Abstract
We used a PCR method to quantify the loads of Chlamydia trachomatis organisms in self-collected urine and vulvovaginal swab (VVS) samples from 93 women and 30 men participating in the Chlamydia Screening Studies Project, a community-based study of individuals not seeking health care. For women, self-collected VVS had a higher mean chlamydial load (10,405 organisms/ml; 95% confidence interval [95% CI], 5,167 to 21,163 organisms/ml) than did first-void urines (FVU) (503 organisms/ml; 95% CI, 250 to 1,022 organisms/ml; P < 0.001). Chlamydial loads in female and male self-collected FVU specimens were similar (P = 0.634). The mean chlamydial load in FVU specimens decreased with increasing age in females and males. There was no strong statistical evidence of differences in chlamydial load in repeat male and female FVU specimens taken when patients attended for treatment a median of 23.5 (range, 14 to 62) and 28 (range, 13 to 132) days later, respectively, or in VVS taken a median of 35 (range, 14 to 217) days later. In this study, chlamydial load values for infected persons in the community who were not seeking treatment were lower than those published in other studies involving symptomatic patients attending clinical settings. This might have implications for estimates of the infectiousness of chlamydia. The results of this study provide a scientific rationale for preferring VVS to FVU specimens from women.
Nucleic acid amplification tests to detect Chlamydia trachomatis have revolutionized the diagnosis and management of this common treatable sexually transmitted infection (6). Increased test sensitivity has enabled the use of noninvasive specimens, such as first-void urine specimens for men and women and vulvovaginal swabs for women, thus dispensing with the need for a genital examination and making it easier to perform screening tests on people without symptoms suggestive of chlamydial infection.
The burden of C. trachomatis organisms in the genital tract (chlamydial load) can vary from 10 to over a million organisms per milliliter of genital tract secretions (4, 8). This is likely to influence the performance of different nucleic acid amplification tests, which do not routinely distinguish between people with high and low chlamydial loads (4, 8). Differences in chlamydial load have been reported to be associated with the presence of clinical symptoms (2, 8), the transmissibility and persistence of infection (3, 10), and the risk of developing chronic sequelae (2). Studies investigating the relevance of chlamydial load so far have been conducted only with patients attending health care settings (2, 4, 8). The objectives of this study were to measure chlamydial loads in individuals in a community setting and to investigate factors associated with chlamydial load.
MATERIALS AND METHODS
We used specimens from people participating in the Chlamydia Screening Studies (ClaSS) project, which has been described in detail elsewhere (5, 7, 11). In brief, specimens were collected at home from February 2000 to August 2001 by women and men selected at random from the catchment populations of 27 family practices in the Bristol and Birmingham areas of the United Kingdom. Male and female respondents were asked to provide a first-void urine specimen, and female respondents were also asked for a vulvovaginal swab according to written and pictorial instructions provided in the test pack. Chlamydial diagnosis was made using the Cobas Amplicor CT test PCR assay (Roche Diagnostics, Basel, Switzerland) at the Bristol laboratory and by Becton Dickinson ProbeTec ET DNA strand displacement amplification (SDA; Becton Dickinson and Company, Franklin Lakes, NJ) at the Birmingham laboratory. In addition, female vulvovaginal swab specimens at both centers and male first-void urine specimens in Bristol were tested using the IDEIA polymer conjugate enhanced enzyme immunoassay (PCE EIA; Dako, Ely, Cambridgeshire, United Kingdom) with negative-gray-zone testing as described elsewhere (5, 11). The manufacturers' instructions were followed, except that specimens with initially inhibitory results were retested with a different nucleic acid amplification test. At the Birmingham laboratory, Roche Cobas PCR was used for retesting. At the Bristol laboratory, retesting was done using either Becton Dickinson SDA or an in-house real-time PCR, owing to technical problems with the SDA assay which we have described before (5). Infected persons were defined according to an algorithm that required concordant results in two separate tests. A person was considered infected if a reactive nucleic acid amplification test result was confirmed by a reactive PCE EIA or other nucleic acid amplification test or if a reactive PCE EIA result was confirmed by a nucleic acid amplification test. The algorithm was applied to results for the first-void urine specimen for men and for either specimen type for women (5, 11). A person was considered chlamydia negative if the results of the initial nucleic acid amplification test and PCE EIA test were negative. Individuals with a confirmed positive result were asked to provide a second specimen before receiving treatment with azithromycin (1 g as a single directly observed dose) and prior to partner notification. We tested both specimens to examine the concordance of positivity over time and to determine whether or not postal transport of specimens was likely to have affected test performance. All positive specimens were stored at −20°C for the duration of the study and then moved to −80°C freezers at the Health Protection Agency, Bristol, United Kingdom (7).
We invited all participants with positive chlamydia tests and the next two individuals with a negative test who were of the same sex and in the same broad age band (16 to 24 or 25 to 39 years) as the case patient to take part in a case-control study comparing the characteristics of individuals with and without chlamydial infection (7). Participants were asked to complete a questionnaire before they obtained their test result. This included questions about whether they had had symptoms that can be associated with chlamydia in the month before completing the questionnaire, including vaginal discharge, intermenstrual bleeding, postcoital bleeding, urinary frequency, and lower abdominal pain in women and urethral discharge, pain passing urine, penile irritation, and testicular pain in men. For this study, we used specimens from patients with chlamydia infection in the case-control study aged 16 to 24 years because we stopped enrolling 25- to 39-year-olds owing to the small number of infections in this age group. This study was approved by the South West Multicenter Research Ethics Committee.
Quantitative real-time PCR.
We tested stored specimens in 2005. Briefly, the methods were as follows. Nucleic acids were extracted from 1 ml of thawed first-void urine or vulvovaginal swab eluate by use of a column-based system containing a silica gel (QIAamp Viral RNA Mini kit; Qiagen Ltd., Crawley, Sussex, United Kingdom). Quantitative PCR (qPCR) was undertaken using a RealArt C. trachomatis PCR kit (Qiagen). Ten microliters of column eluate from each sample was placed in duplicate in a 96-well PCR plate (Genetic Research Instrumentation Ltd., Essex, United Kingdom) and mixed with the RealArt master mix. Detection of a section of the C. trachomatis major outer membrane protein gene was undertaken against a standard curve taken from kit quantitation standards, using a TaqMan probe-based system with an Applied Biosystems 7500 series real-time PCR instrument. Samples were multiplexed with an internal control (C. trachomatis plasmid) provided by the kit manufacturer to control for the DNA isolation procedure and to check for PCR inhibition. The reaction was considered successful if the concentration threshold value for the internal control was 22 ± 3 cycles. All specimens were tested in duplicate. A negative control containing no C. trachomatis DNA was included in each reaction setup.
Chlamydial load.
We calculated the number of C. trachomatis organisms per milliliter of first-void urine and vulvovaginal specimen (i.e., per swab, as these were eluted in 1 ml), assuming that one organism is equivalent to one genomic copy because there is one copy of the major outer membrane gene per elementary body (EB). We used a detection limit for the assay of 10 organisms per ml (copies/ml). This is lower than that stated in the Artus C. trachomatis kit product insert, which states that the lower limit of detection for the assay, with 95% accuracy, is 200 genome copies/ml of specimen. However, we obtained some results between 20 and 200 genome copies/ml. We considered these valid results after discussion with the Artus technical support team (Tobias Ruckes, personal communication) because the original samples were confirmed to be chlamydia positive prior to freezing and the amplification curves of the duplicate samples demonstrated the same sigmoid profile as samples containing over 200 copies/ml and the Artus concentration standards. Contamination was discounted, as the negative controls used as part of the protocol demonstrated no C. trachomatis-specific amplification. There were also some specimens in which no DNA could be detected by quantitative real-time PCR. A result of zero copies does not necessarily mean that there was no chlamydial DNA in the original sample but that none was retrieved at the time of analysis, which took place more than 2 years after specimen collection.
Statistical analysis.
We normalized distributions of chlamydial loads by converting values to natural logarithms. We then used standard parametric statistical tests for analysis. Results for specimens for which no chlamydial DNA was present were treated as missing values because no logarithm could be obtained. First, we compared chlamydial loads over time among women and men who had specimens for both time points, using paired t tests. We then compared chlamydial loads between specimen types, using unpaired t tests for comparisons of chlamydial loads in first-void urine specimens between women and men and paired t tests for comparisons between vulvovaginal swabs and first-void urines from women. If specimens were available from both time points, we used the value for the first specimen. We also repeated the comparisons using average values to minimize the effect of variation over time, but this did not affect the results (data not shown). For ease of interpretation and comparability, we displayed geometric mean values as both logarithms and absolute values.
We investigated associations between chlamydial load and the presence of symptoms in female and male specimens separately, using logistic regression models. We examined the association between a 1-unit increase in the logarithm of the chlamydial load and the presence of each symptom or the presence of any symptom, expressing the results as odds ratios (with 95% confidence intervals [CI]) before and after controlling for age. Given the small sample size, we did not conduct multivariable analyses. All analyses were performed using STATA (release 8.2; StataCorp LP, TX).
RESULTS
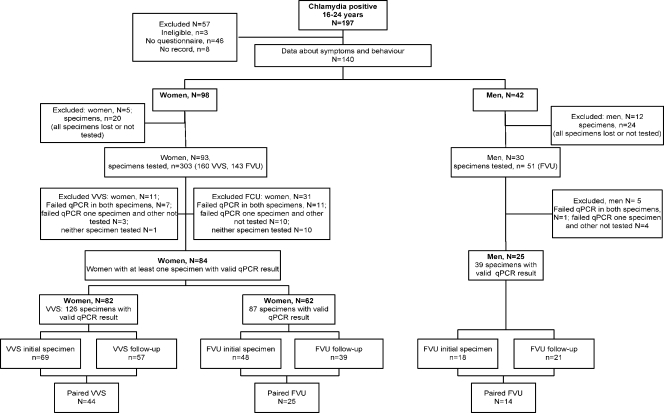
There were 219 people with an initial confirmed chlamydia-positive specimen, 197 of whom were 16 to 24 years old (Fig. 1). Of these, 140 (98 women and 42 men) took part in the case-control study. Of a possible maximum total of 476 specimens (98 first-void urine and vulvovaginal swabs at two time points from women and 42 first-void urine specimens at two time points from men), qPCR could be conducted on 354 (74.4%; 93 women and 30 men), and valid results were obtained for 252 (52.9%). There were 102 (21.4%) specimens for which no DNA could be detected, and these were excluded from subsequent analyses. The other 122 specimens either could not be found or were not tested due to a lack of resources. The demographic characteristics, sexual behavior, and reported clinical symptoms of participants are shown in Table 1. There were no marked differences between individuals in whom DNA could or could not be detected.
FIG. 1.
Flow chart of those participating in the ClaSS project. VVS, vulvovaginal swab; FVU, first-void urine.
TABLE 1.
Characteristics of the study population
| Characteristic | Men (n = 30)
|
Women (n = 93)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Valid qPCR available (n = 25)
|
Valid qPCR not available (n = 5)
|
Valid qPCR availablea (n = 84)
|
Valid qPCR not available (n = 9)
|
|||||
| n | % | n | % | n | % | n | % | |
| Age Group (yr) | ||||||||
| 16-19 | 7 | 28 | 0 | 0 | 37 | 44.0 | 4 | 44.4 |
| 20-24 | 18 | 72 | 5 | 100 | 47 | 56.0 | 5 | 55.6 |
| Marital status | ||||||||
| Single | 20 | 80 | 5 | 100 | 76 | 90.5 | 8 | 88.9 |
| Married/living as married | 4 | 16 | 0 | 0 | 6 | 7.1 | 1 | 11.1 |
| Not specified | 1 | 4 | 0 | 0 | 2 | 2.4 | ||
| Ethnic group | ||||||||
| White | 21 | 84 | 3 | 60 | 72 | 85.7 | 9 | 100 |
| Black Caribbean/Black African | 3 | 12 | 2 | 40 | 7 | 8.3 | 0 | 0 |
| Asian/Chinese/other | 0 | 0 | 0 | 0 | 5 | 6.0 | 0 | 0 |
| Not specified | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Age at leaving school | ||||||||
| 17 yr+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16 yr and below | 19 | 76 | 3 | 60 | 48 | 57.1 | 7 | 77.8 |
| Still in school | 5 | 20 | 1 | 20 | 25 | 29.8 | 1 | 11.1 |
| Not specified | 1 | 4 | 1 | 20 | 11 | 13.1 | 1 | 11.1 |
| Employment status | ||||||||
| Full-time education | 4 | 16 | 1 | 20 | 13 | 15.5 | 1 | 11.1 |
| Paid employment | 18 | 72 | 3 | 60 | 47 | 56.0 | 4 | 44.4 |
| Unemployed/other | 3 | 12 | 1 | 20 | 21 | 25.0 | 4 | 44.4 |
| Not specified | 0 | 0 | 0 | 0 | 3 | 3.6 | 0 | 0 |
| Current smoker (not specified) | 14 (0) | 56 | 3 (0) | 60 | 39 (1) | 4 (0) | 44.4 | |
| No. of opposite sex partners in the last 12 mo | ||||||||
| 0 | 0 | 0 | 0 | 0 | 25 | 29.8 | 3 | 33.3 |
| 1 | 1 | 4 | 0 | 0 | 20 | 23.8 | 3 | 33.3 |
| ≥2 | 23 | 92 | 4 | 80 | 36 | 42.9 | 3 | 33.3 |
| Not specified | 1 | 4 | 1 | 20 | 3 | 3.6 | 0 | 0 |
| Symptoms in the past month, women | ||||||||
| Vaginal discharge (not specified) | 19 (12) | 22.6 | 3 (0) | 33.3 | ||||
| Intermenstrual bleeding (not specified) | 10 (17) | 11.9 | 1 (2) | 11.1 | ||||
| Postcoital bleeding (not specified) | 9 (17) | 10.7 | 1 (2) | 11.1 | ||||
| Lower abdominal pain (not specified) | 25 (11) | 29.8 | 3 (1) | 33.3 | ||||
| Urinary frequency (not specified) | 20 (12) | 23.8 | 5 (0) | 55.6 | ||||
| Symptoms in the past month, men | ||||||||
| Urethral discharge (not specified) | 4 (5) | 16 | 0 (3) | 0 | ||||
| Pain passing urine (not specified) | 4 (6) | 16 | 4 (0) | 80 | ||||
| Penile irritation (not specified) | 7 (3) | 28 | 2 (1) | 40 | ||||
| Testicular pain (not specified) | 1 (8) | 4 | 0 (3) | 0 | ||||
A valid qPCR result was available from either a vulvovaginal swab or a first-void urine sample.
Chlamydial loads in first-void urine specimens and vulvovaginal swabs over time.
There were 44 women with vulvovaginal swabs, 25 women with first-void urines (31 women with both specimens), and 14 men with first-void urine specimens in which chlamydial DNA could be measured at both time points (Table 2). The median time between specimen collection times for men was 23.5 days (range, 14 to 62 days), that for female urine was 28 days (range, 13 to 132 days), and that for swabs was 35 days (range, 13 to 217 days). There was no evidence of a difference in chlamydial load in urine specimens from either men or women. Chlamydial load values from the initial female vulvovaginal swab specimens (geometric mean, 10,829 copies/ml; 95% CI, 5,115 to 22,697 copies/ml) were slightly higher than those in the second specimens (geometric mean, 5,115 copies/ml; 95% CI, 2,515 to 10,199 copies/ml) (P = 0.077).
TABLE 2.
Mean chlamydial loads at two time points, by specimen type and sex of participants
| Specimen type, group | Time point | n | Chlamydial load (95% CI)
|
P value | |
|---|---|---|---|---|---|
| Log mean copies/ml | Geometric mean copies/ml | ||||
| First-void urine, men | 1 | 14 | 7.47 (5.54-9.39) | 1,755 (255-11,968) | 0.538 |
| 2 | 14 | 7.99 (6.23-9.74) | 2,951 (508-16,983) | ||
| Difference | −0.52 (−2.29-1.25) | ||||
| First-void urine, women | 1 | 25 | 6.96 (6.08-7.84) | 1,054 (437-2,540) | 0.394 |
| 2 | 25 | 6.57 (5.64-7.50) | 713 (281-1,808) | ||
| Difference | 0.39 (−0.53-1.32) | ||||
| Vulvovaginal swab, women | 1 | 44 | 9.29 (8.54-10.03) | 10,829 (5,115-22,697) | 0.077 |
| 2 | 44 | 8.54 (7.83-9.23) | 5,115 (2,515-10,199) | ||
| Difference | 0.75 (−0.08-1.59) | ||||
Chlamydial loads in different specimens.
There were 87 people (62 women and 25 men) with measurable chlamydial loads in first-void urine specimens (Table 3). The geometric mean chlamydial loads in these samples were similar in women (446 copies/ml; 95% CI, 219 to 916 copies/ml) and men (626 copies/ml; 95% CI, 156 to 2,515 copies/ml) (P = 0.634). For women, the chlamydial load was higher in vulvovaginal swabs (geometric mean, 10,405 copies/ml; 95% CI, 5,167 to 21,163 copies/ml) than in first-void urine (503 copies/ml; 95% CI, 250 to 1,022 copies/ml) (P < 0.001).
TABLE 3.
Mean chlamydial loads, by specimen type and sex and age of participants
| Specimen, study group | n | Mean chlamydial load (95% CI)
|
P value | |
|---|---|---|---|---|
| Log copies/ml | Absolute copies/ml | |||
| First-void urine, by sex | 0.634 | |||
| Men | 25 | 6.44 (5.05-7.83) | 626 (156-2,515) | |
| Women | 62 | 6.10 (5.39-6.82) | 446 (219-916) | |
| Vagina vs first-void urine, women | <0.0001 | |||
| First-void urine | 60 | 6.22 (5.52-6.93) | 503 (250-1,022) | |
| Vulvovaginal swab | 60 | 9.25 (8.55-9.96) | 10,405 (5,167-21,163) | |
| Differences by age group | ||||
| First-void urine, women and men | 0.006 | |||
| 16-19 yr | 33 | 7.31 (6.24-8.37) | 1,495 (513-4,316) | |
| 20-24 yr | 54 | 5.52 (4.76-6.28) | 250 (117-534) | |
| First-void urine, women | 0.013 | |||
| 16-19 yr | 26 | 7.13 (6.09-8.17) | 1,248 (441-3,533) | |
| 20-24 yr | 36 | 5.35 (4.41-6.30) | 211 (82-545) | |
| First-void urine, men | 0.169 | |||
| 16-19 yr | 7 | 7.95 (3.81-12.08) | 2,836 (45-176,310) | |
| 20-24 yr | 18 | 5.85 (4.47-7.24) | 347 (87-1,394) | |
| Vulvovaginal swab, women | 0.430 | |||
| 16-19 yr | 36 | 9.11 (8.00-10.23) | 9,045 (2,980-27,723) | |
| 20-24 yr | 46 | 8.63 (7.97-9.29) | 5,597 (2,893-10,829) | |
There was some evidence that chlamydial loads were higher in the younger than in the older age group for all specimen types (Table 3). The difference was most marked for first-void urine specimens from women, as the geometric mean chlamydial load in 16- to 19-year-olds was 1,248 copies/ml (95% CI, 441 to 3,533 copies/ml), compared with 211 copies/ml (95% CI, 82 to 545 copies/ml) in 20- to 24-year-old women (P = 0.013). The age difference was least marked for female vulvovaginal swabs (P = 0.430).
Associations between genital symptoms and chlamydial load.
Among women with information about symptoms and a measurable chlamydial load on a vulvovaginal swab specimen, the presence of vaginal discharge was associated with a higher chlamydial load after controlling for age (Table 4). The odds of having reported any symptom in the past month (46 women [49% of all women and 58% of those responding to specific questions]) increased 28% (95% CI, 6 to 54%) for every 1-logarithm rise in chlamydial load. The strength of this association did not change after controlling for age. There was no statistical evidence of an association between symptoms and chlamydial loads in first-void urine specimens for women (age-adjusted odds ratio, 1.12; 95% CI, 0.93 to 1.35; P = 0.503) or men (age-adjusted odds ratio, 0.94; 95% CI, 0.73 to 1.20; P = 0.850).
TABLE 4.
Associations between chlamydial load and presence of genital symptoms, by participant sex and specimen type
| Group and symptom | n | No. (%) of participants with symptom | Odds ratio (95% CI) for a 1-logarithm increase in chlamydial load
|
|||
|---|---|---|---|---|---|---|
| Unadjusted | P value | Adjusted for age | P value | |||
| Women, vulvovaginal swabs | ||||||
| Vaginal discharge | 70 | 19 (27.1) | 1.29 (1.03-1.63) | 0.016 | 1.37 (1.05-1.77) | 0.023 |
| Intermenstrual bleeding | 65 | 10 (15.4) | 1.23 (0.93-1.62) | 0.132 | 1.34 (0.94-1.91) | 0.108 |
| Postcoital bleeding | 65 | 9 (13.8) | 1.15 (0.87-1.51) | 0.309 | 1.15 (0.87-1.53) | 0.594 |
| Urinary frequency | 70 | 20 (28.6) | 1.27 (1.02-1.59) | 0.002 | 1.26 (1.01-1.58) | 0.064 |
| Lower abdominal pain | 71 | 25 (35.2) | 1.15 (0.95-1.39) | 0.142 | 1.17 (0.96-1.43) | 0.222 |
| Any symptom | 79 | 46 (58.2) | 1.28 (1.06-1.54) | 0.006 | 1.29 (1.07-1.57) | 0.015 |
| Women, first-void urine | ||||||
| Vaginal discharge | 54 | 13 (24.1) | 0.96 (0.78-1.19) | 0.734 | 0.97 (0.78-1.21) | 0.884 |
| Intermenstrual bleeding | 50 | 6 (12.0) | 1.25 (0.86-1.81) | 0.213 | 1.26 (0.85-1.89) | 0.432 |
| Postcoital bleeding | 49 | 6 (12.2) | 0.97 (0.73-1.30) | 0.846 | 0.95 (0.71-1.28) | 0.806 |
| Urinary frequency | 55 | 17 (30.9) | 1.06 (0.87-1.31) | 0.540 | 1.05 (0.85-1.29) | 0.665 |
| Lower abdominal pain | 57 | 19 (33.3) | 0.93 (0.77-1.13) | 0.475 | 0.94 (0.77-1.14) | 0.767 |
| Any symptom | 60 | 33 (55.0) | 1.11 (0.93-1.34) | 0.251 | 1.12 (0.93-1.35) | 0.503 |
| Men, first-void urine | ||||||
| Penile irritation | 22 | 7 (31.8) | 0.76 (0.55-1.04) | 0.061 | 0.76 (0.56-1.04) | 0.171 |
| Pain or burning on passing urine | 19 | 4 (21.1) | 0.82 (0.57-1.17) | 0.244 | 0.82 (0.57-1.17) | 0.502 |
| Urethral discharge | 20 | 4 (20.0) | 1.01 (0.72-1.43) | 0.935 | 1.01 (0.71-1.45) | 0.996 |
| Any symptom | 22 | 10 (45.5) | 0.95 (0.74-1.21) | 0.667 | 0.94 (0.73-1.20) | 0.850 |
DISCUSSION
This population-based study found that the burden of C. trachomatis organisms in noninvasively collected specimens from women was higher in vulvovaginal swabs than in first-void urine specimens. The chlamydial loads in female first-void urines were similar to those in male specimens. Chlamydial loads might be higher in urine specimens from 16- to 19-year-olds than in those from 20- to 24-year-olds. Chlamydial loads in female vulvovaginal swabs were higher for women with vaginal discharge. There was no association between chlamydial load in first-void urine specimens and symptoms in men.
The main strength of this study is that it is population based and provides information about chlamydial loads in people with a low prevalence of chlamydia and few or mild symptoms who were not attending health care settings. We used robust and reproducible methods to measure chlamydial load. qPCR was performed in duplicate, using a commercially available kit that has a four-point standard curve for reference and an internal control for the identification of inhibition and/or assay efficiency. Our choice of detection limit was made following discussion with the assay manufacturer. Nevertheless, over 20% of specimens had no measurable C. trachomatis DNA. We consider false-positive results for the original specimens to be very unlikely because all of the original reactive samples underwent confirmatory testing using a different methodology (5, 6, 11). There are three possible explanations for these results. First, some of the negative specimens might have had very low copy numbers of chlamydial DNA, as the reliability of the assay decreases below 200 copies/ml (9). Second, it is possible that some DNA suffered degradation following direct sample storage. Testing in duplicate would not exclude the presence of chlamydial DNA, particularly at lower copy numbers. Third, because the assay targets the single-copy OmpA gene, rather than the 8- to 10-copy chlamydial plasmid, the sensitivity of the test is less than that of, for example, the Roche Amplicor system.
The main weakness of this study was the small sample size, particularly for men, owing to the number of specimens that could not be included in the analysis. We do not think that excluding the specimens without detectable DNA resulted in differential bias because the distributions of measured characteristics were similar for individuals with and without valid qPCR results. Due to a lack of statistical power, we might, however, have underestimated the importance of differences in chlamydial load over time. The lack of strong associations between symptoms and chlamydial loads in first-void urine specimens might also have been influenced by the sample size.
We are aware of only two other published studies using qPCR to examine associations between chlamydial load and specimen type (4, 8). Other studies which have looked at the association between chlamydial burden and clinical presentation or partner numbers used quantitative culture (1, 2) or a surrogate marker for chlamydial load based on the greater sensitivity of nucleic acid amplification tests than those of enzyme immunoassay and culture (10). The values reported in these studies cannot be compared directly with those of qPCR. All former studies involved patients attending clinical settings, many of whom would have had symptoms that prompted consultation. Our study, in contrast, is more likely to be representative of chlamydial infection in the general population (6).
Michel et al., who studied attendees at a sexually transmitted disease clinic, found that the average chlamydial loads were 20 times higher in male (11,999 EB/ml [equivalent to copies/ml]) than female first-void urine specimens (470 EB/ml), whereas we found them to be similar (8). Male patients attending departments of sexually transmitted diseases often have symptomatic disease. However, when one excludes those with significant clinical disease in the study by Michel et al., the observed chlamydial loads in female vulvovaginal swabs and male and female first-void urine specimens are similar to those obtained in this study (8). Michel et al. observed mean chlamydial loads of 14,950 EB/ml in vulvovaginal swabs and 270 EB/ml in urine specimens from women with up to one symptom (vaginal irritation, abnormal vaginal discharge, irregular vaginal bleeding, dysuria, and pelvic or lower abdominal pain) and no signs, compared to 36,050 EB/ml and 11,400 EB/ml, respectively, for women with two or more symptoms or signs, and saw a load of 1,130 EB/ml for men without urethritis, compared to 21,430 EB/ml for those with urethritis (8). This suggests that the symptoms reported by men in our study were not severe enough to have prompted treatment seeking and that, in a community setting, chlamydial loads in male and female first-void urine specimens are similar.
Patient-reported symptoms in women appear to be inconsistently associated with chlamydial load. In our study, only the presence of vaginal discharge was associated with higher chlamydial loads in vulvovaginal swabs. Geisler et al. also reported an association with intermenstrual bleeding and a lack of association with abdominal pain (2), but they found that reported vaginal discharge was not associated with chlamydial load. Michel et al. found no association with reported irregular vaginal bleeding but did find an association with vaginal discharge and abdominal pain (8).
Our finding of higher chlamydial loads in first-void urine specimens from younger patients supports the observations of Eckert et al., who used quantitative culture on cervical swabs from women and urethral swabs from men (1). The time between acquisition of C. trachomatis and clinical presentation was not known in any study. The association with age, however, is compatible with younger adults having acquired their infection more recently and with the development of acquired immunity in older adults, resulting in a lower detectable burden of infection. However, chlamydial load was not associated with age for vulvovaginal specimens from women in our study. As with the difference demonstrated between symptoms and chlamydial load for vulvovaginal swabs but not urine, caution must be applied in interpreting these results, as the qPCR did not detect a measurable chlamydial load in all samples which had previously been classified as positive.
In this study, chlamydial load values for infected persons in the community who were not seeking treatment were lower than those published in other studies involving symptomatic patients attending clinical settings. Age might also be an important determinant of chlamydial load. This requires further investigation, as it is possible that the observed differences in chlamydial load might influence the epidemiology of chlamydia, with patients with lower loads being less infectious and at lower risk of developing disease. The results of this study provide a scientific rationale for preferring vulvovaginal swabs to first-void urine specimens from women.
Acknowledgments
John Macleod and Nicola Low were funded during the ClaSS project by National Health Service career scientist awards. Paddy Horner was supported by Jefferiss Trust grant 2002-7 and by a clinical senior lectureship award, cofunded by the Higher Education Funding Council for England (HEFCE) and the NHS through local NHS trusts.
Members of the ClaSS Project group are as follows: Matthias Egger, Pelham Barton, Stirling Bryan, Rona Campbell, E. Owen Caul, George Davey Smith, Gavin Daker-White, Anna Graham, Deborah Hawkings, Alan Herring, F. D. Richard Hobbs, Aisha Holloway, Paddy Horner, Mia Huengsberg, Fowzia Ibrahim, Nicola Low, John Macleod, Anne McCarthy, Nicola Mills, Andrea Morcom, Rita Patel, Ian Paul, Tim Peters, Karl Pye, Tracy Roberts, Suzanne Robinson, Jonathan Ross, Chris Salisbury, Emma Sanford, Joanne Sell, Sue Skidmore, Jonathan Sterne, Jane Thomas, and Mark Young.
Footnotes
Published ahead of print on 8 April 2009.
REFERENCES
- 1.Eckert, L. O., R. J. Suchland, S. E. Hawes, and W. E. Stamm. 2000. Quantitative Chlamydia trachomatis cultures: correlation of chlamydial inclusion-forming units with serovar, age, sex, and race. J. Infect. Dis. 182540-544. [DOI] [PubMed] [Google Scholar]
- 2.Geisler, W. M., R. J. Suchland, W. L. Whittington, and W. E. Stamm. 2001. Quantitative culture of Chlamydia trachomatis: relationship of inclusion-forming units produced in culture to clinical manifestations and acute inflammation in urogenital disease. J. Infect. Dis. 1841350-1354. [DOI] [PubMed] [Google Scholar]
- 3.Geisler, W. M., C. Wang, S. G. Morrison, C. M. Black, C. I. Bandea, and E. W. Hook. 2008. The natural history of untreated Chlamydia trachomatis infection in the interval between screening and returning for treatment. Sex. Transm. Dis. 35119-123. [DOI] [PubMed] [Google Scholar]
- 4.Herring, A., D. Longhurst, K. Eastick, I. Paul, J. Leece, O. Caul, and P. Horner. 2002. Which is the best specimen for chlamydial screening? Chlamydial ‘loads’ in urine and vulvo/vaginal swabs, p. 421-424. In J. Schachter, G. Christiansen, I. N. Clarke, M. R. Hammerschlag, B. Kaltenboeck, C. C. Kuo, R. Rank, G. Ridgway, P. Saikku, W. E. Stamm, R. S. Stephens, J. T. Summersgill, P. Timms, and P. B. Wyrick (ed.), Proceedings of the Tenth International Symposium on Human Chlamydial Infections. International Chlamydia Symposium, San Francisco, CA.
- 5.Horner, P., S. Skidmore, A. Herring, J. Sell, I. Paul, O. Caul, M. Egger, A. McCarthy, E. Sanford, C. Salisbury, J. MacLeod, J. Sterne, N. Low, et al. 2005. Enhanced enzyme immunoassay with negative-gray-zone testing compared to a single nucleic acid amplification technique for community-based chlamydial screening of men. J. Clin. Microbiol. 432065-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horner, P., and F. Boag. 2006. UK national guideline for the management of genital tract infection with Chlamydia trachomatis. BASHH, London, United Kingdom. http://www.bashh.org/documents/61/61.pdf.
- 7.Low, N., A. McCarthy, J. Macleod, C. Salisbury, R. Campbell, T. E. Roberts, P. Horner, S. Skidmore, J. A. Sterne, E. Sanford, F. Ibrahim, A. Holloway, R. Patel, P. M. Barton, S. M. Robinson, N. Mills, A. Graham, A. Herring, E. O. Caul, G. Davey Smith, F. D. Hobbs, J. D. Ross, M. Egger, et al. 2007. Epidemiological, social, diagnostic and economic evaluation of population screening for genital chlamydial infection. Health Technol. Assess. (Winchester, England) 111-184. [DOI] [PubMed] [Google Scholar]
- 8.Michel, C. E., C. Sonnex, C. A. Carne, J. A. White, J. P. Magbanua, E. C. Nadala, and H. H. Lee. 2007. Chlamydia trachomatis load at matched anatomic sites: implications for screening strategies. J. Clin. Microbiol. 451395-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiagen. 2008. C. trachomatis PCR kit handbook. Qiagen, Hilden, Germany. http://www1.qiagen.com/literature/handbooks/literature.aspx?id=1000839.
- 10.Rogers, S. M., W. C. Miller, C. F. Turner, J. Ellen, J. Zenilman, R. Rothman, M. A. Villarroel, A. Al-Tayyib, P. Leone, C. Gaydos, L. Ganapathi, M. Hobbs, and D. Kanouse. 2008. Concordance of Chlamydia trachomatis infections within sexual partnerships. Sex. Transm. Infect. 8423-28. [DOI] [PubMed] [Google Scholar]
- 11.Skidmore, S., P. Horner, A. Herring, J. Sell, I. Paul, J. Thomas, E. O. Caul, M. Egger, A. McCarthy, E. Sanford, C. Salisbury, J. MacLeod, J. A. C. Sterne, N. Low, et al. 2006. Vulvovaginal-swab or first-catch urine specimen to detect Chlamydia trachomatis in women in a community setting? J. Clin. Microbiol. 444389-4394. [DOI] [PMC free article] [PubMed] [Google Scholar]