Abstract
Cellular and humoral immune responses of healed cutaneous leishmaniasis and Mediterranean visceral leishmaniasis patients were evaluated against results for Leishmania major virulence proteins L. major protein disulfide isomerase (LmPDI) and mitogen-activated protein kinase kinase (MAPKK). Only MAPKK induces significant peripheral blood mononuclear cell proliferation with gamma interferon production as well as antibody responses. Thus, MAPKK may be of interest in Leishmania vaccination and serodiagnosis.
The leishmaniases are diseases caused by vector-borne pathogens that represent a major public health problem affecting the lives of millions of people worldwide (http://www.who.int/whr/en). Depending on the parasite species and on the immunological response of the human host, leishmaniasis ranges from an asymptomatic infection to a self-limiting cutaneous lesion(s) or a fatal visceral form.
No anti-Leishmania vaccine is available at the moment. Different studies showed that development of Th1- and Leishmania-specific cytotoxic immune responses correlate with healing of patients with cutaneous leishmaniasis (CL) (3, 12). An intense effort is being made to identify antigens that could induce an immune state similar to that developed by individuals who recover from symptomatic infection and are resistant to a subsequent natural challenge. Such antigens may contribute to the development of an anti-Leishmania vaccine.
Diagnostic tools targeting leishmaniasis are available. However, parasite detection is invasive and poorly sensitive. Serological diagnosis using various techniques based on detection of Leishmania-specific antibodies that are developed during acute disease are often more sensitive, less time-consuming, and more user friendly (4, 11, 15).
In an attempt to identify new antigens to be used as vaccines or for serodiagnosis, we focused on Leishmania virulence factors. Indeed, several studies described these factors as potentially immunogenic in humans, mice, and, more recently, dogs (6, 8, 9, 13). Here, cellular and humoral immune responses of healed CL (hCL) and Mediterranean visceral leishmaniasis (MVL) patients were evaluated against results for Leishmania major protein disulfide isomerase (LmPDI) and mitogen-activated protein kinase kinase (MAPKK), which we and others previously described as potential virulence factors (2, 10).
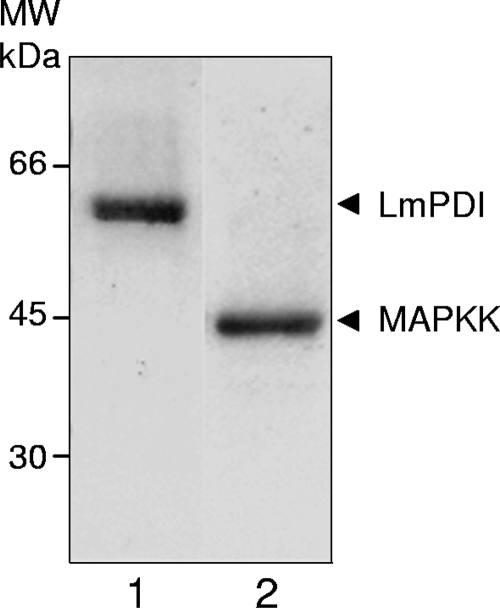
We produced LmPDI (55 kDa) and MAPKK (40 kDa) in the prokaryotic expression pET system (Novagen, Gibbstown, NJ) and then purified them by affinity chromatography (Fig. 1).
FIG. 1.
Expression of recombinant proteins in Escherichia coli. Recombinant LmPDI (lane 1) and MAPKK (lane 2) were synthesized in BL21, purified by affinity chromatography over Ni-nitrilotriacetic acid resin, and analyzed on 14% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel, followed by Coomassie blue staining. MW, molecular mass markers (kDa).
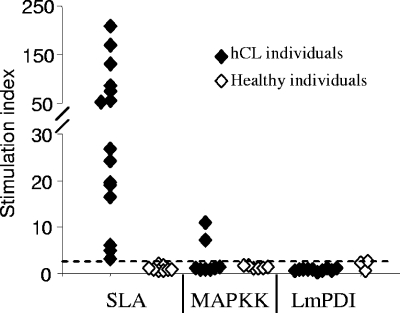
Lymphoproliferative responses (Fig. 2) and gamma interferon (IFN-γ) and interleukin-10 (IL-10) production (Fig. 3) induced by recombinant LmPDI and MAPKK (10 μg/ml) were characterized in vitro using peripheral blood mononuclear cells (PBMC) from two groups. The first group consisted of 18 hCL patients (age range, 17 to 42 years; mean ± standard deviation [SD], 28 ± 10.9 years) living in an area of L. major infection endemicity (the governorate of Sidi Bouzid, Tunisia). Diagnosis of leishmaniasis was based on the presence of specific scars, leishmanin skin test positivity, and a positive lymphoproliferative response to soluble Leishmania antigen (SLA). The second group consisted of 12 healthy individuals (negative controls) living outside areas of endemicity and having no history of CL. All healed patients showed positive proliferation in response to SLA, with stimulation indices (SI) ranging from 3.04 to 207.76 (mean ± SD, 56.83 ± 58.03) (Fig. 2). While no proliferation was observed with LmPDI, MAPKK induced significant PBMC proliferation levels (SI > 2.5) in two of eight hCL individuals. No proliferative responses against SLA and the two recombinant proteins were observed in PBMC from healthy individuals (SI < 2.5) (Fig. 2). Polymyxin B treatment of PBMC cultures stimulated with recombinant proteins had very little or no effect on proliferative responses, showing that observed stimulation could not be due to lipopolysaccharide contamination (data not shown).
FIG. 2.
Lymphoproliferative response induced by MAPKK or LmPDI. Levels of lymphocyte proliferation in response to either SLA (10 μg/ml) or recombinant proteins MAPKK (10 μg/ml) and LmPDI (5 μg/ml) incubated for 5 days at 37°C and 5% CO2 are expressed as SI. The cutoff value (SI = 2.5) is indicated by a horizontal bar.
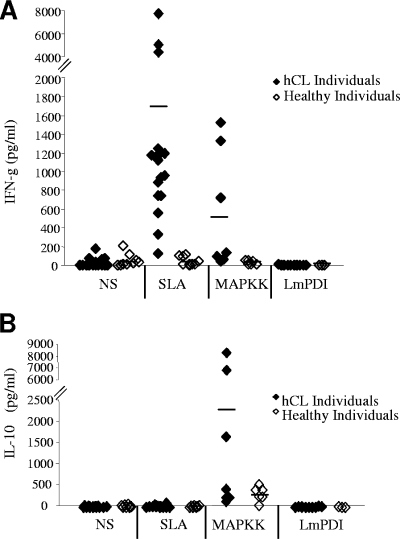
FIG. 3.
Immune response induced by MAPKK or LmPDI: IFN-γ (A) and IL-10 (B) induction. PBMC from hCL or healthy subjects were stimulated with either SLA (10 μg/ml) or recombinant proteins MAPKK (10 μg/ml) and LmPDI (5 μg/ml). Supernatants were harvested at 48 h and 72 h and were used for quantification of IL-10 and IFN-γ. The dashes indicate the mean cytokine values obtained for the different groups of individuals. NS, nonstimulated cultures.
IFN-γ and IL-10 levels in culture supernatants of PBMC stimulated with either SLA (10 μg/ml), MAPKK (10 μg/ml), or LmPDI (5 μg/ml) were determined using an enzyme-linked immunosorbent assay (ELISA) (Fig. 3A and B). As expected, SLA induced significantly high levels of IFN-γ but no IL-10 in hCL individuals (mean IFN-γ production levels ± SD of 1,695 ± 1,983 pg/ml and 39.4 ± 49.7 pg/ml, respectively; P = 0.03) (Fig. 3A and B). Interestingly, MAPKK induced high IFN-γ and IL-10 levels in three of eight hCL individuals tested (mean IFN-γ production levels ± SD of 559 ± 642.5 pg/ml and 26.29 ± 25.33 pg/ml and mean IL-10 production levels ± SD of 2,486 ± 3,394 pg/ml and 316.3 ± 174.8 pg/ml in hCL and healthy individuals, respectively) (Fig. 3A and B). Interestingly, the difference in production between the two groups was highly significant only for IFN-γ (P = 0.007) (Fig. 3A). However, no IFN-γ or IL-10 productions were observed after stimulation of PBMC with LmPDI (Fig. 3A and B). A positive correlation was found between IFN-γ and IL-10 levels (Spearman rank correlation r = 0.594) and between PBMC proliferation and IFN-γ or IL-10 production (Spearman rank correlation r values of 0.6 [P = 0.03] and 0.583 [P = 0.036] for IFN-γ and IL-10, respectively) in response to MAPKK.
These results show that MAPKK could constitute a potential vaccine candidate. It is well established that IFN-γ is a key cytokine in resistance against CL and is implicated in parasite killing by activated macrophages (14). However, IL-10 is the main downregulating cytokine of the Th1 immune response and exhibits macrophage-deactivating properties (5, 7). Interestingly, the MAPKK IL-10-inducing capacity was not sufficient for suppression of significant proliferation and high, significant levels of IFN-γ in hCL patients. The IL-10 production by PBMC from hCL as well as healthy individuals might be due to the high level of conservation of MAPKK proteins in eukaryotic species. Although MAPKK stimulates high IL-10 levels, it could constitute a potential vaccine candidate since it was recently reported that the human immune response to crude and defined Leishmania antigens generated during immunization can differ from that induced by natural infection (1). Similarly, MAPKK used as a vaccine might induce a dominant Th1 response compatible with protection.
For the second part of the study, we chose to analyze humoral responses to LmPDI and MAPKK in MVL patients rather than CL patients for the following reasons: (i) human sera from CL patients generally show low-level reactivity against Leishmania antigens, compared to sera from MVL patients; (ii) diagnosis of MVL is more problematic than that of CL; and (iii) LmPDI and MAPKK are highly conserved with their Leishmania infantum homologue, with 92% and 97% identities, respectively.
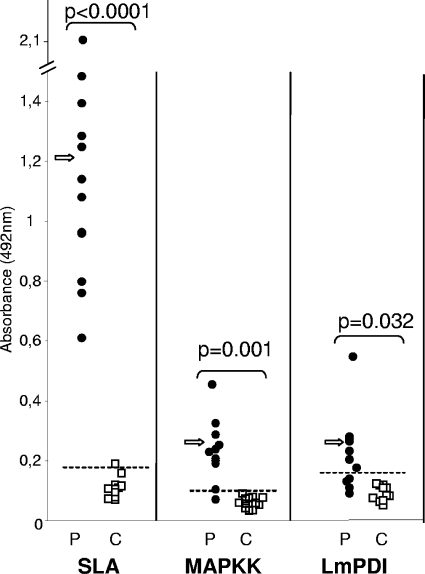
The reactivities of MVL patients to LmPDI (10 μg/ml), MAPKK (5 μg/ml), and SLA (2 μg/ml) were assayed by ELISAs with two groups. The first group consisted of 12 MVL children (age range, 1 to 5 years; mean age ± SD, 35 ± 21 months) living in the governorate of Kairouan (a region of MVL endemicity), blood sampled before treatment. Diagnosis of MVL was established on clinical criteria (fever, anemia, splenomegaly, and weight loss) and on demonstration of Leishmania parasites in Giemsa-stained bone marrow smears and/or culture in biphasic Nicolle-Novy-McNeal medium. The second group consisted of 13 healthy volunteers living outside areas of endemicity as negative controls (Fig. 4). The cutoff value of reactivity with each antigen was defined as the mean optical density (OD) + 2 SDs obtained with healthy individuals, and these values were equal to 0.18, 0.1, and 0.14 for SLA, MAPKK, and LmPDI, respectively. These cutoff values allowed us to identify positive and negative sera and consequently to estimate the performance parameters of each ELISA (Table 1). ELISAs based on SLA and MAPKK had the best sensitivities, with 100% and 91.7%, respectively. Interestingly, the best specificities and positive predictive values were obtained with MAPKK (100% for MAPKK versus 92.3% for SLA). However, SLA gave the best results for negative predictive values (100% versus 92.9% for MAPKK). The ELISA using LmPDI showed only 66.7% sensitivity, with a specificity of 100%. For all proteins, significant differences in measured OD were observed between MVL patients and healthy controls, with P values of <0.05. Taken together, these results indicate that only MAPKK constitutes a major target of humoral response during MVL.
FIG. 4.
ELISA reactivity of sera from patients with MVL (P) and healthy controls (C) with SLA (2 μg/ml) and recombinant Leishmania proteins MAPKK (5 μg/ml) and LmPDI (10 μg/ml). Bars show the cutoff value for each ELISA, which is defined as the mean OD + 2 SDs for the values obtained with sera from healthy controls. The significance of differences between P and C groups was evaluated by the Mann-Whitney test. P values of <0.05 were considered significant. Arrows indicate mean OD values.
TABLE 1.
Sensitivities and specificities of ELISAs using crude SLAs and recombinant Leishmania proteins MAPKK and LmPDIa
| Antigen | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| Crude SLA | 100 | 92.3 | 92.3 | 100 |
| MAPKK | 91.7 | 100 | 100 | 92.9 |
| LmPDI | 66.7 | 100 | 100 | 76.5 |
PPV, positive predictive value; NPV, negative predictive value.
This is the first step in the determination of MAPKK as an interesting antigen for MVL serodiagnosis. Indeed, recruitment of individuals must be improved by (i) increasing the sizes of MVL patient and control groups and (ii) increasing the numbers of control groups with healthy individuals living outside the area of endemicity, healthy individuals living inside the area of endemicity, and patients infected by other pathogens and showing similar symptoms.
Acknowledgments
To our knowledge, this is the first description of MAPKK from Leishmania as a potential vaccine candidate and an MVL serodiagnosis antigen.
Footnotes
Published ahead of print on 1 April 2009.
REFERENCES
- 1.Azeredo-Coutinho, R. B., D. C. Matos, G. G. Armoa, R. M. Maia, A. Schubach, W. Mayrink, and S. C. Mendonca. 2008. Contrasting human cytokine responses to promastigote whole-cell extract and the Leishmania analogue receptor for activated C kinase antigen of L. amazonensis in natural infection versus immunization. Clin. Exp. Immunol. 153369-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben Achour, Y., M. Chenik, H. Louzir, and K. Dellagi. 2002. Identification of a disulfide isomerase protein of Leishmania major as a putative virulence factor. Infect. Immun. 703576-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bousoffara, T., H. Louzir, A. Ben Salah, and K. Dellagi. 2004. Analysis of granzyme B activity as a surrogate marker of Leishmania-specific cell-mediated cytotoxicity in zoonotic cutaneous leishmaniasis. J. Infect. Dis. 1891265-1273. [DOI] [PubMed] [Google Scholar]
- 4.Chappuis, F., S. Rijal, U. K. Jha, P. Desjeux, B. M. Karki, S. Koirala, L. Loutan, and M. Boelaert. 2006. Field validity, reproducibility and feasibility of diagnostic tests for visceral leishmaniasis in rural Nepal. Trop. Med. Int. Health 1131-40. [DOI] [PubMed] [Google Scholar]
- 5.de Waal Malefyt, R., J. Abrams, B. Bennett, C. G. Figdor, and J. E. de Vries. 1991. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1741209-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandes, A. P., M. M. Costa, E. A. Coelho, M. S. Michalick, E. de Freitas, M. N. Melo, W. Luiz Tafuri, M. Resende, V. Hermont, F. Abrantes, and R. T. Gazzinelli. 2008. Protective immunity against challenge with Leishmania (Leishmania) chagasi in beagle dogs vaccinated with recombinant A2 protein. Vaccine 265888-5895. [DOI] [PubMed] [Google Scholar]
- 7.Fiorentino, D. F., A. Zlotnik, T. R. Mosmann, M. Howard, and A. O'Garra. 1991. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1473815-3822. [PubMed] [Google Scholar]
- 8.Jaafari, M. R., A. Ghafarian, A. Farrokh-Gisour, A. Samiei, M. T. Kheiri, F. Mahboudi, F. Barkhordari, A. Khamesipour, and W. R. McMaster. 2006. Immune response and protection assay of recombinant major surface glycoprotein of Leishmania (rgp63) reconstituted with liposomes in BALB/c mice. Vaccine 245708-5717. [DOI] [PubMed] [Google Scholar]
- 9.Khoshgoo, N., F. Zahedifard, H. Azizi, Y. Taslimi, M. J. Alonso, and S. Rafati. 2008. Cysteine proteinase type III is protective against Leishmania infantum infection in BALB/c mice and highly antigenic in visceral leishmaniasis individuals. Vaccine 265822-5829. [DOI] [PubMed] [Google Scholar]
- 10.Kuhn, D., and M. Wiese. 2005. LmxPK4, a mitogen-activated protein kinase kinase homologue of Leishmania mexicana with a potential role in parasite differentiation. Mol. Microbiol. 561169-1182. [DOI] [PubMed] [Google Scholar]
- 11.Pedras, M. J., L. de Gouvea Viana, E. J. de Oliveira, and A. Rabello. 2008. Comparative evaluation of direct agglutination test, rK39 and soluble antigen ELISA and IFAT for the diagnosis of visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 102172-178. [DOI] [PubMed] [Google Scholar]
- 12.Reithinger, R., J. C. Dujardin, H. Louzir, C. Pirmez, B. Alexander, and S. Brooker. 2007. Cutaneous leishmaniasis. Lancet Infect. Dis. 7581-596. [DOI] [PubMed] [Google Scholar]
- 13.Resende, D. M., B. C. Caetano, M. S. Dutra, M. L. Penido, C. F. Abrantes, R. M. Verly, J. M. Resende, D. Pilo-Veloso, S. A. Rezende, O. Bruna-Romero, A. P. Fernandes, and R. T. Gazzinelli. 2008. Epitope mapping and protective immunity elicited by adenovirus expressing the Leishmania amastigote specific A2 antigen: correlation with IFN-gamma and cytolytic activity by CD8+ T cells. Vaccine 264585-4593. [DOI] [PubMed] [Google Scholar]
- 14.Sacks, D., and N. Noben-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2845-858. [DOI] [PubMed] [Google Scholar]
- 15.Schallig, H. D., G. J. Schoone, C. C. Kroon, A. Hailu, F. Chappuis, and H. Veeken. 2001. Development and application of ‘simple’ diagnostic tools for visceral leishmaniasis. Med. Microbiol. Immunol. 19069-71. [DOI] [PubMed] [Google Scholar]