951324-1331. [DOI] [PubMed] [Google Scholar]
- 29.Moraes, M. P., J. Chinsangaram, M. C. Brum, and M. J. Grubman. 2003. Immediate protection of swine from foot-and-mouth disease: a combination of adenoviruses expressing interferon alpha and a foot-and-mouth disease virus subunit vaccine. Vaccine 22268-279. [DOI] [PubMed] [Google Scholar]
- 30.Moraes, M. P., T. de Los Santos, M. Koster, T. Turecek, H. Wang, V. G. Andreyev, and M. J. Grubman. 2007. Enhanced antiviral activity against foot-and-mouth disease virus by a combination of type I and II porcine interferons. J. Virol. 817124-7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mori, S., A. Jewett, M. Cavalcanti, K. Murakami-Mori, S. Nakamura, and B. Bonavida. 1998. Differential regulation of human NK cell-associated gene expression following activation by IL-2, IFN-α and PMA/ionomycin. Int. J. Oncol. 121165-1170. [DOI] [PubMed] [Google Scholar]
- 31a.Nfon, C. K., G. S. Ferman, F. N. Toka, and W. T. Golde. 2008. Inhibition of interferon alpha from circulating dendritic cells by foot-and-mouth disease virus (FMDV) during acute infection. Viral Immunol. 2168-77. [DOI] [PubMed] [Google Scholar]
- 32.Pintaric, M., W. Gerner, and A. Saalmuller. 2008. Synergistic effects of IL-2, IL-12 and IL-18 on cytolytic activity, perforin expression and IFN-γ production of porcine natural killer cells. Vet. Immunol. Immunopathol. 12168-82. [DOI] [PubMed] [Google Scholar]
- 33.Pirhonen, J., T. Sareneva, I. Julkunen, and S. Matikainen. 2001. Virus infection induces proteolytic processing of IL-18 in human macrophages via caspase-1 and caspase-3 activation. Eur. J. Immunol. 31726-733. [DOI] [PubMed] [Google Scholar]
- 34.Pompei, L., S. Jang, B. Zamlynny, S. Ravikumar, A. McBride, S. P. Hickman, and P. Salgame. 2007. Disparity in IL-12 release in dendritic cells and macrophages in response to Mycobacterium tuberculosis is due to use of distinct TLRs. J. Immunol. 1785192-5199. [DOI] [PubMed] [Google Scholar]
- 35.Roda, J. M., R. Parihar, and W. E. Carson III. 2005. CpG-containing oligodeoxynucleotides act through TLR9 to enhance the NK cell cytokine response to antibody-coated tumor cells. J. Immunol. 1751619-1627. [DOI] [PubMed] [Google Scholar]
- 36.Sawaki, J., H. Tsutsui, N. Hayashi, K. Yasuda, S. Akira, T. Tanizawa, and K. Nakanishi. 2007. Type 1 cytokine/chemokine production by mouse NK cells following activation of their TLR/MyD88-mediated pathways. Int. Immunol. 19311-320. [DOI] [PubMed] [Google Scholar]
- 37.Scheel, B., R. Teufel, J. Probst, J. P. Carralot, J. Geginat, M. Radsak, D. Jarrossay, H. Wagner, G. Jung, H. G. Rammensee, I. Hoerr, and S. Pascolo. 2005. Toll-like receptor-dependent activation of several human blood cell types by protamine-condensed mRNA. Eur. J. Immunol. 351557-1566. [DOI] [PubMed] [Google Scholar]
- 38.Schlaepfer, E., and R. F. Speck. 2008. Anti-HIV activity mediated by natural killer and CD8+ cells after toll-like receptor 7/8 triggering. PLoS ONE 3e1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seya, T., T. Akazawa, J. Uehori, M. Matsumoto, I. Azuma, and K. Toyoshima. 2003. Role of toll-like receptors and their adaptors in adjuvant immunotherapy for cancer. Anticancer Res. 234369-4376. [PubMed] [Google Scholar]
- 40.Sivori, S., S. Carlomagno, L. Moretta, and A. Moretta. 2006. Comparison of different CpG oligodeoxynucleotide classes for their capability to stimulate human NK cells. Eur. J. Immunol. 36961-967. [DOI] [PubMed] [Google Scholar]
- 41.Stary, G., C. Bangert, M. Tauber, R. Strohal, T. Kopp, and G. Stingl. 2007. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J. Exp. Med. 2041441-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Summerfield, A., L. Guzylack-Piriou, A. Schaub, C. P. Carrasco, V. Tache, B. Charley, and K. C. McCullough. 2003. Porcine peripheral blood dendritic cells and natural interferon-producing cells. Immunology 110440-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toka, F. N., C. K. Nfon, H. Dawson, D. Mark Estes, and W. T. Golde. 2009. Activation of porcine natural killer (NK) cells and lysis of foot-and-mouth disease virus (FMDV) infected cells. J. Interferon Cytokine Res. 29179-192. [DOI] [PubMed] [Google Scholar]
- 44.Trinchieri, G., and A. Sher. 2007. Cooperation of Toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. 7179-190. [DOI] [PubMed] [Google Scholar]
- 45.Tsujimoto, H., T. Uchida, P. A. Efron, P. O. Scumpia, A. Verma, T. Matsumoto, S. K. Tschoeke, R. F. Ungaro, S. Ono, S. Seki, M. J. Clare-Salzler, H. V. Baker, H. Mochizuki, R. Ramphal, and L. L. Moldawer. 2005. Flagellin enhances NK cell proliferation and activation directly and through dendritic cell-NK cell interactions. J. Leukoc. Biol. 78888-897. [DOI] [PubMed] [Google Scholar]
- 46.Ulevitch, R. J. 2004. Therapeutics targeting the innate immune system. Nat. Rev. Immunol. 4512-520. [DOI] [PubMed] [Google Scholar]
- 47.Weck, M. M., F. Grunebach, D. Werth, C. Sinzger, A. Bringmann, and P. Brossart. 2007. TLR ligands differentially affect uptake and presentation of cellular antigens. Blood 1093890-3894. [DOI] [PubMed] [Google Scholar]
- 48.Zhang, B., J. Zhang, and Z. Tian. 2008. Comparison in the effects of IL-2, IL-12, IL-15 and IFN-α on gene regulation of granzymes of human NK cell line NK-92. Int. Immunopharmacol. 8989-996. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, Y., Y. Guo, K. Lv, K. Wang, and S. Sun. 2008. Molecular cloning and functional characterization of porcine toll-like receptor 7 involved in recognition of single-stranded RNA virus/ssRNA. Mol. Immunol. 451184-1190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.