Abstract
Discrimination of soft tissue infection from osteomyelitis in diabetic foot infections is a common clinical problem. Staphylococcus aureus isolates from patients with osteomyelitis express bone sialoprotein-binding protein (Bbp) that binds the bone matrix protein bone sialoprotein. The serological assay with Bbp discriminated cases of osteomyelitis from soft tissue infections in patients with diabetic foot ulcers.
Staphylococcus aureus is the most prevalent bacterium in human skeletal infections, causing osteomyelitis and septic arthritis. S. aureus is frequently found in diabetic foot infections, including osteomyelitis and soft tissue infections, and in addition occurs in endocarditis, pneumonia, and septicemia (1, 5, 7, 11, 25, 26, 29). S. aureus from osteomyelitis and septic arthritis binds bone sialoprotein (BSP) via a cell wall glycoprotein, BSP-binding protein (Bbp), belonging to the Sdr family (20). The Sdr family comprises several microbial surface components recognizing adhesive matrix molecules (16, 19, 28). Bbp from S. aureus strain O24 is a 97-kDa protein, having an A and a B domain with 76% and 96% identity, respectively, with the corresponding domains in SdrE (8, 12). SdrE is associated with platelet activation (14) but negatively correlated with bone infection (24). Expression of the S. aureus gene bbp correlates with genes for methicillin (meticillin) resistance and Panton-Valentine leukocidin, but its geographical distribution varies (2, 13, 15).
The present study was prompted by the desire to evaluate levels of antibodies to Bbp in serum samples from patients with different types of infection and to confirm whether the correlation between the location of infection and the BSP-binding ability of staphylococci previously reported (18, 22) is reflected by the ability of the bacteria to evoke an immune response against Bbp. We investigated the immunological response as levels of immunoglobulin G (IgG) antibodies to Bbp in sera from patients suffering from infections caused by S. aureus by using an enzyme-linked immunosorbent assay (21) based on recombinant Bbp (28). Microtiter plates were coated with Bbp-glutathione S-transferase (GST) fusion protein (28) (5 μg/ml), recombinant SdrE (14) (5 μg/ml), or GST (5 μg/ml) alone or with the commercially available antigens (PhPlate, Stockholm, Sweden) (4, 6, 9, 23) alpha-toxin (4 μg/ml) or teichoic acid (4 μg/ml). Plates were incubated with patient sera serially diluted in phosphate-buffered saline with Tween 20. Bound human IgG was detected by phosphatase-conjugated mouse anti-human antibodies, and the optical density at 405 nm was recorded at 30-min intervals over 2 h (21). The titers of IgG antibodies for teichoic acid and alpha-toxin were within the range seen by using a commercial enzyme-linked immunosorbent assay (4, 6, 9, 23). Titers of IgG antibodies against the recombinant Bbp protein were lower by 1 order of magnitude within the 2-h development time (Table 1) . Cutoff values were set at 2 standard deviations (SD) above the mean titer for healthy blood donors (1:90 for Bbp, 1:1,000 for alpha-toxin, 1:1,050 for teichoic acid, and 1:68 for SdrE) for the selected time of development. The Mann-Whitney test was applied for statistical analysis of the results for two-group analyses, and the Kruskal-Wallis post hoc Dunn procedure was applied in multiple-group analyses (Table 2).
TABLE 1.
Titers of antibodies to Bbp, alpha-toxin, teichoic acid, and SdrE
| Patient group | Titer (mean ± SD) fora:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Cases (patients with S. aureus infection)
|
References (patients with no S. aureus infection)
|
|||||||
| Bbp | Alpha-toxin | Teichoic acid | SdrE | Bbp | Alpha-toxin | Teichoic acid | SdrE | |
| Total osteomyelitis | 149 ± 128 | 1,150 ± 737 | 1,288 ± 987 | 77 ± 44 | 89 ± 28 | 991 ± 579 | 795 ± 220 | 66 ± 9 |
| Vertebral osteitis | 144 ± 70 | 1,153 ± 493 | 1,702 ± 1,307 | 86 ± 60 | 89 ± 39 | 1,292 ± 1,194 | 862 ± 278 | 64 ± 3 |
| Diabetic foot infection | 199 ± 209 | 1,214 ± 981 | 972 ± 377 | 61 ± 5 | 74 ± 4 | 740 ± 50 | 716 ± 33 | |
| Presence of foreign body | 130 ± 120 | 1,030 ± 511 | 1,018 ± 502 | 92 ± 60 | 86 ± 26 | 907 ± 378 | 764 ± 182 | 67 ± 12 |
| Other osteomyelitis | 129 ± 69 | 1,222 ± 913 | 1,475 ± 1,262 | 70 ± 23 | 102 ± 29 | 1,054 ± 272 | 869 ± 333 | 64 ± 11 |
| Septic arthritis | 93 ± 36 | 1,178 ± 1,105 | 1,155 ± 643 | 74 ± 24 | 102 ± 55 | 808 ± 178 | 825 ± 372 | 90 ± 38 |
| Endocarditis | 98 ± 72 | 968 ± 407 | 1,528 ± 1,187 | 57 ± 1 | 84 ± 24 | 715 ± 84 | 686 ± 107 | 66 |
| Septicemia | 80 ± 38 | 775 ± 272 | 878 ± 390 | 75 ± 11 | 78 ± 28 | 664 ± 63 | 618 ± 58 | 56 |
| Soft tissue infection | 72 ± 8 | 746 ± 266 | 812 ± 416 | 63 ± 9 | 67 ± 6 | 748 ± 5 | 664 ± 108 | 60 ± 1 |
| Respiratory tract infection | NA | NA | NA | NA | 67 ± 7 | 614 ± 32 | 644 ± 72 | |
| Healthy blood donor | NA | NA | NA | NA | 70 ± 9 | 727 ± 140 | 716 ± 164 | 66 ± 1 |
| Cutoff valueb | 90 | 1,000 | 1,050 | 68 | 90 | 1,000 | 1,050 | 68 |
NA, not applicable.
Cutoff values for the different protein antigens were based on titers for healthy blood donors. Cutoff values were set at +2 SD above the mean titer for these controls for the individual antigens, resulting in values for Bbp of 1:90, for alpha-toxin of 1:1,000, for teichoic acid of 1:1,050, and for SdrE of 1:68.
TABLE 2.
Significant differences between groups of diagnoses as calculated by the Kruskal-Wallis post hoc Dunn procedurea
| Comparison between groups | Significance of difference of results for:
|
||
|---|---|---|---|
| Bbp | Alpha-toxin | Teichoic acid | |
| Cases of osteitis and septic arthritis vs: | |||
| Cases of endocarditis and septicemia | Significant | Significant | Not significant |
| Cases of soft tissue infection | Significant | Significant | Significant |
| Reference cases of osteitis and septic arthritis | Significant | Not significant | Significant |
| Reference cases of endocarditis and septicemia | Significant | Significant | Significant |
| Controls with respiratory tract infections and healthy blood donors | Significant | Significant | Significant |
| Cases of endocarditis and septicemia vs: | |||
| Reference cases of endocarditis and septicemia | Not significant | Not significant | Significant |
| Controls with respiratory tract infections and healthy blood donors | Not significant | Not significant | Significant |
| Reference cases of osteitis and septic arthritis vs: | |||
| Cases of soft tissue infection | Not significant | Significant | Not significant |
| Reference cases of endocarditis and septicemia | Not significant | Significant | Not significant |
| Controls with respiratory tract infections and healthy blood donors | Significant | Significant | Not significant |
Results for subgroups of staphylococcal skeletal infections (osteitis and septic arthritis), invasive infections (endocarditis and septicemia), and soft tissue infections, all denoted cases, were compared with one another and with reference cases of nonstaphylococcal infections as well as with controls, yielding 7 groups and 21 comparisons altogether for the Kruskal-Wallis post hoc Dunn procedure calculation. Only those with any calculations showing significance for any antigen were included.
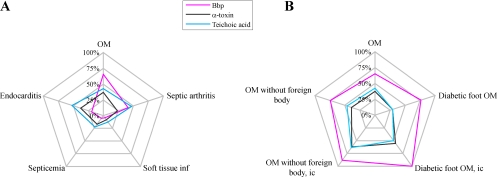
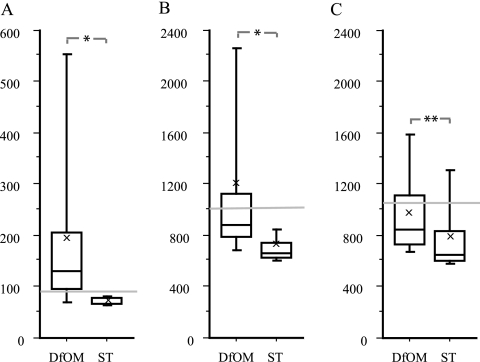
Serum samples from patients suffering from infections affecting bone tissue had higher titers of IgG to the Bbp fusion protein than serum samples from patients with other types of diseases (Table 1; Fig. 1A). Patients suffering from diabetic osteomyelitis had significantly higher titers of IgG antibodies to recombinant Bbp than patients with S. aureus soft tissue infections (Fig. 2A) (P < 0.0001). Anti-Bbp titers above the cutoff were found in 13/17 patients with diabetic foot infections (Table 3). The presence of serum antibodies to recombinant Bbp antigen, as well as to alpha-toxin, differentiated skeletal infections (osteomyelitis and septic arthritis) from other types of invasive disease, whereas teichoic acid antibody titers did not when analyzed by the Kruskal-Wallis post hoc Dunn procedure (Table 2). The presence of anti-alpha-toxin IgG did not distinguish between actual cases and reference cases of invasive osteitis and septic arthritis. Foreign-body-related osteomyelitis was not reliably detected by the Bbp assay, with only 9/21 patients with titers above cutoff levels (sensitivity, 43%). Within the group of osteomyelitis patients without foreign material, i.e., no prosthesis or other osteosynthetic material 40/54 had IgG titers to the Bbp antigen above the cutoff (Table 3). The 14 negative serum samples included 6 samples from patients who had been treated with immunosuppressive drugs.
FIG. 1.
(A and B) Radargraphs showing percentages of patients with indicated diagnoses of S. aureus infection with titers above cutoff values for Bbp, alpha-toxin, and teichoic acid. OM, osteomyelitis; inf, infection; ic, immunocompetent.
FIG. 2.
Box plot of IgG titers among patient sera against Bbp (A), alpha-toxin (B), and teichoic acid (C). Sera were from patients with diabetic foot osteomyelitis (DfOM) and soft tissue infections (ST) with S. aureus. Cutoff levels were set at +2 SD above the mean titer value in healthy controls for each antigen and are indicated by horizontal lines. Mean values are indicated by ×. *, P < 0.0001; **, P = 0.005, both analyzed by the Mann-Whitney test.
TABLE 3.
Number of cases and control samples positive for anti-Bbp IgG, indicating S. aureus infectiona
| Patient group | No. of cases
|
No. of controls
|
||
|---|---|---|---|---|
| Total | Positive | Total | Positive | |
| Total osteomyelitis | 75 | 49 | 34b | 12 |
| Vertebral osteitis | 18 | 15 | 6 | 2 |
| Diabetic foot infection | 17 | 13 | 2 | 0 |
| Presence of foreign body | 21 | 9 | 17 | 6 |
| Other osteomyelitis | 19 | 12 | 6 | 4 |
| Septic arthritis | 17 | 7 | 29c | 10 |
| Endocarditis | 21 | 4 | 23d | 7 |
| Septicemia | 18 | 1 | 12 | 1 |
| Soft tissue infection | 24 | 1 | 3 | 0 |
| Respiratory tract infection | NA | 9 | 0 | |
| Healthy blood donor | NA | 29 | 1 | |
| Total | 155 | 110 | 139 | 53 |
Control samples were from individuals with other defined infections or healthy blood donors. Patients with titers above the cutoff for anti-Bbp IgG are indicated as positive. NA, not applicable.
Coagulase-negative staphylococci were found in positive samples among control patients in 8/17 patients of the osteomyelitis group.
Coagulase negative-staphylococci were found in positive samples among control patients in 4/10 patients of the septic arthritis group.
Coagulase-negative staphylococci were found in positive samples among control patients in 2/3 patients of the endocarditis group.
Sera from patients with endocarditis had low IgG titers to Bbp levels (Table 1; Fig. 1A) and, in only a few cases (4/21), anti-Bbp IgG levels rose to the levels detected in osteomyelitis patients. In patients suffering from S. aureus endocarditis, the level of IgG antibodies to teichoic acid was higher than the level of IgG to Bbp or to alpha-toxin. Our Bbp assay results showed elevated IgG responses in 11/44 patients with endocarditis. Four of these patients were culture positive for S. aureus, and two yielded coagulase-negative staphylococci in blood cultures.
IgG to SdrE was found with much lower frequency and at lower concentrations than that to Bbp in most samples tested. The levels of IgG to SdrE were above the cutoff in 20/79 sera. Titers were generally low, with the highest levels of anti-SdrE IgG found in cases of osteomyelitis and in reference cases of septic arthritis (Table 1). There was no correlation between IgG levels to Bbp and to SdrE. Only 2/15 patients with IgG concentrations above the cutoff for both Bbp and SdrE had high levels of IgG to both antigens (data not shown). The genes encoding Bbp and SdrE are both commonly found in isolates from patients with musculoskeletal infections but rarely occur in the same strain (3), contradicting earlier findings (17). The present findings showing that SdrE antibodies were present in the highest titers in septic arthritis patients (Table 1) and that titers of antibodies to Bbp and SdrE did not correlate indicate that the proteins are immunologically different (data not shown). Control patients with raised titers of antibodies to Bbp included 14 patients with coagulase-negative staphylococci, 9 with streptococci, and 3 with enterococci. There were no antibodies found in sera tested with empty GST vector as the antigen (data not shown).
The study results could be relevant for diagnosing S. aureus osteomyelitis, particularly in cases of diabetic foot infections, since neuropathy leads to an extended delay before patients consult a physician. The elevated IgG levels found in sera from diabetic patients at their first clinical visits for presumed osteomyelitis probably reflect the neuropathy and delay rather than a truly acute IgG rise. IgG to recombinant Bbp antigen could aid in early diagnosis of osteomyelitis when radiological changes have not yet appeared, as well as in culture-negative patients, and thus could be helpful in deciding the treatment regimen, including the duration of antibiotic treatment. The low levels of antibodies to Bbp in S. aureus soft tissue infections in diabetic patients may be attributed to an impaired local immune response due to diabetes. Patients with diabetes mellitus have an impaired antibody response when vaccine antigen is given intradermally, whereas intramuscular injection induces a normal antibody response (10). The absence of antibodies, however, strongly indicates that staphylococcal infection does not affect bone tissue. Several studies support our previous finding that the presence of bbp in staphylococcal cells is associated with osteomyelitis (15, 27), and data presented here support our earlier hypothesis that Bbp expression is associated with bone tissue infection. We conclude from this study that detection of serum IgG directed against Bbp can serve as a marker of osteomyelitis, especially in diabetic foot infections.
Acknowledgments
This work was supported by Konung G:V 80-årsfond, Olinder-Nielsens stiftelse, and Uppsala University.
We thank Kristofer Rubin for assistance in preparing the manuscript as well as scientific discussions, Timothy Foster for kindly providing the SdrE recombinant protein, and Lars Wesslén for assistance with the serological data bank. Serum samples were kindly provided by Åsa Ljungh (serum samples from 17 coded patients), Department of Medical Microbiology, University of Lund, Lund, Sweden, and by Eva Hjelm, Department of Bacteriology, Uppsala Academic Hospital (29 samples from adult blood donors with full anonymity). We thank Jesper Rydén, Department of Mathematics, Uppsala University, for assistance in selecting statistical methods.
Footnotes
Published ahead of print on 15 April 2009.
REFERENCES
- 1.Abdelnour, A., S. Arvidson, T. Bremell, C. Rydén, and A. Tarkowski. 1993. The accessory gene regulator (agr) controls Staphylococcus aureus virulence in a murine arthritis model. Infect. Immun. 613879-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell, S. J., H. S. Deshmukh, C. L. Nelson, I.-G. Bae, M. E. Stryjewski, J. J. Federspiel, G. T. Tonthat, T. H. Rude, S. L. Barriere, R. Corey, and V. G. Fowler, Jr. 2008. Genotypic characteristics of Staphylococcus aureus isolates from a multinational trial of complicated skin and skin structure infections. J. Clin. Microbiol. 46678-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassat, J. E., P. M. Dunman, F. McAleese, E. Murphy, S. J. Projan, and M. S. Smeltzer. 2005. Comparative genomics of Staphylococcus aureus musculoskeletal isolates. J. Bacteriol. 187576-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowder, J. G., and A. White. 1972. Teichoic acid antibodies in staphylococcal and nonstaphylococcal endocarditis. Ann. Intern. Med. 7787-90. [DOI] [PubMed] [Google Scholar]
- 5.Eder, L., D. Zisman, M. Rozenbaum, and I. Rosner. 2005. Clinical features and aetiology of septic arthritis in northern Israel. Rheumatology 441559-1563. [DOI] [PubMed] [Google Scholar]
- 6.Granström, M., I. G. Julander, S.-A. Hedström, and R. Möllby. 1983. Enzyme-linked immunosorbent assay for antibodies against teichoic acid in patients with staphylococcal infections. J. Clin. Microbiol. 17640-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartemann-Heurtier, A., and E. Senneville. 2008. Diabetic foot osteomyelitis. Diabetes Metab. 3487-95. [DOI] [PubMed] [Google Scholar]
- 8.Josefsson, E., K. W. McCrea, D. Ni Eidhin, D. O'Connell, J. Cox, M. Höök, and T. J. Foster. 1998. Three new members of the serine-aspartate repeat protein multigene family of Staphylococcus aureus. Microbiology (Reading) 1443387-3395. [DOI] [PubMed] [Google Scholar]
- 9.Julander, I. G., M. Granström, S. A. Hedström, and R. Möllby. 1983. The role of antibodies against α-toxin and teichoic acid in the diagnosis of staphylococcal infections. Infection 1177-83. [DOI] [PubMed] [Google Scholar]
- 10.Li Volti, S., M. Caruso-Nicoletti, F. Biazzo, A. Sciacca, G. Mandara, M. Mancuso, and F. Mollica. 1998. Hyporesponsiveness to intradermal administration of hepatitis B vaccine in insulin dependent diabetes mellitus. Arch. Dis. Child. 7854-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mandal, S., A. R. Berendt, and S. J. Peacock. 2002. Staphylococcus aureus bone and joint infection. J. Infect. 44143-151. [DOI] [PubMed] [Google Scholar]
- 12.McCrea, K. W., O. Hartford, S. Davis, D. N. Eidhin, G. Lina, P. Speziale, T. J. Foster, and M. Höök. 2000. The serine-aspartate repeat (Sdr) protein family in Staphylococcus epidermidis. Microbiology (Reading) 1461535-1546. [DOI] [PubMed] [Google Scholar]
- 13.Moumile, K., C. Cadilhac, G. Lina, P. Berche, C. Glorion, and A. Ferroni. 2006. Severe osteoarticular infection associated with Panton-Valentine leukocidin-producing Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 5695-97. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien, L., S. W. Kerrigan, G. Kaw, M. Hogan, J. Penadés, D. Litt, D. J. Fitzgerald, T. J. Foster, and D. Cox. 2002. Multiple mechanisms for the activation of human platelet aggregation by Staphylococcus aureus: roles for the clumping factors ClfA and ClfB, the serine-aspartate repeat protein SdrE and protein A. Mol. Microbiol. 441033-1044. [DOI] [PubMed] [Google Scholar]
- 15.Otsuka, T., K. Saito, S. Dohmae, T. Takano, W. Higuchi, Y. Takizawa, T. Okubo, N. Iwakura, and T. Yamamoto. 2006. Key adhesin gene in community-acquired methicillin-resistant Staphylococcus aureus. Biochem. Biophys. Res. Commun. 3461234-1244. [DOI] [PubMed] [Google Scholar]
- 16.Patti, J. M., and M. Höök. 1994. Microbial adhesins recognizing extracellular matrix macromolecules. Curr. Opin. Cell Biol. 6752-758. [DOI] [PubMed] [Google Scholar]
- 17.Peacock, S. J., C. E. Moore, A. Justice, M. Kantzanou, L. Story, K. Mackie, G. O'Neill, and N. P. Day. 2002. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect. Immun. 704987-4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rydén, C., I. Maxe, A. Franzen, A. Ljungh, D. Heinegård, and K. Rubin. 1987. Selective binding of bone matrix sialoprotein to Staphylococcus aureus in osteomyelitis. Lancet ii515. [DOI] [PubMed] [Google Scholar]
- 19.Rydén, C., K. Rubin, P. Speziale, M. Höök, M. Lindberg, and T. Wadström. 1983. Fibronectin receptors from Staphylococcus aureus. J. Biol. Chem. 2583396-3401. [PubMed] [Google Scholar]
- 20.Rydén, C., H. S. Tung, V. Nikolaev, A. Engström, and Å. Oldberg. 1997. Staphylococcus aureus causing osteomyelitis binds to a nonapeptide sequence in bone sialoprotein. Biochem. J. 327825-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rydén, C., A. Yacoub, A. Kvarnström, T. Wadström, I. Maxe, G. Friman, and K. Rubin. 1990. Detection of Staphylococcus aureus infection by enzyme-linked immunosorbent assay and immunoblotting, using high molecular weight staphylococcal proteins. FEMS Microbiol. Immunol. 265-73. [DOI] [PubMed] [Google Scholar]
- 22.Rydén, C., A. I. Yacoub, I. Maxe, D. Heinegård, Å. Oldberg, A. Franzén, Å. Ljungh, and K. Rubin. 1989. Specific binding of bone sialoprotein to Staphylococcus aureus isolated from patients with osteomyelitis. Eur. J. Biochem. 184331-336. [DOI] [PubMed] [Google Scholar]
- 23.Ryding, U., F. Espersen, B. Söderquist, and B. Christensson. 2002. Evaluation of seven different enzyme-linked immunosorbent assays for serodiagnosis of Staphylococcus aureus bacteremia. Diagn. Microbiol. Infect. Dis. 429-15. [DOI] [PubMed] [Google Scholar]
- 24.Sabat, A., D. C. Melles, G. Martirosian, H. Grundmann, A. van Belkum, and W. Hryniewicz. 2006. Distribution of the serine-aspartate repeat protein-encoding sdr genes among nasal-carriage and invasive Staphylococcus aureus strains. J. Clin. Microbiol. 441135-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shirtliff, M. E., and J. T. Mader. 2002. Acute septic arthritis. Clin. Microbiol. Rev. 15527-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trampuz, A., and W. Zimmerli. 2008. Diagnosis and treatment of implant-associated septic arthritis and osteomyelitis. Curr. Infect. Dis. Rep. 10394-403. [DOI] [PubMed] [Google Scholar]
- 27.Tristan, A., L. Ying, M. Bes, J. Etienne, F. Vandenesch, and G. Lina. 2003. Use of multiplex PCR to identify Staphylococcus aureus adhesins involved in human hematogenous infections. J. Clin. Microbiol. 414465-4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tung, H., B. Guss, U. Hellman, L. Persson, K. Rubin, and C. Rydén. 2000. A bone sialoprotein-binding protein from Staphylococcus aureus: a member of the staphylococcal Sdr family. Biochem. J. 345611-619. [PMC free article] [PubMed] [Google Scholar]
- 29.Zeller, J. L., C. Lynm, and R. M. Glass. 2007. JAMA patient page. Septic arthritis. JAMA 2971510. [DOI] [PubMed] [Google Scholar]