Abstract
The present study demonstrates that the subcutaneous administration of Neospora caninum dense granule protein 7 (NcGRA7) entrapped in liposomes coated with mannotriose strongly induces the parasite-specific T-helper type 1 immune response and humoral antibody in mice. Although anti-NcGRA7 immunoglobulin G1 antibody production was induced in mice injected with NcGRA7 alone, the dams and offspring were never protected from N. caninum infection. The immunization of mice with liposome-entrapped NcGRA7 before pregnancy resulted in increased offspring survival and decreased the infection rates in the brains of dams after parasite infection at 6 to 9 days of gestation. In conclusion, oligomannose-coated liposome-entrapped NcGRA7 can be used as a new type of effective vaccine to control neosporosis.
Neosporosis, caused by an apicomplexan protozoan parasite, Neospora caninum, is a cause of infectious abortion and congenital disease in cattle worldwide (11). Transplacental transmission is the major route of N. caninum infection (7, 39), and the parasite persists in the herd over successive generations, causing significant economic losses due to abortion, decreased milk production, and the resultant culling (6, 10, 21, 46, 47). Horizontal transmission of N. caninum by oocysts that are shed by dogs has also been documented in cattle, but this transmission is not considered a major route of infection (7).
Various mouse models have been utilized to understand the host protective immune responses to N. caninum infection. A Th1-type immune response appears to be important in protection against N. caninum infection (2, 34). Gamma interferon (IFN-γ) and interleukin-12 (IL-12), known to be crucial cytokines for the development of Th1-type immunity, are important for protective immunity against acute N. caninum infection (2). Furthermore, CD4+ T-cell-depleted BALB/c mice were more highly susceptible to parasite infection than were CD8+ T-cell-depleted mice (34, 45). Studies of IFN-γ knockout mice indicated the importance of macrophage activation by IFN-γ for protective immunity (34). On the other hand, a Th2-type immune response with predominant production of humoral antibody specific for the parasite antigens is also capable of mediating protection against neosporosis (17, 18, 30, 38, 40). These observations suggest that a suitable balance in the production of Th1- and Th2-type cytokines has a crucial role in the control of N. caninum infection (33).
Oligomannose-coated liposomes have been shown to be a safe adjuvant to induce Th1-type immunity because no skin damage by the liposomes is caused at the injection site (16). A previous study showed that liposomes coated with a neoglycolopid consisting of mannotriose and dipalmitoylphosphatidylethanolamine (Man3-DPPE) were specifically and rapidly incorporated into intraperitoneal macrophages when injected into the peritoneal cavity and that the liposome-incorporating macrophages smoothly accumulated in nearby lymphoid tissue (23). The effect of Man3-coated liposome as an effective adjuvant has been confirmed with Leishmania major infection (41) and with tumors (23, 25). Administration of soluble leishmanial antigens entrapped within the Man3-coated liposomes to BALB/c mice strongly induced the antigen-specific Th1 immune response, as evidenced by a higher level of IFN-γ production and a lower level of IL-4 production than those in mice receiving the antigens alone (41).
There is accumulating evidence that some N. caninum-infected cows develop a degree of protective immunity against abortion and/or congenital transmission, indicating the advantage of vaccine development (31). Although the prevention of abortion might be a realistic goal for a vaccine, the ultimate objective for the control of neosporosis must be to prevent the vertical transmission of the parasite. Evidence has shown that cattle which abort due to neosporosis have higher levels of N. caninum-specific antibody than do infected but nonaborting cattle (9). In addition, our previous study showed that a higher level of bovine antibody specific for N. caninum dense granule protein 7 (NcGRA7) was detected in aborting than in nonaborting cows and heifers, while levels of specific antibodies against parasite surface proteins NcSAG1 and NcSRS2 exhibited no significant difference between the aborting and nonaborting cows (22). To control N. caninum infection, a suitable balance of Th1- and Th2-type immune responses is important (33). We speculated that an NcGRA7-specific Th2-type immune response might be predominant in aborting cows. Therefore, induction of the NcGRA7-specific Th1-type immune response could play a crucial role in the control of N. caninum infection, since antibodies against the parasites did not prevent vertical transmission (32). Thus, the present study was conducted to evaluate the vaccine efficacy of oligomannose-coated liposome-entrapped NcGRA7 on N. caninum infection in dams and offspring, using a BALB/c mouse model. Our results suggest that the Th1-type immune response against NcGRA7 plays a crucial role in the control of N. caninum infection.
MATERIALS AND METHODS
Cultures and purification of parasites.
Neospora caninum tachyzoites of the Nc-1 isolate (12) were maintained in monkey kidney adherent fibroblasts (Vero cells) cultured in Eagle's minimum essential medium (Sigma, St. Louis, MO) supplemented with 8% heat-inactivated fetal bovine serum. For the purification of tachyzoites, the parasites and host cell debris were washed in cold phosphate-buffered saline (PBS), and the final pellet was resuspended in cold PBS and then passed through a 27-gauge needle and a 5.0-μm-pore-size filter (Millipore, Bedford, MA).
Preparation of recombinant proteins.
The cDNAs of the coding region of NcGRA7 mRNA were obtained by reverse transcription-PCR amplification using specifically designed primer pairs, with the extracted RNA as the template. The truncated NcGRA7 (NcGRA7t) gene (26), without the sequence encoding a hydrophobic signal peptide (amino acids 1 to 25), was amplified from the cDNA by a PCR using the oligonucleotide primers 5′-ACG AAT TCC GCT GGA GAC TTG GCA-3′ and 5′-ACG AAT TCC TAT TCG GTG TCT ACT TCC-3′, which contain an EcoRI cleavage site. The PCR product was digested with EcoRI and cloned into an EcoRI site of the bacterial expression vector pGEX-4T-3 (Amersham Biosciences, Piscataway, NJ). The recombinant protein of NcGRA7t was expressed in Escherichia coli as a glutathione S-transferase (GST) fusion protein (NcGRA7t-GST) and then purified using glutathione Sepharose 4B beads (Amersham Pharmacia Biotech, Uppsala, Sweden) as described previously (19). Furthermore, endotoxins were removed from the purified protein fraction by using Detoxi-Gel endotoxin removal gel (Pierce, Rockford, IL).
Preparation of liposomes.
Liposomes were prepared as described previously (41). Briefly, a chloroform-methanol (2:1 [vol/vol]) solution containing 1.5 μmol of DPPE and 1.5 μmol of cholesterol was placed in a conical flask and then dried by rotary evaporation. Subsequently, 2 ml of chloroform containing 0.15 μmol of Man3-DPPE was added to the flask and evaporated to prepare a lipid film containing the neoglycolipid. Two hundred microliters of PBS containing the indicated recombinant protein (500 μg/ml) was added to the dried lipid film, and multilamellar vesicles were prepared by intense vortex dispersion. The multilamellar vesicles were extruded 10 times through a 1-μm-pore-size polycarbonate membrane (Nuclepore, Pleasanton, CA). Liposomes entrapping the recombinant protein were separated from free recombinant protein by three successive rounds of washing in PBS with centrifugation (20,000 × g, 30 min) at 4°C. The amount of entrapped antigen was measured using a modified Lowry protein assay reagent (Pierce) in the presence of 0.3% (wt/vol) sodium dodecyl sulfate, using bovine serum albumin as the standard.
Immunization and infection.
BALB/c mice of 6 to 7 weeks of age were purchased from Clea Japan (Tokyo, Japan). Until their use at 7 to 8 weeks of age, mice were housed under specific-pathogen-free conditions in the animal facility of the National Research Center for Protozoan Diseases at Obihiro University of Agriculture and Veterinary Medicine, Obihiro, Japan. All mice used in the present study were treated under the guiding principles for the care and use of research animals promulgated by Obihiro University of Agriculture and Veterinary Medicine. Female mice were inoculated subcutaneously with 40 nmol NcGRA7t-GST entrapped within Man3-coated liposomes (M3-NcGRA7), 40 nmol GST entrapped within Man3-coated liposomes (M3-GST), 40 nmol NcGRA7t-GST in PBS (NcGRA7), or PBS alone (100 μl each). Booster immunizations were administered 7 and 14 days after the first immunization. Seven days after the third immunization, the female mice were housed with male mice for 3 days (one female with one male per cage) and inspected twice daily for the presence of vaginal plugs. The first day on which a plug was noted was designated day 0 of pregnancy for each individual. All pregnant dams were challenged on the same day with 1 × 105 Nc-1 tachyzoites of N. caninum at 6 to 9 days of gestation. Numbers and survival rates of the offspring were measured for 30 days after birth. Sera (20 μl) were obtained via the tail vein from mice 7, 14, and 21 days after the immunization for measurements of N. caninum-specific antibodies by enzyme-linked immunosorbent assay (ELISA). To confirm the lack of an antibody response in unvaccinated and uninfected mice, control sera were taken from all animals on day 0, before the immunization.
DNA isolation and PCR analysis.
For DNA preparation, the brain of each dam was thawed in 10 times its volume of DNA extraction buffer (0.1 M Tris-HCl, pH 9.0, 1% sodium dodecyl sulfate, 0.1 M NaCl, and 1 mM EDTA) with 1 mg/ml of proteinase K at 55°C. The DNA was purified by phenol-chloroform extraction and subsequent ethanol precipitation. The DNA concentration was adjusted to 100 μg/ml for each brain, and the DNA was used as a template DNA for PCR analysis. The template DNA (2.5 μl) was suspended in 10 μl of the final reaction mixture, containing 1 μl of a 10× PCR buffer with 15 mM MgCl2 (Perkin Elmer, Waltham, MA), 1 μl of 10 mM deoxynucleoside triphosphate mix (Invitrogen, Carlsbad, CA), 0.1 μl of 5-U/μl Ampli GoldTaq DNA polymerase (Perkin Elmer), and 2 μl of 10-pmol/μl N. caninum-specific primers Np6 and Np21 (29). PCR amplification was conducted in a GeneAmp PCR 2400 thermal cycler (Perkin Elmer), employing 40 cycles of denaturation (94°C, 1 min), annealing (63°C, 1 min), and primer extension (74°C, 3.5 min). At the end of the reaction cycles, primer extension was continued for 10 min at 74°C, and products were then kept at 4°C. The PCR products were visualized by electrophoresis of agarose gels. To confirm the specificity of PCR, DNAs from the brain of an uninfected mouse and from purified N. caninum tachyzoites were used as negative and positive controls, respectively.
Measurement of N. caninum-specific antibodies.
N. caninum-specific immunoglobulin G (IgG) levels were measured in mouse sera by ELISA. A total lysate antigen of N. caninum tachyzoites (NLA), prepared as previously reported (27), was adjusted to a concentration of 5 μg/ml with a carbonate-bicarbonate buffer (pH 9.6), and 50 μl of the NLA solution was used to coat the ELISA plates (Nunc, Roskilde, Denmark) overnight at 4°C. After being blocked with PBS containing 3% skim milk (PBS-SM) for 1 h at 37°C, the plates were washed twice with PBS containing 0.05% Tween 20 (PBS-T), and 100 μl of the serum samples, diluted 1:100 with PBS-SM, was added to duplicate wells. Plates were incubated at 37°C for 1 h. After being washed five times with PBS-T, the plates were incubated with horseradish peroxidase-conjugated goat anti-mouse IgG1 and IgG2a (Bethyl Laboratories, Montgomery, TX) diluted 1:4,000 with PBS-SM at 37°C for 1 h. The plates were washed five times, and then a substrate solution (0.1 M citric acid, 0.2 M sodium phosphate, 0.003% H2O2, and 0.3 mg/ml 2,2′-azide-bis [3-ethylbenzthiazoline-6-sulfonic acid]; Sigma) was added to each well in 100-μl aliquots. The absorbance at 415 nm was read after 1 h of incubation at room temperature by using an ELISA reader (Corona MTP-120 microplate reader; Corona, Tokyo, Japan). The ELISA result was determined by measuring the mean optical density at a wavelength of 415 nm. To confirm the specificity of ELISA, sera from uninfected and N. caninum-infected mice were used as negative and positive controls, respectively.
In vitro stimulation of spleen cells.
A single-cell suspension was prepared from a spleen and hemolysed in a lysing buffer (0.83% NH4Cl and 0.01 M Tris-HCl, pH 7.2). After being washed with PBS, the cells were plated into 96-well microplates at 3 × 105/200 μl/well in RPMI 1640 medium (Sigma) supplemented with 5% fetal bovine serum. The cells were stimulated by adding 10 μg/ml of purified NcGRA7t-GST or 50 μg/ml of NLA. As a control heterogeneous protein, 10 μg/ml of purified recombinant N. caninum apical membrane antigen 1 (NcAMA1) fused with GST (49) was also used. After incubation for 48 h at 37°C, the supernatants of cultures were collected and assayed for cytokines. The levels of IFN-γ and IL-4 were quantified using commercial ELISA kits (Pierce). To confirm the specificity of ELISA, culture medium and recombinant cytokines were used as negative and positive controls, respectively.
RESULTS
Measurement of N. caninum-specific antibody production in immunized dams.
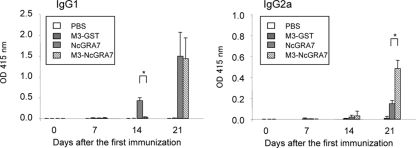
To investigate the effects of Man3-coated liposome on N. caninum-specific antibody production, ELISA using NLA was carried out (Fig. 1). Mice immunized with NcGRA7 alone immediately produced parasite-specific IgG1 antibody after the second immunization. The parasite-specific IgG1 antibody was detected in mice immunized with M3-NcGRA7 only after the third immunization. Twenty-one days after the first immunization, the level of the parasite-specific IgG2a antibody was higher in mice immunized with M3-NcGRA7 than in mice immunized with NcGRA7 alone, while similar levels of the parasite-specific IgG1 antibody were detected in those two groups. Production of parasite-specific antibody was not observed in the groups receiving PBS and M3-GST immunization. These results suggested that immunization with M3-NcGRA7 induced both Th1 and Th2 immune responses against N. caninum, while NcGRA7 immunization showed a predominantly Th2 immune response.
FIG. 1.
N. caninum-specific antibody responses of mice vaccinated with Man3-coated liposomes. Five mice per group were injected subcutaneously with PBS, NcGRA7t-GST in PBS (NcGRA7), NcGRA7t-GST entrapped within Man3-coated liposomes (M3-NcGRA7), or GST entrapped within Man3-coated liposomes (M3-GST) at days 0, 7, and 14. ELISA using NLA measured the parasite-specific IgG1 and IgG2a antibodies in sera. The values are expressed as optical densities at 415 nm. Each bar represents the mean ± standard deviation (SD) for five mice per group. Results are representative of two independent experiments. *, statistically significant differences were observed between the two groups immunized with NcGRA7 and M3-NcGRA7 by Student's t test on the same day after immunization (P < 0.01).
N. caninum-specific cytokine responses of spleen cells.
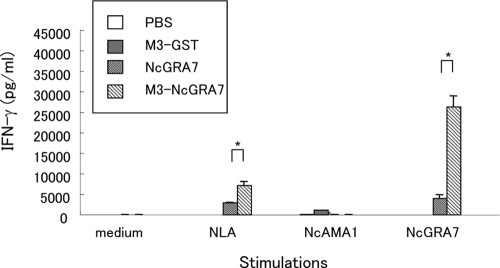
To confirm whether immunization with M3-NcGRA7 induced the Th1 immune response, the production of Th1 (IFN-γ) and Th2 (IL-4) cytokines was measured following in vitro stimulation of spleen cells obtained from mice after the third immunization (Fig. 2). The production of IFN-γ was higher in the spleen cells of mice immunized with M3-NcGRA7 than in cells of animals immunized with NcGRA7 alone for stimulation with NLA or NcGRA7 (P < 0.005). Although IL-4 production was also detectable in the spleen cells of mice immunized with M3-NcGRA7, the amount of this cytokine was relatively low (<50 pg/ml) (data not shown). These results indicated that immunization with M3-NcGRA7 triggered parasite- and antigen-specific Th1 immune responses in mice.
FIG. 2.
Production of IFN-γ from spleen cells. Three mice per group were injected subcutaneously with PBS, NcGRA7t-GST in PBS (NcGRA7), NcGRA7t-GST entrapped within Man3-coated liposomes (M3-NcGRA7), or GST entrapped within Man3-coated liposomes (M3-GST) at days 0, 7, and 14 and then sacrificed at day 21. Single-cell suspensions were prepared from the spleens of individual mice in the groups and cultured for 48 h in the presence of 50 μg/ml NLA, 10 μg/ml NcAMA1, or 10 μg/ml NcGRA7 or without any stimulator (medium). Culture supernatants were assayed for IFN-γ production by ELISA. Each bar represents the mean ± SD for three mice per group. Results are representative of two independent experiments. *, statistically significant differences were observed between the two groups immunized with NcGRA7 and M3-NcGRA7 by Student's t test on the same day of immunization (P < 0.01).
Survival rates of offspring in vaccination trials.
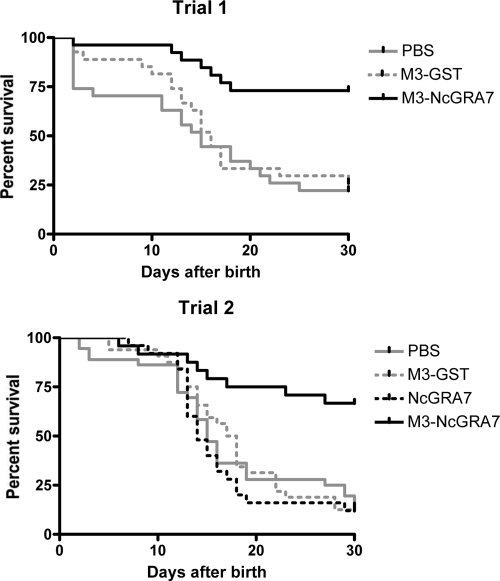
The numbers of offspring in two vaccination trials are summarized in Table 1. No difference in the number of offspring was seen between the group receiving immunization with NcGRA7 alone or with M3-NcGRA7 and the group receiving PBS. These results indicate that immunization with M3-NcGRA7 has no effect on the birth rate. Next, the survival rates of offspring were examined for 30 days after birth (Table 1). The survival rates for the groups immunized with M3-GST and NcGRA7 (18.6 and 12.0%, respectively) were similar to that for the group receiving PBS (17.5%). However, the survival rates were increased by vaccination with M3-NcGRA7 (68.6%; P < 0.01). For the groups receiving PBS, M3-GST, and NcGRA7 immunizations, mice succumbed to infection about 10 to 20 days after birth (Fig. 3). The dying offspring showed clinical neosporosis, such as walking disorders, a rounded back, paralysis of the hind limbs, and hypoplasia, while animals surviving for more than 30 days after birth had no symptoms. These results showed that a combination of Man3-coated liposome and NcGRA7 protein induced protective efficacy to prevent neonatal mortality.
TABLE 1.
Survival rates of offspringa
| Group | Trial | No. of litters | Mean no. of offspring/litter (SD) | No. of surviving offspring/no. of offspring in litter | Total no. of surviving offspring/total no. of offspring (%) |
|---|---|---|---|---|---|
| PBS | 1 | 4 | 6.8 (2.6) | 2/3, 2/7, 0/8, 2/9 | 6/27 (22.2) |
| 2 | 5 | 7.2 (2.2) | 1/5, 0/8, 0/10, 4/8, 0/5 | 5/36 (13.4) | |
| Total | 9 | 7.0 (2.3) | 11/63 (17.5) | ||
| M3-GST | 1 | 4 | 6.8 (1.7) | 0/7, 3/6, 1/9, 3/5 | 7/27 (25.9) |
| 2 | 5 | 6.4 (1.3) | 0/7, 3/5, 0/7, 1/8, 0/5 | 4/32 (12.5) | |
| Total | 9 | 6.6 (1.4) | 11/59 (18.6) | ||
| NcGRA7 | 2 | 4 | 6.3 (2.6) | 2/8, 0/9, 1/4, 0/4 | 3/25 (12.0) |
| M3-NcGRA7 | 1 | 4 | 6.5 (1.3) | 5/7, 8/8, 3/6, 3/5 | 19/26 (73.1) |
| 2 | 5 | 5.0 (2.6) | 2/4, 0/2, 5/6, 7/9, 2/4 | 16/25 (64.0) | |
| Total | 9 | 5.7 (2.2) | 35/51 (68.6)* |
Female mice were immunized subcutaneously with NcGRA7t-GST entrapped within Man3-coated liposomes (M3-NcGRA7), GST entrapped within Man3-coated liposomes (M3-GST), NcGRA7t-GST in PBS (NcGRA7), or PBS. Booster immunizations were administered 7 and 14 days after the first immunization. Seven days after the third immunization, the mice were housed with males for 3 days. All pregnant dams were challenged on the same day with 1 × 105 Nc-1 tachyzoites, at 6 to 9 days of gestation. For the survival rates of offspring at 30 days after birth, statistical differences versus the PBS group were determined by χ2 test (*, P < 0.01).
FIG. 3.
Survival rates of offspring in trials 1 and 2. Female mice were immunized subcutaneously with NcGRA7t-GST entrapped within Man3-coated liposomes (M3-NcGRA7), GST entrapped within Man3-coated liposomes (M3-GST), NcGRA7t-GST in PBS (NcGRA7), or PBS. Booster immunizations were administered 7 and 14 days after the first immunization. Seven days after the third immunization, the mice were housed with males for 3 days. All pregnant dams were challenged on the same day with 1 × 105 tachyzoites, at 6 to 9 days of gestation. Numbers of offspring were monitored for 30 days after birth.
Detection of parasite DNA in the brains of dams.
PCR detection allows for examination of tissue localization of N. caninum. Although N. caninum DNA was detected in the brains, lungs, livers, and spleens of BALB/c mice until 6 days after the infection, parasite DNA was found almost exclusively in the brain 10 and 26 days after the infection (35). To confirm the existence of the parasite, we examined the brains of dams 40 days after the infection, using PCR. Immunization with M3-NcGRA7 inhibited the N. caninum burden in the brains of dams (33.3%), while the parasite was detected in all animals of the other groups (Table 2) (P < 0.01). For the group receiving M3-NcGRA7 immunization, the survival rates of offspring from dams with a negative N. caninum PCR (73.5%) were higher than those of offspring from PCR-positive animals (58.8%), though there were no statistical differences (P = 0.286 by χ2 test). Thus, M3-NcGRA7-induced immune responses could control parasite infection in dams.
TABLE 2.
PCR detection of N. caninum in damsa
| Group | Trial | No. of positive dams/total no. of dams (%) |
|---|---|---|
| PBS | 1 | 4/4 (100) |
| 2 | 5/5 (100) | |
| Total | 9/9 (100) | |
| M3-GST | 1 | 4/4 (100) |
| 2 | 5/5 (100) | |
| Total | 9/9 (100) | |
| NcGRA7 | 2 | 4/4 (100) |
| M3-NcGRA7 | 1 | 1/4 (25) |
| 2 | 2/5 (40) | |
| Total | 3/9 (33.3)* |
Female mice were immunized subcutaneously with NcGRA7t-GST entrapped within Man3-coated liposomes (M3-NcGRA7), GST entrapped within Man3-coated liposomes (M3-GST), NcGRA7tGST in PBS (NcGRA7), or PBS. Booster immunizations were administered 7 and 14 days after the first immunization. Seven days after the third immunization, the mice were housed with males for 3 days. All pregnant dams were challenged on the same day with 1 × 105 Nc-1 tachyzoites, at 6 to 9 days of gestation. DNAs were extracted from the brains of dams 40 days after infection and were then analyzed by PCR. Statistical differences versus the PBS group were determined by χ2 test (*, P < 0.01).
DISCUSSION
After apicomplexan parasites, including N. caninum, penetrate host cells, they establish a parasitophorous vacuole (PV) in the infected cells, and the protein contents of the parasite's dense granule organelles, named GRA proteins, are released into the PV (4). The GRA proteins are specially targeted to the PV membrane or the vacuolar space. NcGRA7, identified as a dense granule-associated protein in N. caninum (20, 26), was also targeted to the PV membrane and found in the PV space (20). In another apicomplexan parasite, Toxoplasma gondii, the host is immunologically exposed to the GRA proteins during infection because they are excreted from the infected cells or the infected cells rupture as a result of overwhelming parasite infection (15). Consequently, the GRA proteins often function as immunodominant antigens, and the specific antibodies are detected in the sera of infected animals. In fact, antibody against NcGRA7 was also detected in naturally infected cows (22).
In the present study, we demonstrated that subcutaneous immunization with M3-NcGRA7 induces a protective response against N. caninum infection in dams and that this achievement is caused by a strong induction of an NcGRA7-specific Th1 immune response. Induction of the Th1 immune response is the key event in the control of neosporosis (2, 42). In the present study, the administration of NcGRA7 protein alone induced the production of antigen-specific humoral antibody. However, these mice could not control the subsequent infection in both dams and offspring. This result suggests that anti-NcGRA7 IgG1 antibody does not contribute to protective immunity against N. caninum infection. In contrast, when the same amount of NcGRA7 was entrapped in Man3-coated liposomes, the immune response dramatically leaned toward a Th1-type immune response, as assessed by in vitro cytokine production, resulting in the effective control of parasite infection. Moreover, the higher level of parasite-specific IgG2a in the mice immunized with M3-NcGRA7 than in those immunized with NcGRA7 alone also demonstrated the induction of a Th1 immune response, as Th1 cells are known to induce IgG2a secretion (43).
A previous study has shown that oligomannose-coated liposomes are preferentially incorporated into macrophages (41). Since the macrophage mannose receptor (CD206) is known to be expressed mainly on macrophages (13), the action of oligomannose-coated liposomes is believed to be caused by their facilitation of antigen delivery to macrophages as a result of interaction between CD206 and oligomannose exposed on the liposomes. In addition, a recent study showed that specific ICAM-3-grabbing nonintegrin-related protein 1 (SIGNR1) and complement receptor type 3 (CR3) play a crucial role in the uptake of oligomannose-coated liposomes by macrophages (44). Uptake of the antigen-entrapping oligomannose-coated liposomes by macrophages would have been an initial key event in the induction of the antigen-specific Th1 immune response. Because the activation of macrophages with IFN-γ plays a crucial role in eliciting the N. caninum-specific Th1 response (34), administration of oligomannose-coated liposome-entrapped NcGRA7 induced protective immunity against subsequent infection.
Previous studies have shown that immune responses to NcGRA7 have a protective role in preventing the vertical transmission of N. caninum in a pregnant mouse model using plasmid DNA, as well as acute fatal infection of N. caninum in a gerbil model using a recombinant NcGRA7 protein expressed by E. coli (5, 24, 28). In contrast, Brucella abortus vaccine strain RB51 expressing the NcGRA7 gene did not show any vaccine efficacy against cerebral infection with N. caninum (48). These results suggest that suitable vaccine vehicles and/or adjuvants are necessary to induce protective immunity against N. caninum infection. In addition to these trials of recombinant vaccines, our group has demonstrated the induction of protective immunity by using a recombinant vaccinia virus expressing the NcSRS2 gene (35, 36). Searching for suitable vaccine antigens and adjuvants to control neosporosis, other groups have also examined the effects of a microneme protein, NcMIC3, mixed with a Ribi adjuvant system (3); NcSRS2; a rhoptry protein, NcROP2, mixed with Freund's incomplete adjuvant (8, 18); NcSRS2 in ISCOMs, which are submicroscopic cage-like particles comprising the target antigen, the saponin Quil A, cholesterol, and phospholipids (37, 38); NcGRA7 plus CpG (24); and GRA1, GRA2, MIC10, and p24B in VSA3 (14). In order to optimize dendritic cell processing, the NcSRS2 DNA vaccine was delivered with granulocyte-macrophage colony-stimulating factor and Flt3 ligand adjuvant. In addition, the synthesized NcSRS2 peptides were coupled with a palmitic acid molecule and delivered with Freund's adjuvant. Cattle vaccinated with the NcSRS2 DNA vaccine and boosted with NcSRS2 lipopeptide induced a Th1-type immune response against N. caninum antigen (1). For the application of a vaccine for animal use, however, we must consider the pathogenicity and side effects of the recombinant vaccines and adjuvants. Since oligomannose-coated liposomes consist of innocuous materials distributed ubiquitously throughout the body and cause no damage to the skin at the injection site, they would be highly suitable for use as a safe adjuvant and vehicle in a vaccine (16).
The present study indicated that immunization with oligomannose-coated, liposome-entrapped NcGRA7 could elicit a Th1 immune response against subsequent infection with N. caninum. Our previous study showed that parasite DNA was not detected in surviving pups with no clinical symptoms at 30 days after birth, while the rate of PCR-positive results was 84.3% for dead pups for 30 days after birth (36). Therefore, our vaccination regimen could prevent N. caninum burdens in dams, resulting in a reduction of neonatal mortality by parasite vertical transmission. In conclusion, oligomannose-coated, liposome-entrapped NcGRA7 could be used as a novel effective vaccine to control the vertical transmission of neosporosis.
Acknowledgments
We thank J. P. Dubey (United States Department of Agriculture, Agriculture Research Service, Livestock and Poultry Sciences Institute, Parasite Biology and Epidemiology Laboratory) for the gift of the Neospora caninum NC-1 isolate.
This work was supported by a grant-in-aid for young scientists (start-up) from the Japan Society for the Promotion of Science (18880003) and by a grant for the Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN), Japan.
Footnotes
Published ahead of print on 8 April 2009.
REFERENCES
- 1.Baszler, T. V., V. Shkap, W. Mwangi, C. J. Davies, B. A. Mathison, M. Mazuz, D. Resnikov, L. Fish, B. Leibovitch, L. M. Staska, and I. Savitsky. 2008. Bovine immune response to inoculation with Neospora caninum surface antigen SRS2 lipopeptides mimics immune response to infection with live parasites. Clin. Vaccine Immunol. 15659-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baszler, T. V., M. T. Long, T. F. McElwain, and B. A. Mathison. 1999. Interferon-gamma and interleukin-12 mediate protection to acute Neospora caninum infection in BALB/c mice. Int. J. Parasitol. 291635-1646. [DOI] [PubMed] [Google Scholar]
- 3.Cannas, A., A. Naguleswaran, N. Müller, B. Gottstein, and A. Hemphill. 2003. Reduced cerebral infection of Neospora caninum-infected mice after vaccination with recombinant microneme protein NcMIC3 and ribi adjuvant. J. Parasitol. 8944-50. [DOI] [PubMed] [Google Scholar]
- 4.Cesbron-Delauw, M. F. 1994. Dense-granule organelles of Toxoplasma gondii: their role in the host-parasite relationship. Parasitol. Today 10293-296. [DOI] [PubMed] [Google Scholar]
- 5.Cho, J. H., W. S. Chung, K. J. Song, B. K. Na, S. W. Kang, C. Y. Song, and T. S. Kim. 2005. Protective efficacy of vaccination with Neospora caninum multiple recombinant antigens against experimental Neospora caninum infection. Korean J. Parasitol. 4319-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cramer, G., D. Kelton, T. F. Duffield, J. C. Hobson, K. Lissemore, S. K. Hietala, and A. S. Peregrine. 2002. Neospora caninum serostatus and culling of Holstein cattle. J. Am. Vet. Med. Assoc. 2211165-1168. [DOI] [PubMed] [Google Scholar]
- 7.Davison, H. C., A. Otter, and A. J. Trees. 1999. Estimation of vertical and horizontal transmission parameters of Neospora caninum infections in dairy cattle. Int. J. Parasitol. 291683-1689. [DOI] [PubMed] [Google Scholar]
- 8.Debach, K., C. Guionaud, F. Alaeddine, M. Mevissen, and A. Hemphill. 2008. Vaccination of mice with recombinant NcROP2 antigen reduces mortality and cerebral infection in mice infected with Neospora caninum tachyzoites. Int. J. Parasitol. 381455-1463. [DOI] [PubMed] [Google Scholar]
- 9.Dubey, J. P., and G. Schares. 2006. Diagnosis of bovine neosporosis. Vet. Parasitol. 1401-34. [DOI] [PubMed] [Google Scholar]
- 10.Dubey, J. P. 1999. Neosporosis—the first decade of research. Int. J. Parasitol. 291485-1488. [DOI] [PubMed] [Google Scholar]
- 11.Dubey, J. P., and D. S. Lindsay. 1996. A review of Neospora caninum and neosporosis. Vet. Parasitol. 671-59. [DOI] [PubMed] [Google Scholar]
- 12.Dubey, J. P., J. L. Carpenter, C. A. Speer, M. J. Topper, and A. Uggla. 1988. Newly recognized fatal protozoan disease of dogs. J. Am. Vet. Med. Assoc. 1921269-1285. [PubMed] [Google Scholar]
- 13.East, L., and C. M. Isacke. 2002. The mannose receptor family. Biochim. Biophys. Acta 1572364-386. [DOI] [PubMed] [Google Scholar]
- 14.Ellis, J., C. Miller, H. Quinn, C. Ryce, and M. P. Reichel. 2008. Evaluation of recombinant proteins of Neospora caninum as vaccine candidates (in a mouse model). Vaccine 265989-5996. [DOI] [PubMed] [Google Scholar]
- 15.Fischer, H. G., S. Stachelhaus, M. Sahm, H. E. Meyer, and G. Reichmann. 1998. GRA7, an excretory 29 kDa Toxoplasma gondii dense granule antigen released by infected host cells. Mol. Biochem. Parasitol. 91251-262. [DOI] [PubMed] [Google Scholar]
- 16.Fukasawa, M., Y. Shimizu, K. Shikata, M. Nakata, R. Sakakibara, N. Yamamoto, M. Hatanaka, and T. Mizuochi. 1998. Liposome oligomannose-coated with neoglycolipid, a new candidate for a safe adjuvant for induction of CD8+ cytotoxic T lymphocytes. FEBS Lett. 441353-356. [DOI] [PubMed] [Google Scholar]
- 17.Haldorson, G. J., J. B. Stanton, B. A. Mathison, C. E. Suarez, and T. V. Baszler. 2006. Neospora caninum: antibodies directed against tachyzoite surface protein NcSRS2 inhibit parasite attachment and invasion of placental trophoblasts in vitro. Exp. Parasitol. 112172-178. [DOI] [PubMed] [Google Scholar]
- 18.Haldorson, G. J., B. A. Mathison, K. Wenberg, P. A. Conrad, J. P. Dubey, A. J. Trees, I. Yamane, and T. V. Baszler. 2005. Immunization with native surface protein NcSRS2 induces a Th2 immune response and reduces congenital Neospora caninum transmission in mice. Int. J. Parasitol. 351407-1415. [DOI] [PubMed] [Google Scholar]
- 19.Hara, A. O., M. Liao, W. Baticados, H. Bannai, G. Zhang, S. Zhang, E. G. Lee, Y. Nishikawa, F. Claveria, M. Igarashi, H. Nagasawa, and X. Xuan. 2006. Expression of recombinant dense granule protein 7 of Neospora caninum and evaluation of its diagnostic potential for canine neosporosis. J. Protozool. Res. 1634-41. [Google Scholar]
- 20.Hemphill, A., N. Gajendran, S. Sonda, N. Fuchs, B. Gottstein, B. Hentrich, and M. Jenkins. 1998. Identification and characterisation of a dense granule-associated protein in Neospora caninum tachyzoites. Int. J. Parasitol. 28429-438. [DOI] [PubMed] [Google Scholar]
- 21.Hobson, J. C., T. F. Duffield, D. Kelton, K. Lissemore, S. K. Hietala, K. E. Leslie, B. McEwen, G. Cramer, and A. S. Peregrine. 2002. Neospora caninum serostatus and milk production of Holstein cattle. J. Am. Vet. Med. Assoc. 2211160-1164. [DOI] [PubMed] [Google Scholar]
- 22.Huang, P., M. Liao, H. Zhang, E. G. Lee, Y. Nishikawa, and X. Xuan. 2007. Dense-granule protein NcGRA7, a new marker for the serodiagnosis of Neospora caninum infection in aborting cows. Clin. Vaccine Immunol. 141640-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikehara, Y., N. Shiuchi, S. Kabata-Ikehara, H. Nakanishi, N. Yokoyama, H. Takagi, T. Nagata, Y. Koide, K. Kuzushima, T. Takahashi, K. Tsujimura, and N. Kojima. 2008. Effective induction of anti-tumor immune responses with oligomannose-coated liposome targeting to intraperitoneal phagocytic cells. Cancer Lett. 260137-145. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins, M., C. Parker, W. Tuo, B. Vinyard, and J. P. Dubey. 2004. Inclusion of CpG adjuvant with plasmid DNA coding for NcGRA7 improves protection against congenital neosporosis. Infect. Immun. 721817-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kojima, N., L. Biao, T. Nakayama, M. Ishii, Y. Ikehara, and K. Tsujimura. 2008. Oligomannose-coated liposomes as a therapeutic antigen-delivery and an adjuvant vehicle for induction of in vivo tumor immunity. J. Control Release 12926-32. [DOI] [PubMed] [Google Scholar]
- 26.Lally, N., M. Jenkins, S. Liddell, and J. P. Dubey. 1997. A dense granule protein (NCDG1) gene from Neospora caninum. Mol. Biochem. Parasitol. 87239-243. [DOI] [PubMed] [Google Scholar]
- 27.Liao, M., X. Xuan, X. Huang, H. Shirafuji, S. Fukumoto, H. Hirata, H. Suzuki, and K. Fujisaki. 2005. Identification and characterization of cross-reactive antigens from Neospora caninum and Toxoplasma gondii. Parasitology 130481-488. [DOI] [PubMed] [Google Scholar]
- 28.Liddell, S., C. Parker, B. Vinyard, M. Jenkins, and J. P. Dubey. 2003. Immunization of mice with plasmid DNA coding for NcGRA7 or NcsHSP33 confers partial protection against vertical transmission of Neospora caninum. J. Parasitol. 89496-500. [DOI] [PubMed] [Google Scholar]
- 29.Liddell, S., M. C. Jenkins, C. M. Collica, and J. P. Dubey. 1999. Prevention of vertical transfer of Neospora caninum in BALB/c mice by vaccination. J. Parasitol. 851072-1075. [PubMed] [Google Scholar]
- 30.Lundén, A., S. Wright, J. E. Allen, and D. Buxton. 2002. Immunisation of mice against neosporosis. Int. J. Parasitol. 32867-876. [DOI] [PubMed] [Google Scholar]
- 31.McAllister, M. M., C. Björkman, R. Anderson-Sprecher, and D. G. Rogers. 2000. Evidence of point-source exposure to Neospora caninum and protective immunity in a herd of beef cows. J. Am. Vet. Med. Assoc. 217881-887. [DOI] [PubMed] [Google Scholar]
- 32.Nishikawa, Y., H. Zhang, P. Huang, G. Zhang, and X. Xuan. 2009. Effects of a transferring antibody against Neospora caninum infection in a murine model. Vet. Parasitol. 16060-65. [DOI] [PubMed] [Google Scholar]
- 33.Nishikawa, Y., N. Inoue, L. Makala, and H. Nagasawa. 2003. A role for balance of interferon-gamma and interleukin-4 production in protective immunity against Neospora caninum infection. Vet. Parasitol. 116175-184. [DOI] [PubMed] [Google Scholar]
- 34.Nishikawa, Y., K. Tragoolpua, N. Inoue, L. Makala, H. Nagasawa, H. Otsuka, and T. Mikami. 2001. In the absence of endogenous gamma interferon, mice acutely infected with Neospora caninum succumb to a lethal immune response characterized by inactivation of peritoneal macrophages. Clin. Diagn. Lab. Immunol. 8811-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishikawa, Y., N. Inoue, X. Xuan, H. Nagasawa, I. Igarashi, F. Fujisaki, H. Otsuka, and T. Mikami. 2001. Protective efficacy of vaccination by recombinant vaccinia virus against Neospora caninum infection. Vaccine 191381-1390. [DOI] [PubMed] [Google Scholar]
- 36.Nishikawa, Y., X. Xuan, H. Nagasawa, I. Igarashi, K. Fujisaki, H. Otsuka, and T. Mikami. 2001. Prevention of vertical transmission of Neospora caninum in BALB/c mice by recombinant vaccinia virus carrying NcSRS2 gene. Vaccine 191710-1716. [DOI] [PubMed] [Google Scholar]
- 37.Pinitkiatisakul, S., M. Friedman, M. Wikman, J. G. Mattsson, K. Lövgren-Bengtsson, S. Ståhl, and A. Lundén. 2007. Immunogenicity and protective effect against murine cerebral neosporosis of recombinant NcSRS2 in different iscom formulations. Vaccine 253658-3668. [DOI] [PubMed] [Google Scholar]
- 38.Pinitkiatisakul, S., J. G. Mattsson, M. Wikman, M. Friedman, K. L. Bengtsson, S. Ståhl, and A. Lundén. 2005. Immunisation of mice against neosporosis with recombinant NcSRS2 iscoms. Vet. Parasitol. 12925-34. [DOI] [PubMed] [Google Scholar]
- 39.Schares, G., M. Peters, R. Wurm, A. Bärwald, and F. J. Conraths. 1998. The efficiency of vertical transmission of Neospora caninum in dairy cattle analysed by serological techniques. Vet. Parasitol. 8087-98. [DOI] [PubMed] [Google Scholar]
- 40.Shibahara, T., T. Kokuho, M. Eto, M. Haritani, T. Hamaoka, K. Shimura, K. Nakamura, Y. Yokomizo, and I. Yamane. 1999. Pathological and immunological findings of athymic nude and congenic wild type BALB/c mice experimentally infected with Neospora caninum. Vet. Pathol. 36321-327. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu, Y., H. Takagi, T. Nakayama, K. Yamakami, T. Tadakuma, N. Yokoyama, and N. Kojima. 2007. Intraperitoneal immunization with oligomannose-coated liposome-entrapped soluble leishmanial antigen induces antigen-specific T-helper type immune response in BALB/c mice through uptake by peritoneal macrophages. Parasite Immunol. 29229-239. [DOI] [PubMed] [Google Scholar]
- 42.Staska, L. M., C. J. Davies, W. C. Brown, T. C. McGuire, C. E. Suarez, J. Y. Park, B. A. Mathison, J. R. Abbott, and T. V. Baszler. 2005. Identification of vaccine candidate peptides in the NcSRS2 surface protein of Neospora caninum by using CD4+ cytotoxic T lymphocytes and gamma interferon-secreting T lymphocytes of infected Holstein cattle. Infect. Immun. 731321-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevens, T. L., A. Bossie, V. M. Sanders, R. Fernandez-Botran, R. L. Coffman, T. R. Mosmann, and E. S. Vitetta. 1988. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature 334255-258. [DOI] [PubMed] [Google Scholar]
- 44.Takagi, H., M. Numazaki, T. Kajiwara, Y. Abe, M. Ishii, C. Kato, and K. Kojima. 2008. Cooperation of specific ICAM-3 grabbing nonintegrin related 1 (SIGNR1) and complement receptor type 3 (CR3) in uptake of oligomannose-coated liposomes by macrophages. Glycobiology 211-9. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka, T., T. Hamada, N. Inoue, H. Nagasawa, K. Fujisaki, N. Suzuki, and T. Mikami. 2000. The role of CD4(+) or CD8(+) T cells in the protective immune response of BALB/c mice to Neospora caninum infection. Vet. Parasitol. 90183-191. [DOI] [PubMed] [Google Scholar]
- 46.Thurmond, M. C., and S. K. Hietala. 1997. Effect of Neospora caninum infection on milk production in first-lactation dairy cows. J. Am. Vet. Med. Assoc. 210672-674. [PubMed] [Google Scholar]
- 47.Thurmond, M. C., and Hietala. 1996. Culling associated with Neospora caninum infection in dairy cows. Am. J. Vet. Res. 571559-1562. [PubMed] [Google Scholar]
- 48.Vemulapalli, R., N. Sanakkayala, J. Gulani, G. G. Schurig, S. M. Boyle, D. S. Lindsay, and N. Sriranganathan. 2007. Reduced cerebral infection of Neospora caninum in BALB/c mice vaccinated with recombinant Brucella abortus RB51 strains expressing N. caninum SRS2 and GRA7 proteins. Vet. Parasitol. 148219-230. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, H., M. K. Compaore, E. G. Lee, M. Liao, G. Zhang, C. Sugimoto, K. Fujisaki, Y. Nishikawa, and X. Xuan. 2007. Apical membrane antigen 1 is a cross-reactive antigen between Neospora caninum and Toxoplasma gondii, and the anti-NcAMA1 antibody inhibits host cell invasion by both parasites. Mol. Biochem. Parasitol. 151205-212. [DOI] [PubMed] [Google Scholar]