Abstract
A cDNA expression library of Babesia gibsoni was screened with the serum collected from a dog experimentally infected with B. gibsoni. A novel antigen sharing homology with secreted antigen 1 of B. gibsoni was isolated. The genomic analysis indicated that the BgSA3 gene exists as multicopies in the genome of B. gibsoni. The putative peptide encoded by the BgSA3 gene showed some characteristics of secreted proteins. The serum raised in mice immunized with the recombinant BgSA3 expressed in Escherichia coli could recognize a native parasite protein with a molecular mass of 70 kDa. Moreover, a sandwich enzyme-linked immunosorbent assay with anti-BgSA3 antibodies could detect the circulating BgSA3 in the blood plasma of dogs experimentally infected with B. gibsoni. The identification of BgSA3 provided a useful target for the development of a diagnostic test for detecting specific antibodies and circulating antigens.
Babesia canis and Babesia gibsoni are recognized as the two species that cause canine babesiosis, a clinically significant hemolytic disease of dogs. Individual Babesia species are typically classified by the size and morphological appearance of the intraerythrocytic forms, often referred to as piroplasms. B. gibsoni is considered to be a small babesial parasite with round or oval intraerythrocytic piroplasms. The infection caused by B. gibsoni is characterized by serious clinical problems, including remittent fever, progressive anemia, hemoglobinuria, and sometimes death (4, 6). This disease is epidemic in many areas of Asia, North America, and Northern and Eastern Africa but rarely so in Europe (4, 12, 18).
For the diagnosis of this disease, several serological methods have been developed, such as indirect fluorescent antibody tests (IFAT) (24), enzyme-linked immunosorbent assays (ELISAs) (13, 22), and immunochromatographic tests (14). However, the detection of antibodies is unreliable for determining the infection status of dogs because the titer of the antibodies against the parasites can remain very high for a long time, even when the parasites have been completely eliminated. However, the secreted proteins, which include a broad variety of antigens released by the parasites and circulating in the bloodstream of the hosts during the asexual stage, can be used as diagnostic targets in antigen detection tests in order to avoid such problems. In an attempt to identify these circulating antigens, a method to screen a cDNA library was designed in a previous study (13). Several antigen candidates were isolated, and one of them, named secreted antigen 1 of B. gibsoni (BgSA1), was identified and evaluated as a useful antigen for further serologically diagnostic tests (13). Circulating BgSA1 could be detected in the plasma of a dog infected with B. gibsoni. In addition, the ELISA using the recombinant BgSA1 expressed in Escherichia coli showed advantages in sensitivity and specificity when it was used in field samples.
In this study, we describe the identification of another member of secreted antigens, which share homology with BgSA1, here designated as secreted antigen 3 of B. gibsoni (BgSA3). The gene encoding BgSA3 was isolated from a cDNA library by immunoscreening with serum from a dog experimentally infected with B. gibsoni. As expected, the native BgSA3 also circulated in the bloodstream of the dogs infected with B. gibsoni. Our data indicate that BgSA3 could be useful as an antigenic marker of active B. gibsoni infection.
MATERIALS AND METHODS
Immunoscreening of a cDNA expression library.
A cDNA expression library constructed from B. gibsoni merozoite mRNA was used for immunoscreening (9). The library was plated on a total of 15 plates at a concentration of approximately 20,000 PFU per plate to lift plaques. The plaques were transferred to nitrocellulose membranes and screened with serum from a dog infected with B. gibsoni according to the protocol of the picoBlue immunoscreening kit (Stratagene, San Diego, CA). After an in vivo excision, the cDNA inserts in the positive clones were transferred into pBluescript phagemids and then sequenced with M13 forward, reverse, and internal DNA primers by using an automated sequencer (ABI PRISM 3100 genetic analyzer; Applied Biosystems, Foster City, CA). A cDNA clone encoding a protein sharing homology with the previously identified BgSA1 was chosen and designated as secreted BgSA3 and subjected to further analysis.
Southern blotting.
Southern blot analysis was performed as described previously (5). Briefly, 10 μg of B. gibsoni genomic DNA extracted from the infected red blood cells was digested with relative restriction enzymes and then separated on a 0.8% agarose gel. The DNA fragments were transferred to a nylon membrane (Hybond-N+; Amersham-Buchler, Munich, Germany) and hybridized with a cDNA probe labeled with alkaline phosphate using an AlkPhos Direct kit (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom).
Expression and purification of recombinant BgSA3.
The cDNA fragment of the BgSA3 without a signal peptide was inserted into E. coli expression vector pGEX-4T-3 (Amersham Pharmacia Biotech, Piscataway, NJ). The resulting plasmid was designated as pGEX-4T-3/BgSA3 after it was identified by restriction enzyme analysis and sequencing. The recombinant protein fused with a glutathione S-transferase (GST) tag was expressed in the E. coli BL21 strain according to the manufacturer's instructions (Amersham Pharmacia Biotech, Piscataway, NJ). Purification of recombinant BgSA3 (rBgSA3) was performed with glutathione-Sepharose 4B beads (Amersham Pharmacia Biotech, Piscataway, NJ) according to the manufacturer's instructions.
Preparation of rabbit and mouse sera against BgSA3.
Two Japanese white rabbits were immunized subcutaneously with 1 mg of purified rBgSA3 or rGST in Freund's complete adjuvant (Difco Laboratories, Detroit, MI) for the first injection. Five hundred micrograms of the same antigen in Freund's incomplete adjuvant (Difco) was subcutaneously injected into the rabbits on days 14 and 28. For the preparation of mouse antiserum (DDY mice, 6 weeks old), 100 μg and 50 μg of rBgSA3 were used for the first immunization intraperitoneally and for boosting on days 14 and 28, respectively. Sera samples were collected 14 days after the last immunization.
IFAT and confocal laser microscopic observation.
A thin blood smear prepared with B. gibsoni-infected red blood cells was fixed with a mixture of methanol and acetone (vol/vol, 1:20) at −20°C for 40 min as described previously (13). Briefly, the anti-rBgSA3-specific mouse serum was applied as the first antibody on the fixed smears and incubated for 30 min at 37°C. After three washings with phosphate-buffered saline (PBS), Alexa Fluor 488-conjugated goat anti-mouse immunoglobulin G (IgG) (Molecular Probes, Inc., Eugene, OR) was subsequently applied as a secondary antibody and incubated for another 30 min at 37°C. The slides were washed three times with PBS and incubated with 6.25 μg/ml propidium iodide (Wako, Osaka, Japan) containing 50 μg/ml RNase A (Qiagen, Gaithersburg, MD) for 10 min at 37°C. After two washings with PBS, the glass slides were mounted by adding 200 μl of a 50% (vol/vol) glycerol-PBS solution and covering with a glass coverslip. The slides were examined under a confocal laser scanning microscope (TCS NT; Leica, Wetzlar, Germany).
Western blotting.
B. gibsoni-infected dog erythrocytes and normal dog erythrocytes were treated with a 0.83% NH4Cl solution and then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The gel was electrically transferred to a nitrocellulose membrane, and Western blotting was carried out as described previously (23).
ELISA.
For indirect ELISA, 50 μl of purified rBgSA3 diluted in a coating buffer (a 0.05 M carbonate-bicarbonate buffer, pH 9.6) was used to coat the individual wells of 96-well microtiter plates. The rGST diluted in the same buffer was also coated on the plates as a control. The mean absorbance values of rGST were subtracted from the mean absorbance values of rBgSA3. The cutoff value of 0.1 was calculated from the results of the ELISA of 27 specific-pathogen-free (SPF) dog sera samples as follows: 0.051 (mean value) + 3 × 0.015 (standard deviation). The ELISA was performed as described previously (13). Sera samples used for ELISA were as follows: 16 samples from dogs experimentally infected with B. gibsoni; 3 samples from dogs experimentally infected with B. canis canis; 3 samples from dogs experimentally infected with B. canis rossi; 2 samples from dogs experimentally infected with B. canis vogeli; 3 samples from dogs experimentally infected with Leishmania infantum; 2 samples from Neospora caninum; 27 samples from SPF dogs; and serial serum samples from a dog experimentally infected with B. gibsoni.
Double-antibody sandwich ELISA.
The sandwich ELISA was performed as previously described (13). Briefly, rabbit anti-rBgSA3 polyclonal IgG was purified using Econo-Pac A columns (Bio-Rad Laboratories, Hercules, CA) following the manufacturer's guidelines. One microgram of the IgG diluted in a 0.05 M carbonate buffer (pH 9.6) was used as the capture antibody to coat microtiter plates at 4°C overnight, and purified rabbit anti-GST IgG was used as the control antibody. Blocking was performed with a blocking solution (3% skim milk in PBS, pH 7.2) at 37°C for 2 h. The plates were incubated at 37°C for 30 min with 50 μl of each of the serial plasma samples from a dog experimentally infected with B. gibsoni. After washing six times with PBS-Tween 20, mouse anti-rBgSA3 polyclonal serum diluted in a blocking solution was added in each well as a detection antibody. After washing six times again, the plates were incubated with 50 μl per well of horseradish peroxidase-conjugated goat anti-mouse IgG (Bethyl Laboratories, Montgomery, TX) diluted in a blocking solution. Binding was visualized with 100 μl per well of a substrate solution [0.3 mg/ml of 2,2′-azino-bis-(3-ethylbenz-thiazoline-6-sulfonic acid), 0.1 M citric acid, 0.2 M sodium phosphate, and 0.003% H2O2]. The absorbance at 415 nm was measured using an MTP-500 microplate reader (Corona Electric, Tokyo, Japan).
All experiments described in this article were conducted in accordance with the Guiding Principles for the Care and Use of Research Animals promulgated by Obihiro University of Agriculture and Veterinary Medicine.
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper was submitted to the DDBJ/EMBL/GenBank nucleotide sequence databases with the accession number AB481149.
RESULTS
Identification of the BgSA3 gene.
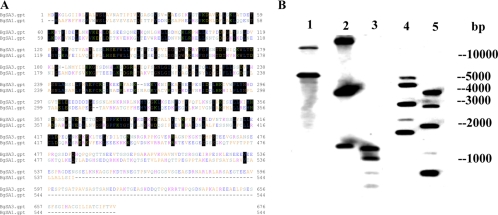
The cDNA sequence of BgSA3 was completely sequenced and analyzed using computer software (GENETYX version 7.0; Software Development, Tokyo, Japan). The full-length of BgSA3 contains a single open reading frame of 2,028 nucleotides encoding a polypeptide of 676 amino acid residues. The hydrophobic region at the N terminus of BgSA3 clearly shows the characteristics of a signal peptide, and the most likely cleavage site was predicted to be between 21 and 22 amino acids. The molecular mass of the mature protein with 655 amino acid residues is 72.2 kDa, as calculated with MacVector. BLAST analysis of the sequence against all nonredundant databases accessed through NCBI revealed significant scores with BgSA1 (13). The identity between the two antigens was 28.57% by alignment with the ClustalW program of GENETYX software version 7.0 (Fig. 1A). Southern blot analysis indicated that the BgSA3 gene seemed to exist in a multicopy form in the genome of B. gibsoni (Fig. 1B). However, further study is still necessary to confirm this point.
FIG. 1.
(A) Comparison of the predicted amino acid sequence of BgSA3 with BgSA1 (GenBank accession no. AB246895). Conservation between amino acids is indicated by boxes. (B) Southern blot analysis. The B. gibsoni genomic DNA was digested with SphI (lane 1), KpnI (lane 2), HinfI (lane 3), AccI (lane 4), and HindII (lane 5). There is one cleavage site each for KpnI, HinfI, AccI, and HindII and no SphI cleavage site within the probes. The blot from lane 1 to lane 3 was hybridized with a 350-bp probe, and that from lane 4 to lane 5 was hybridized with a 1,000-bp probe.
Expression of BgSA3 in E. coli.
The BgSA3 gene was cloned into the prokaryotic expression vector pGEX-4T-3, and the resulting plasmid was transformed into an E. coli BL21 strain. A 99-kDa recombinant protein fused with GST was expressed, as expected (data not shown). Sera samples from dogs experimentally infected with B. gibsoni could recognize the GST-fused BgSA3 in Western blotting (data not shown). This result suggested that the rBgSA3 expressed in E. coli maintained its antigenicity. In addition, specific antibodies against rBgSA3 were induced in both mice and rabbits with immunization of rBgSA3 expressed in E. coli (data not shown).
Identification of the native BgSA3 of B. gibsoni.
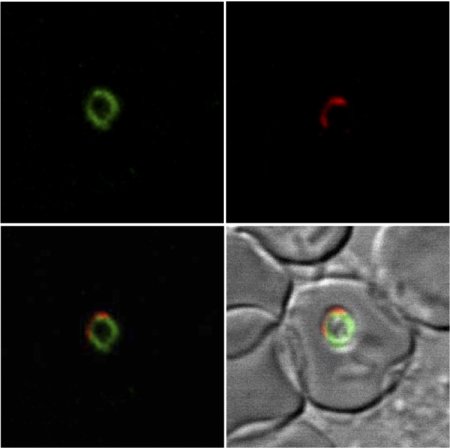
A mouse anti-rBgSA3 polyclonal serum was used to identify the native BgSA3 in the lysate of B. gibsoni parasites. As shown in Fig. 2, a specific band with a size of around 70 kDa was detected in B. gibsoni-infected red blood cells by Western blotting but not in normal ones. In order to determine the cellular localization of BgSA3, a thin blood smear was used to perform IFAT with the mouse anti-rBgSA3 serum and observed under a confocal laser microscope; the specific fluorescence seemed to localize in the cytoplasm of B. gibsoni merozoites (Fig. 3).
FIG. 2.
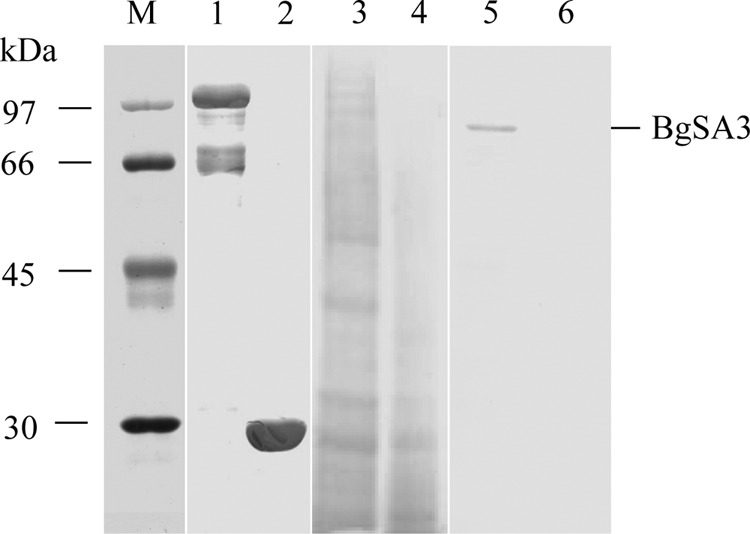
SDS-PAGE and Western blot analysis of BgSA3. The samples were separated on a 12% polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane. Lanes 1 and 2, purified rBgSA3 and rGST; lane 3, B. gibsoni-infected dog erythrocyte lysate; lane 4, normal dog erythrocyte lysate; and lanes 5 and 6, the same sample with lanes 3 and 4. Lanes 1 to 4 were SDS-PAGE, while lanes 5 and 6 were Western blot analysis probed with an anti-rBgSA3 mouse serum.
FIG. 3.
Observation of an antigen recognized by a mouse anti-rBgSA3 serum in confocal laser micrographs. (Top left) Immunofluorescent staining of B. gibsoni merozoites with a mouse anti-rBgSA3 serum. (Top right) Propidium iodide staining of B. gibsoni merozoite nuclei. (Bottom left) The top left panel overlaid on the top right panel. (Bottom right) The top left and top right panels overlaid on phase-contrast images of B. gibsoni merozoites. The images were derived from a single section.
Evaluation of recombinant BgSA3 in an ELISA for the detection of a specific antibody.
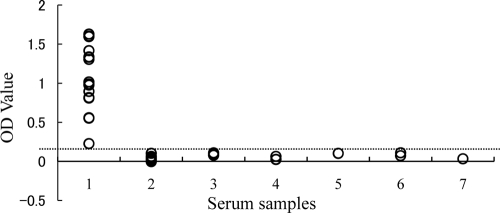
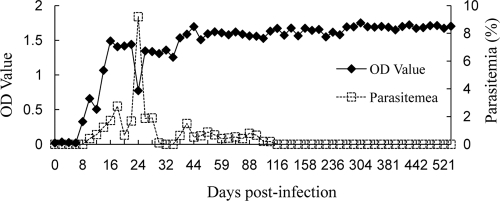
The potential of recombinant BgSA3 as a diagnostic antigen was evaluated in an indirect ELISA (BgSA3-ELISA). All 16 serum samples from B. gibsoni-infected dogs were positive (optical density of >0.1), whereas the serum samples from the uninfected dogs and the dogs infected with B. canis canis, B. canis vogeli, B. canis rossi, N. caninum, and L. infantum were negative (optical density of <0.1) (Fig. 4). A specific antibody against BgSA3 could be detected on the eighth day postinfection. The antibody level was maintained until 541 days postinfection, even when the infection was in a chronic stage, which is characterized by a recovering hematocrit value (data not shown) and a significantly low level of parasitemia (Fig. 5).
FIG. 4.
Values of the ELISA with experimentally infected dog sera. Serum samples: 1, sera from B. gibsoni-infected dogs (n = 16); 2, SPF dog sera (n = 27); 3, sera from B. canis canis-infected dogs (n = 3); 4, sera from B. canis rossi-infected dogs (n = 3); 5, sera from B. canis vogeli-infected dogs (n = 2); 6, sera from N. caninum-infected dogs (n = 2); and 7, sera from L. infantum-infected dogs (n = 3). OD, optical density.
FIG. 5.
Detection of antibody against BgSA3 in a dog experimentally infected with B. gibsoni by ELISA using the rBgSA3. OD, optical density.
Evaluation of double-antibody sandwich ELISA for the detection of circulating BgSA3.
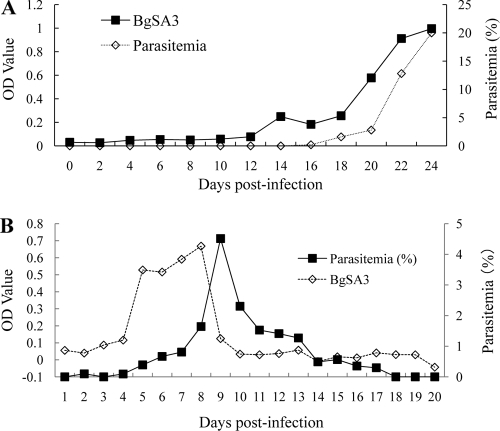
Serial blood plasma samples from an asplenic dog and a nonsplenectomized dog experimentally infected with B. gibsoni were detected using a double-antibody sandwich ELISA. The circulating BgSA3 was detectable in the infected plasma of the asplenic dog with a parasitemia of 0.2%, and the sandwich ELISA titer of the samples was well associated with the parasitemia postinfection (Fig. 6A). As expected, the circulating BgSA3 was also detectable in the plasma of the nonsplenectomized dog infected with B. gibsoni (Fig. 6B).
FIG. 6.
Detection of circulating BgSA3 in plasma from a splenectomized dog (A) and a nonsplenectomized dog (B) experimentally infected with B. gibsoni by a double-antibody sandwich ELISA.
DISCUSSION
During the asexual stage, a broad variety of soluble antigens are probably secreted into the bloodstreams of hosts. These circulating antigens released by parasites might be employed as diagnostic targets in serological tests to predict the total parasite biomass or the infection status of the infected dogs. In malaria, circulating antigens have been successfully used to develop serological tests to detect antigens, and several of them have already been used in the development of a model to estimate the total parasite biomass (1, 7, 8) and in a commercial ELISA kit to test the sensitivity of a drug against Plasmodium in a sandwich ELISA (19).
In a previous study, BgSA1 was demonstrated to be circulating in the plasma of B. gibsoni-infected dogs and evaluated as a diagnostic target for the detection of antibodies or antigen in serological tests (13). In a double-antibody sandwich ELISA, the native BgSA1 could be detected in the plasma of an asplenic dog infected with B. gibsoni when the parasitemia reached 0.2% after infection. However, only the samples in the acute phase of infection in an asplenic dog were used. In the present study, samples in both the acute and chronic stages of infection from a nonsplenectomized dog were used in the sandwich ELISA to determine whether the antigen in plasma can reflect the infection status or parasite burden in infected dogs. Our data indicated that the secreted BgSA3 could also be detected in the plasma from an infected nonsplenectomized dog, although the sandwich ELISA data did not match the parasitemia very well. The peak of circulating BgSA3 appeared earlier than the peak of parasitemia. This might be because BgSA3 could be secreted into the plasma when the parasite growth was not inhibited by any factors in the early stage but the expression or secretion of BgSA3 was inhibited by the immune response induced in the late stage.
In our study, the same samples from the asplenic dog were used in both sandwich ELISAs, which were used to detect BgSA1 and BgSA3. Although higher optical density values were obtained from the latter one than from the former one, it is still necessary to determine which antigen is better to be used as a target for a detection assay in further studies. In addition, the sensitivity of the sandwich ELISA might be improved by using monoclonal antibodies instead of polyclonal antibodies or combining this system with an avidin/biotin system (2, 16).
The recombinant BgSA3 expressed in E. coli was also evaluated in an ELISA as an antigen for the detection of a specific antibody against B. gibsoni in dogs. The results indicated that the ELISA with recombinant BgSA3 could be a useful method for the detection of the antibody in both acutely and chronically infected dogs. However, more comprehensive studies are still necessary to optimize this assay, including standardization of antigen preparation and determination of the cutoff value with a large amount of samples.
In short, the sensitivity and specificity of the secreted BgSA3 indicated its advantages as a target in a serodiagnostic test for the detection of both antibodies and antigens. On the other hand, the released proteins from rhoptries and micronemes of Babesia parasites, such as RAP-1, BbAMA-1, and BbTRAP, play crucial roles in the process of attachment and invasion (10, 11, 15, 25). Therefore, they might be a source of potential vaccine components, although the composition of secreted antigens is largely unknown. The culture-derived soluble parasite antigens of several Babesia species could provide protective immunity against parasite infection (3, 17, 20, 21). The secretion peak of BgSA3 that appears before the peak of parasitemia might indicate that this protein is necessary in the merozoite stage of the parasites. Our next step is to determine whether recombinant BgSA3 could be used as a vaccine to control canine B. gibsoni infection. Moreover, the characterization of the novel secreted molecule might be helpful for understanding the complex conversation between the host cells and the parasites.
Acknowledgments
This work was supported by a grant from the 21st Century COE Program (A-1) and a Grant-in-Aid for Scientific Research, both from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
Published ahead of print on 22 April 2009.
REFERENCES
- 1.Alifrangis, M., F. T. Ihristensen, C. S. Jorgensen, A. M. Ronn, J. E. Weng, M. Chen, I. C. Bygjerg, W. Sirawaraporn, Y. Palarasah, and C. Koch. 2004. Homology building as a means to define antigenic epitopes on dihydrofolate reductase (DHFR) from Plasmodium falciparum. Malar. J. 123-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benito, A., and D. Carmena. 2005. Double-antibody sandwich ELISA using biotinylated antibodies for the detection of Echinococcus granulosus coproantigens in dogs. Acta Trop. 959-15. [DOI] [PubMed] [Google Scholar]
- 3.Beniwal, R. P., A. K. Nichani, N. K. Rakha, R. D. Sharma, and S. Sarup. 1997. An immunization trial with in vitro produced Babesia bigemina exoantigens. Trop. Anim. Health Prod. 29124s-126s. [DOI] [PubMed] [Google Scholar]
- 4.Boozer, A. L., and D. K. Macintire. 2003. Canine babesiosis. Vet. Clin. North Am. Small Anim. Pract. 33855-904. [DOI] [PubMed] [Google Scholar]
- 5.Bork, S., M. Okamura, S. Boonchit, H. Hirata, N. Yokoyama, and I. Igarashi. 2004. Identification of Babesia bovis l-lactate dehydrogenase as a potential chemotherapeutical target against bovine babesiosis. Mol. Biochem. Parasitol. 2165-172. [DOI] [PubMed] [Google Scholar]
- 6.Casapulla, R., L. Baldi, V. Avallone, R. Sannino, L. Pazzanese, and V. Mizzoni. 1998. Canine piroplasmosis due to Babesia gibsoni: clinical and morphological aspects. Vet. Rec. 142168-169. [DOI] [PubMed] [Google Scholar]
- 7.De Arruda, M. E., K. M. Collins, L. P. Hochberg, P. R. Ryan, R. A. Wirtz, and J. R. Ryan. 2004. Quantitative determination of sporozoites and circumsporozoite antigen in mosquitoes infected with Plasmodium falciparum or P. vivax. Ann. Trop. Med. Parasitol. 98121-127. [DOI] [PubMed] [Google Scholar]
- 8.Dondorp, A. M., V. Desakorn, W. Pongtavornpinyo, D. Sahassananda, K. Silamut, K. Chotivanich, P. N. Newton, P. Pitisuttithum, A. M. Smithyman, N. J. White, and N. P. Day. 2005. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med. 8e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukumoto, S., X. Xuan, Y. Nishikawa, N. Inoue, I. Igarashi, H. Nagasawa, K. Fujisaki, and T. Mikami. 2001. Identification and expression of a 50-kilodalton surface antigen of Babesia gibsoni and evaluation of its diagnostic potential in an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 392603-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaffar, F. R., A. P. Yatsuda, F. F. Franssen, and E. de Vries. 2004. Erythrocyte invasion by Babesia bovis merozoites is inhibited by polyclonal antisera directed against peptides derived from a homologue of Plasmodium falciparum apical membrane antigen 1. Infect. Immun. 722947-2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaffar, F. R., A. P. Yatsuda, F. F. Franssen, and E. de Vries. 2004. A Babesia bovis merozoite protein with a domain architecture highly similar to the thrombospondin-related anonymous protein (TRAP) present in Plasmodium sporozoites. Mol. Biochem. Parasitol. 13625-34. [DOI] [PubMed] [Google Scholar]
- 12.Inokuma, H., Y. Yoshizaki, K. Matsumoto, M. Okuda, T. Onishi, K. Nakagome, R. Kosugi, and M. Hirakawa. 2004. Molecular survey of Babesia infection in dogs in Okinawa. Jpn. Vet. Parasitol. 121341-346. [DOI] [PubMed] [Google Scholar]
- 13.Jia, H., J. Zhou, H. Ikadai, A. Matsuu, H. Suzuki, I. Igarashi, K. Fujisaki, and X. Xuan. 2006. Identification of a novel gene encoding a secreted antigen 1 of Babesia gibsoni and evaluation of its use in serodiagnosis. Am. J. Trop. Med. Hyg. 75843-850. [PubMed] [Google Scholar]
- 14.Jia, H., M. Liao, E. Lee, Y. Nishikawa, H. Inokuma, H. Ikadai, A. Matsuu, I. Igarashi, and X. Xuan. 2007. Development of an immunochromatographic test with recombinant BgSA1 for the diagnosis of Babesia gibsoni infection in dogs. Parasitol. Res. 1001381-1384. [DOI] [PubMed] [Google Scholar]
- 15.Johnson, W. C., L. E. Perryman, and W. L. Goff. 1997. Babesia bovis: identification of immunodominant merozoite surface proteins in soluble culture-derived exoantigen. Parasitol. Res. 83776-780. [DOI] [PubMed] [Google Scholar]
- 16.Khusmith, S., P. Intapan, S. Tharavanij, S. Tuntrakul, K. A. Indravijit, and D. Bunnag. 1992. Two-site sandwich ELISA for detection of Plasmodium vivax blood stage antigens using monoclonal and polyclonal antibodies. Southeast Asian J. Trop. Med. Public Health 4745-751. [PubMed] [Google Scholar]
- 17.Lewis, B. D., B. L. Penzhorn, and L. M. Lopez Rebollar. 1995. Immune responses to South African Babesia canis and the development of a preliminary vaccine. J. S. Afr. Vet. Assoc. 6661-65. [PubMed] [Google Scholar]
- 18.Miyama, T., Y. Sakata, Y. Shimada, S. Ogino, M. Watanabe, K. Itamoto, M. Okuda, A. Verdida, X. Xuan, H. Nagasawa, and H. Inokuma. 2005. Epidemiological survey of Babesia gibsoni infection in dogs in eastern Japan. J. Vet. Med. Sci. 67467-471. [DOI] [PubMed] [Google Scholar]
- 19.Noedl, H., W. H. Wernsdorfer, R. S. Miller, and C. Wongsrichanalai. 2002. Histidine-rich protein II: a novel approach to antimalarial drug susceptibility testing. Antimicrob. Agents Chemother. 461658-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schettes, T. P., J. A. Kleuskens, N. C. Scholte, J. W. Pasman, and H. J. Bos. 1994. Vaccination of dogs against Babesia canis infection using antigens from culture supernatants with emphasis on clinical babesiosis. Vet. Parasitol. 52219-233. [DOI] [PubMed] [Google Scholar]
- 21.Valentin, A., E. Precigout, M. L'Hostis, B. Carcy, A. Gorenflot, and J. Schrevel. 1993. Cellular and humoral immune responses induced in cattle by vaccination with Babesia divergens culture-derived exoantigens correlate with protection. Infect. Immun. 61734-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verdida, R. A., O. A. Hara, X. Xuan, S. Fukumoto, I. Igarashi, S. Zhang, J. Dong, H. Inokuma, H. Kabeya, Y. Sato, T. Moritomo, S. Maruyama, F. Claveria, and H. Nagasawa. 2004. Serodiagnosis of Babesia gibsoni infection in dogs by an improved enzyme-linked immunosorbent assay with recombinant truncated P50. J. Vet. Med. Sci. 661517-1521. [DOI] [PubMed] [Google Scholar]
- 23.Xuan, X., K. Maeda, T. Mikami, and H. Otsuka. 1996. Characterization of canine herpesvirus glycoprotein C expressed in insect cells. Virus Res. 4657-64. [DOI] [PubMed] [Google Scholar]
- 24.Yamane, I., J. W. Thomford, I. A. Gardner, J. P. Dubey, M. Levy, and P. A. Conrad. 1993. Evaluation of the indirect fluorescent antibody test for diagnosis of Babesia gibsoni infections in dogs. Am. J. Vet. Res. 541579-1584. [PubMed] [Google Scholar]
- 25.Yokoyama, N., M. Okamura, and I. Igarashi. 2006. Erythrocyte invasion by Babesia parasites: current advances in the elucidation of the molecular interactions between the protozoan ligands and host receptors in the invasion stage. Vet. Parasitol. 13822-32. [DOI] [PubMed] [Google Scholar]