Abstract
Vancomycin MICs (V-MIC) and the frequency of heteroresistant vancomycin-intermediate Staphylococcus aureus (hVISA) isolates are increasing among methicillin (meticillin)-resistant Staphylococcus aureus (MRSA) isolates, but their relevance remains uncertain. We compared the V-MIC (Etest) and the frequency of hVISA (Etest macromethod) for all MRSA blood isolates saved over an 11-year span and correlated the results with the clinical outcome. We tested 489 isolates: 61, 55, 187, and 186 isolates recovered in 1996-1997, 2000, 2002-2003, and 2005-2006, respectively. The V-MICs were ≤1, 1.5, 2, and 3 μg/ml for 74 (15.1%), 355 (72.6%), 50 (10.2%), and 10 (2.1%) isolates, respectively. We detected hVISA in 0/74, 48/355 (13.5%), 15/50 (30.0%), and 8/10 (80.0%) isolates with V-MICs of ≤1, 1.5, 2, and 3 μg/ml, respectively (P < 0.001). The V-MIC distribution and the hVISA frequency were stable over the 11-year period. Most patients (89.0%) received vancomycin. The mortality rate (evaluated with 285 patients for whose isolates the trough V-MIC was ≥10 μg/ml) was comparable for patients whose isolates had V-MICs of ≤1 and 1.5 μg/ml (19.4% and 27.0%, respectively; P = 0.2) but higher for patients whose isolates had V-MICs of ≥2 μg/ml (47.6%; P = 0.03). However, the impact of V-MIC and hVISA status on mortality or persistent (≥7 days) bacteremia was not substantiated by multivariate analysis. Staphylococcal chromosome cassette mec (SCCmec) typing of 261 isolates (including all hVISA isolates) revealed that 93.0% of the hVISA isolates were SCCmec type II. These findings demonstrate that the V-MIC distribution and hVISA frequencies were stable over an 11-year span. A V-MIC of ≥2 μg/ml was associated with a higher rate of mortality by univariate analysis, but the relevance of the V-MIC and the presence of hVISA remain uncertain. A multicenter prospective randomized study by the use of standardized methods is needed to evaluate the relevance of hVISA and determine the optimal treatment of patients whose isolates have V-MICs of ≥2.0 μg/ml.
The treatment of methicillin (meticillin)-resistant Staphylococcus aureus (MRSA) bacteremia with vancomycin is often associated with a poor clinical outcome (6, 15, 28, 40). Treatment failure was reported among patients infected with isolates whose vancomycin MICs were ≥4 μg/ml (6, 9, 12, 25, 28, 42). This prompted the Clinical and Laboratory Standards Institute to lower the cutoffs for S. aureus susceptibility to ≤2 μg/ml for susceptible, 4 to 8 μg/ml for intermediate (vancomycin-intermediate S. aureus [VISA]), and 16 μg/ml for resistance (39). Within the susceptibility range, the MIC is reported to increase over time (14, 25, 35-40). This is often referred to as MIC creep (38). Additionally, isolates with heteroresistance (heteroresistant vancomycin-intermediate S. aureus [hVISA]) are emerging, and this has uncertain implications for laboratory detection and clinical management (2, 5, 15, 24, 40-42). The first isolate of hVISA to be identified was reported from Japan in 1997 (11). Since then, it has been reported worldwide at frequencies of 0 to 50% (2, 4, 6, 9, 12, 19, 20, 21, 24, 26, 27, 31, 40, 42, 44). This disparity in frequency is probably a result of its variable incidence and the different testing methodologies used. Likewise, the frequency of isolates with MICs of 1.5 to <4 μg/ml varies according to the testing method used (3, 32). The relevance of an MIC on the higher side of the susceptibility range and the presence of hVISA isolates remains uncertain (8, 19, 21). Therapeutic failure was reported in patients infected with isolates with vancomycin MICs of 2 μg/ml (6, 12, 28) and 1.5 or 1 μg/ml (25, 34, 37). Most clinical microbiology laboratories use automated testing methods that are known to underestimate the vancomycin MIC (13, 24). Additionally, most previous studies addressing the relevance of such isolates were observational and usually involved only a few patients and poorly selected controls (1, 4, 7, 9, 12, 14, 25, 35, 38, 42). At our institution, we found the frequency of hVISA isolates among isolates from patients with persistent MRSA bacteremia to be 14%; however, heteroresistance did not correlate with the mortality rate (19). In the current study, we tested all blood MRSA isolates collected over 11 years to determine whether the vancomycin MIC and the prevalence of hVISA have changed over time and to evaluate the effects of increasing vancomycin MICs and the hVISA frequency on patient outcomes.
MATERIALS AND METHODS
Laboratory methods.
All blood MRSA isolates collected from S. aureus bacteremia studies conducted over the previous 11 years and saved at our research laboratory were selected (16-18). They were preserved in skim milk at −80°C until they were tested.
The vancomycin MIC was determined by the standard vancomycin Etest (AB Biodisk, Piscataway, NJ) on Mueller-Hinton agar (Remel, Lenexa, KS). The results were compared to those obtained by broth microdilution for 254 isolates. Screening for hVISA was performed by the vancomycin-teicoplanin Etest macromethod, according to the manufacturer's instructions (AB Biodisk). Briefly, several colonies from an overnight culture were selected and suspended in Mueller-Hinton broth to reach a 2.0 McFarland density standard. A 100-μl aliquot of the suspension was inoculated onto a brain heart infusion agar (Remel) plate and allowed to dry. Vancomycin and teicoplanin Etest strips were applied to each plate, and the plates were incubated for 48 h at 35°C. The plates were then examined and the results were recorded at 24 and 48 h. Control strains (methicillin-susceptible S. aureus ATCC 29213, Mu50, Mu3) were also included in the MIC and hVISA screening tests. Mu3 is a prototype strain of hVISA and is considered a precursor of Mu50 (a VISA strain); both strains are often used as controls in susceptibility tests (14, 19, 31). A strain was considered hVISA if growth in the presence of ≥8 μg for both vancomycin and teicoplanin or ≥12 μg for teicoplanin alone was noted. The results were independently interpreted by two investigators.
Genotyping.
Genotyping of all hVISA isolates and 37 non-hVISA isolates with vancomycin MICs of ≥2 μg/ml was performed with DiversiLab repetitive sequence-based PCR Staphylococcus kits and MoBio UltraClean microbial DNA isolation kits (Bacterial Barcodes, Inc., bioMerieux), according to the manufacturer's instructions (33).
SCCmec typing.
The staphylococcal chromosome cassette mec (SCCmec) types of 261 isolates, which encompassed all isolates recovered from the cases of infection in 2006 and strains with the hVISA phenotype or a vancomycin MIC of ≥2 μg/ml, were determined. DNA was extracted by a standard procedure. Frozen isolates were grown on Trypticase soy agar with 5% sheep blood (Remel) overnight at 35°C. One to three isolated colonies were suspended in 50 μl sterile water, and the mixture was heated to 99°C for 15 min. Cellular debris was cleared by centrifugation at 16,000 × g for 1 min. The DNA in the supernatant was used for SCCmec typing by multiplex PCR and was then stored at −20°C. Briefly, a master mixture was prepared. The master mixture contained 18 primers (Applied Biosystems, Foster City, CA) targeting SCCmec types I, II, III, IVa, IVb, IVc, IVd, and V and the mecA gene (internal control); Platinum Taq DNA polymerase; deoxynucleoside triphosphates; MgCl2; and PCR buffer (Invitrogen, Carlsbad, CA), as described by Zhang et al. (45). Two microliters of the extracted DNA was added to 48 μl master mixture, and the DNA was amplified according to the instructions of the manufacturer. The amplified product was separated by electrophoresis, stained, photographed, and analyzed on a Chemi-Imager 4000 apparatus (Alpha Innotech, San Leandro, CA). Positive and negative controls (four ATCC MRSA strains [strains BAA-39, BAA-41, BAA-42, and BAA-44] and four previously typed MRSA patient strains [strains CA60-1, CA85, T-127, and T-162]) were included in each PCR assay.
Clinical data.
The following patient information was collected: demographics, underlying conditions, organ dysfunction score, source of infection, duration of bacteremia, metastatic foci, vancomycin trough concentrations (when available), and treatment outcome (the patient's status at the time of hospital discharge).
Definitions.
Bacteremia was defined as one or more positive blood cultures with systemic manifestations of infection, such as fever, chills, and sweats with or without local signs and symptoms. It was considered community associated when it appeared within 48 h of admission without any health care-associated risk factors (the presence of an invasive device; a history of surgery, hospitalization, or dialysis; or residence in a long-term care facility within the preceding 12 months) or health care associated (onset ≥48 h after admission or with one or more health care risk factors), as described by Klevens et al. (22). The duration of bacteremia was defined as the number of days between the first and the last positive blood culture (16). The duration among patients who died or who were discharged before clearance was counted until death or discharge from the hospital. The source of bacteremia was identified on the basis of the presence of local signs and the isolation of S. aureus from the implicated source, as described previously (16), and the Duke criteria for endocarditis (23). Metastatic infection was defined as a distant focus anatomically unrelated to the implicated source. Persistent bacteremia was defined as positive blood cultures for ≥7 days. Organ dysfunction score was calculated on the basis of the number of dysfunctional organs and the severity of the dysfunction, as described previously (17). Outcomes included cure or death. The duration of bacteremia, complications, and outcome were stratified according to the vancomycin MIC and hVISA status. Treatment was considered adequate if the vancomycin trough level was ≥10 μg/ml.
Statistical analysis.
We used chi-square analysis for categorical variables, Student's t test or analysis of variance for continuous variables, Spearman's correlation for bivariate associations, and stepwise forward logistic regression for multivariate analysis of dichotomous outcomes. All tests were performed with the computer software SPSS (version 15). A P value of <0.05 was considered to indicate statistical significance.
RESULTS
Four hundred eighty-nine MRSA blood isolates were tested over four time periods spanning 11 years: from 1 February 1996 to 31 December 1996, from 1 October 2000 to 31 March 2001, from 1 January 2002 to 30 June 2003, and from 1 November 2005 to 31 December 2006. The vancomycin MIC determined by Etest was compared with the standard broth microdilution MIC for 254 isolates. The results were concordant for 155 isolates (61.0%). Among the remainder of the isolates, the Etest MIC was 0.5 to 1 μg/ml higher than the standard broth microdilution result for 61 isolates (24.0%) and 0.5 to 1 μg/ml lower for 38 isolates (15.0%). We selected the Etest MIC determination method for our analysis. The vancomycin MICs were 1.5 and ≥2 μg/ml for 355 (72.6%) and 60 (12.3%) isolates, respectively. We detected hVISA in 71 instances (14.5%). The MIC distribution and hVISA frequency were similar in all time periods (Table 1). The frequency of hVISA was MIC dependent (Fig. 1). No hVISA isolate for which the vancomycin MIC was ≤1 μg/ml was detected.
TABLE 1.
Vancomycin MIC and hVISA frequency among 489 MRSA blood isolates collected over an 11-year span
| Characteristic | No. (%) of isolates recovered in the following study period:
|
||||
|---|---|---|---|---|---|
| 1996-1997 (n = 61) | 2000-2001 (n = 55) | 2002-2003 (n = 187) | 2005-2006 (n = 186) | Total (n = 489) | |
| Vancomycin MIC (μg/ml) | |||||
| ≤1 | 11 (18.0) | 10 (18.2) | 26 (13.9) | 27 (14.5) | 74 (15.1) |
| 1.5 | 40 (65.6) | 38 (69.1) | 139 (74.3) | 138 (74.2) | 355 (72.6) |
| 2 | 9 (14.5) | 7 (12.7) | 18 (9.6) | 16 (8.6) | 50 (10.2) |
| 3 | 1 (1.6) | 0 | 4 (2.2) | 5 (2.7) | 10 (2.1) |
| hVISA phenotype | 8 (13.1) | 5 (9.1) | 37 (19.8) | 21 (11.3) | 71 (14.5) |
FIG. 1.
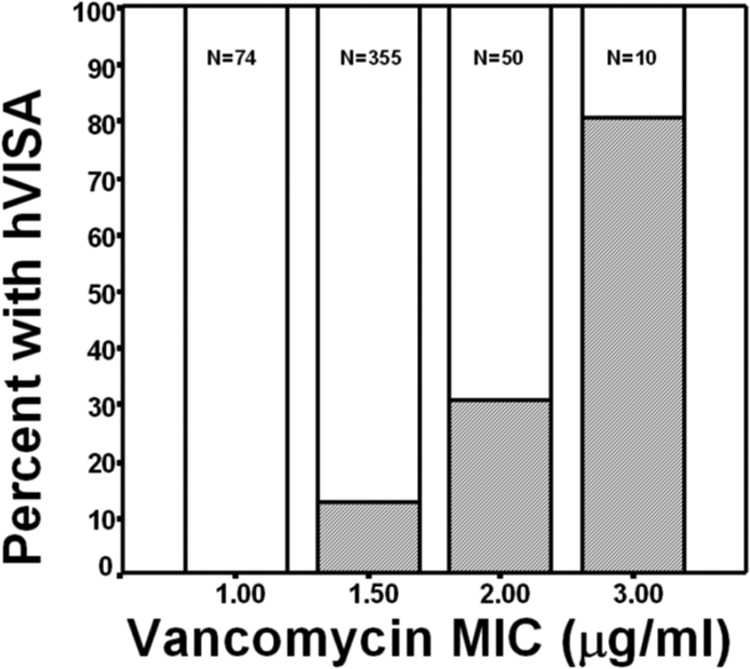
Frequency of hVISA (shaded area) among MRSA blood isolates saved intermittently between 1996 and 2006, stratified according to the vancomycin MIC.
SCCmec typing of 261 isolates revealed that the majority of hVISA isolates (93.0%) were SCCmec type II. The frequencies of hVISA isolates were 38.6% among SCCmec type II isolates and 3.5% among SCCmec type IVa isolates. SCCmec type II isolates had higher MICs than SCCmec type IVa isolates (1.7 ± 0.4 and 1.5 ± 0.4 μg/ml, respectively; P = 0.007).
Genotyping (by repetitive sequence-based PCR) of 108 isolates (all of which had the hVISA phenotype or a vancomycin MIC of ≥2 μg/ml) revealed a large cluster (n = 46; 42.6%) within 97.5% similarity to US100, two other clusters (n = 22 [20.4%] and n = 21 [19.4%]), and four small clusters (2 to 4 isolates each). Sixty-seven hVISA isolates (94.4%) were distributed among the three major clusters.
The majority of patients (n = 435; 89.0%) were treated with vancomycin. The vancomycin trough level was measured in 365 (85.9%) vancomycin-treated patients. The trough level was ≥10 μg/ml in 285 (76.2%) cases. The association between the MIC or hVISA phenotype and the outcome was evaluated for the 285 cases treated with vancomycin who had vancomycin trough levels of ≥10 μg/ml. The remainder of the cases did not meet the treatment selection criteria: the vancomycin trough level was ≤10 μg/ml (n = 80; 16.4%), the vancomycin level was not measured (n = 70; 14.3%), the patients were treated with drugs other than vancomycin (n = 21; 4.3%), treatment information was missing (n = 9; 1.8%), and the patient died or treatment was withdrawn within 1 day (n = 24; 4.9%). The mortality rate correlated with the vancomycin MIC (r = 0.138; P = 0.05) (Fig. 2); however, it did not correlate with the vancomycin trough level. We repeated the analysis for the 365 vancomycin-treated subjects, irrespective of the trough level. We did not detect any correlation between the trough level and mortality (data not shown). Comparison of patients with hVISA infections and patients without hVISA infections revealed that patients infected with hVISA had a slightly longer duration of bacteremia, persistent bacteremia, and a higher mortality rate; but the differences were not significant (Table 2).
FIG. 2.
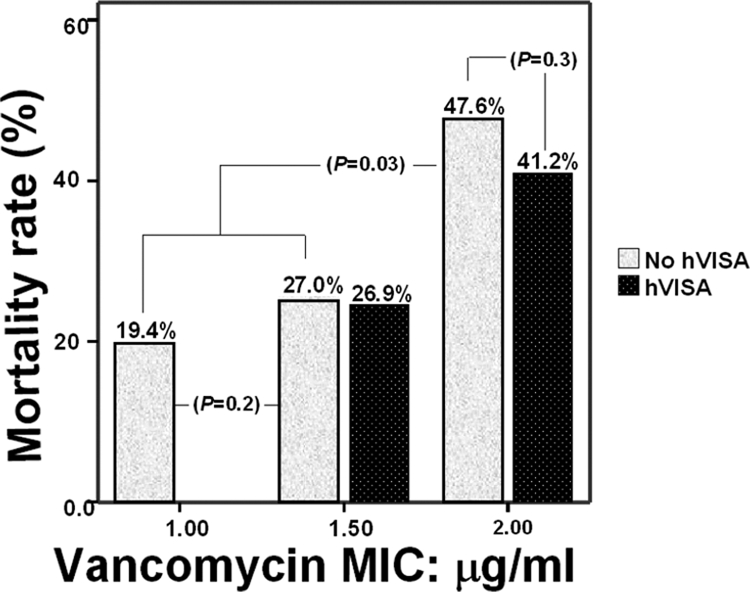
Mortality rate among patients with MRSA bacteremia stratified according to vancomycin MIC and hVISA infection status.
TABLE 2.
Characteristics of patients with MRSA bacteremia stratified according to hVISA infection status
| Characteristic | hVISA (n = 71) | No hVISA (n = 416) | P |
|---|---|---|---|
| Mean age (yr) ± SD | 62.3 ± 17.5 | 62.7 ± 16.4 | 0.8 |
| No. (%) of patients | |||
| Male gender | 33 (46.5) | 226 (54.1) | 0.3 |
| African American | 37 (52.1) | 198 (47.4) | 0.7 |
| On hemodialysis | 12 (16.9) | 95 (22.7) | 0.3 |
| With cardiac comorbidity | 36 (50.7) | 205 (49.0) | 0.8 |
| With diabetes | 34 (47.9) | 165 (39.5) | 0.2 |
| With endovascular source | 32 (45.1) | 185 (44.3) | 0.9 |
| With health care-associated infection | 55 (77.5) | 315 (75.4) | 0.7 |
| With a prosthesis | 13 (18.3) | 66 (15.8) | 0.6 |
| Mean organ dysfunction score ± SD | 3.2 ± 1.8 | 3.2 ± 1.7 | 0.9 |
| Mean duration of bacteremia (days) ± SDa | 6.4 ± 7.7 | 4.9 ± 6.9 | 0.2 |
| No. of patients with the following/total no. (%): | |||
| Persistence (≥7 days)a | 13/43 (30.2) | 60/242 (24.8) | 0.5 |
| Metastatic infectiona | 7/43 (16.3) | 41/242 (16.9) | 0.9 |
| Mortality ratea | 14/43 (32.6) | 67/242 (27.7) | 0.5 |
Determined for 285 patients who received adequate treatment.
Stepwise forward logistic regression analysis with mortality as the dependent variable and all clinically relevant variables as covariates revealed that the predictors of mortality were age, organ dysfunction score, and persistent bacteremia (Table 3). The predictors of persistent bacteremia were metastatic foci (odds ratio [OR], 4.41; 95% confidence interval [CI], 2.21 to 8.80), an endovascular source (OR, 2.86; 95% CI, 1.52 to 5.39), and diabetes (OR, 1.94; 95% CI, 1.08 to 3.48).
TABLE 3.
Predictors of mortality in patients with MRSA bacteremia determined by multivariate analysis with forward logistic regression analysis
| Predictor | P | OR | 95% CI |
|---|---|---|---|
| Age | ≤0.001 | 1.05 | 1.03, 1.07 |
| Organ dysfunction score | ≤0.001 | 1.51 | 1.26, 1.80 |
| Persistent bacteremia | 0.001 | 2.82 | |
| Vancomycin MIC | 0.40 | NAa | |
| hVISA phenotype | 0.99 | NA | |
| Endovascular source | 0.58 | NA | |
| Hemodialysis | 0.12 | NA | |
| Diabetes | 0.30 | NA | |
| Cardiac morbidity | 0.60 | NA | |
| Metastatic infection | 0.82 | NA | |
| Vancomycin trough level | 0.86 | NA |
NA, did not meet model entry criteria.
DISCUSSION
Vancomycin has been the standard treatment for MRSA bacteremia since the emergence of MRSA, but concerns about its efficacy have been increasing. The failure of treatment with vancomycin has been attributed to its slow bactericidal activity, the presence of subtherapeutic concentrations, and the reduced susceptibility of organisms (7, 25, 29, 30, 34, 40). The interpretive criteria for susceptibility were lowered on the basis of the reported failure of treatment of infections caused by organisms with higher MICs (39). Recently, an upward trend in MICs within the susceptibility range, often referred to as “MIC creep,” was reported (38, 39). Additionally, the inadequacy of vancomycin treatment of infections caused by organisms with MICs of 2 μg/ml and lower has been reported (25, 30, 35, 36). Furthermore, hVISA had been proposed as another cause of treatment failure. Despite these reports, the frequency and the relevance of hVISA and isolates with MICs at the higher end of the susceptibility range remain uncertain (10, 24, 36). These uncertainties are due to the various detection methods used (8, 20, 24, 31, 40, 42, 44), the questionable stability of the hVISA phenotype (30, 31, 40, 41, 43), and the small and observational nature of most reported studies (4, 6, 8, 9, 25, 26, 34, 36).
Our study presents data that address most of these shortcomings. We assessed our isolates using a single Etest method. We compared the Etest MIC with the standard broth microdilution MIC and found the results to be concordant for most isolates. We were unable to confirm the findings of Prakash et al. of higher MICs by Etest method (32). We chose to use the Etest macromethod for the detection of hVISA isolates. This is a much less labor-intensive and less costly procedure than the standard broth microdilution method and maintains excellent sensitivity (96.0%) and specificity (97.0%) that are better than those achieved by population analysis (11, 34, 44). We stratified patient outcome measures according to the MIC and hVISA status.
Our data show that the hVISA frequency was MIC dependent. We did not detect any hVISA isolates among isolates with MICs of ≤1 μg/ml. The frequency increased steadily with higher MICs. Similar associations were previously noted (34, 35). Our findings also illustrate that the vancomycin MIC and the hVISA frequency remained stable over an 11-year span. Similar observations were noted for isolates collected between 1997 and 2003 (15). In comparison, a retrospective study of S. aureus isolates collected from two teaching hospitals and one community hospital in the Detroit, MI, metropolitan area over a period of 22 years revealed an increase in the prevalence of hVISA isolates from 2.2% in 1986 to 1993 to 7.6% in 1994 to 2002 and 8.3% in 2003 to 2007 (25, 34). These results suggest that a vancomycin MIC shift may have occurred prior to the time of our study but that continuous vancomycin use did not contribute to additional MIC increases or a higher prevalence of hVISA during the study period. In Michigan, MRSA had been around for many years, and an earlier MIC shift is possible. We also show that hVISA appears to be SCCmec type dependent. It was prevalent among SCCmec type II isolates and was distinctly uncommon among SCCmec type IVa isolates. The association of SCCmec type II with higher MICs and the hVISA phenotype has been noted by others (33).
The impact of hVISA on patient outcomes remains uncertain. Several studies examined the risk factors and their impacts on outcomes (4, 8, 10, 12, 24, 41). A prospective nested case-control study conducted in the United States from 1997 to 2000 revealed an overall rate of mortality of 63% among 19 patients with Staphylococcus aureus infection caused by isolates with reduced vancomycin susceptibilities, including 4 VISA isolates and 15 hVISA isolates (10). Independent risk factors were antecedent vancomycin use and a MRSA infection 2 to 3 months before the current infection. Another study from Hong Kong reported an incidence of hVISA of 8.9% among 203 cases of staphylococcal bacteremia from 1997 to 1998 and a higher rate of mortality among patients infected with hVISA isolates than among those not infected with such isolates (44.4% and 10.0%, respectively) (42). In our study, hVISA status did not affect mortality. The reason for these differences is not clear. It might reflect a difference in case selection, patient populations, or the hVISA screening methods used.
We also failed to show any association between the presence of hVISA and persistent bacteremia, although different results have been reported (2, 4, 26, 29, 34). The reason for these discrepancies is not clear. Our study was not powered to detect a small difference. We suspect that most single-center studies will not be of adequate size for the meaningful assessment of the relevance of the hVISA phenotype.
Our study has limitations. We did not record exposure to vancomycin prior to the bacteremia. In addition, our tests were performed with frozen isolates that had undergone various numbers of passages. Whether freezing leads to a loss of the hVISA phenotype or a change in the MIC is uncertain. Nevertheless, the relevance of unstable hVISA status is uncertain.
Despite these limitations, our study illustrates that the vancomycin MIC and the frequency of hVISA did not change over the last 11 years. We also demonstrated that the relevance of isolates with higher vancomycin MICs and the hVISA phenotype remains undocumented. Vancomycin remains effective against infections caused by highly susceptible isolates (MICs, ≤1 μg/ml and probably 1.5 μg/ml). For isolates with higher MICs and the hVISA phenotype, the treatment of choice remains poorly defined. A multicenter prospective randomized study of the treatment for S. aureus bacteremia is needed to standardize testing methods, evaluate the relevance of the hVISA phenotype and vancomycin MICs of ≥2 μg/ml, and determine the optimal treatment and the benefit of screening for hVISA.
Acknowledgments
The study was supported by St. John Hospital Medical Education Fund.
We are grateful to Susan Szpunar for assistance in statistical analysis and Alice Mar for assistance with figure preparation.
Leonard B. Johnson is on the speakers bureau for Pfizer. None of the other authors has any conflict of interest for disclosure.
Footnotes
Published ahead of print on 15 April 2009.
REFERENCES
- 1.Appelbaum, P. C. 2007. Microbiology of antibiotic resistance in Staphylococcus aureus. Clin. Infect. Dis. 45S165-S170. [DOI] [PubMed] [Google Scholar]
- 2.Benquan, W., T. Yingchun, Z. Kouxing, Z. Tiantuo, Z. Jiaxing, and T. Shuqing. 2002. Staphylococcus heterogeneously resistant to vancomycin in China and antimicrobial activities of imipenem and vancomycin in combination against it. J. Clin. Microbiol. 401109-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle-Vavra, S., S. K. Berke, J. C. Lee, and R. S. Daum. 2000. Revision of glycopeptide resistance phenotype in Staphylococcus aureus clinical isolate. Antimicrob. Agents Chemother. 44272-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charles, P. G. P., P. B. Ward, P. D. R. Johnson, B. P. Howden, and L. Grayson. 2004. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin. Infect. Dis. 38448-451. [DOI] [PubMed] [Google Scholar]
- 5.Chua, T., C. L. Moore, M. B. Perri, S. M. Donabedian, W. Masch, D. Vagar, S. L. Davis, K. Lulek, B. Zimnicki, and M. J. Zervos. 2008. Molecular epidemiology of methicillin-resistant Staphylococcus aureus bloodstream isolates in urban Detroit. J. Clin. Microbiol. 462345-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delgado, A., J. T. Riordan, R. Lamichhane-Khadka, D. C. Winnett, J. Jimenez, K. Robinson, F. G. O'Brien, S. A. Cantore, and G. E. Gustafson. 2007. Hetero-vancomycin-intermediate methicillin-resistant Staphylococcus isolate from medical center in Las Cruces, New Mexico. J. Clin. Microbiol. 451325-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deresinski. S. 2007. Counterpoint: vancomycin and Staphylococcus aureus—an antibiotic enters obsolescence. Clin. Infect. Dis. 441543-1548. [DOI] [PubMed] [Google Scholar]
- 8.Falagas, M. E., G. C. Makris, G. Dimopoulos, and D. K. Matthaiou. 2008. Heteroresistance: a concern of increasing clinical significance? Clin. Microbiol. Infect. 14101-104. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira Nunez, A. P., R. P. Schuenck, C. C. Reis Bastos, M. M. Magnanini, J. B. Long, N. L. Iorio, and K. R. Santos. 2007. Heterogeneous resistance to vancomycin and teicoplanin among Staphylococcus spp. isolated from bacteremia. Brazil. J. Infect. Dis. 11345-350. [DOI] [PubMed] [Google Scholar]
- 10.Fridkin, S. K., J. Hageman, L. K. McDougal, J. Mohammed, W. R. Jarvis, T. M. Perl, F. C. Tenover, and Vancomycin-Intermediate Staphylococcus aureus Epidemiology Study Group. 2003. Epidemiological and microbiological characterization of infection caused by Staphylococcus aureus with reduced susceptibility to vancomycin, United States, 1997-2001. Clin. Infect. Dis. 36429-439. [DOI] [PubMed] [Google Scholar]
- 11.Hiramatsu, K., N. Aritaka, H. Hanaki, S. Kawasaki, Y. Hosoda, S. Hori, Y. Fukuchi, and I. Kobayashi. 1997. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 3501670-1673. [DOI] [PubMed] [Google Scholar]
- 12.Howden, B. P. 2005. Recognition and management of infections caused by vancomycin-intermediate Staphylococcus aureus (VISA) and heterogeneous VISA (hVISA). Int. Med. J. 35S136-S140. [DOI] [PubMed] [Google Scholar]
- 13.Howden, B. P., P. B. Ward, J. Robson, A. J. Morris, B. C. Mayall, and M. L. Grayson. 2004. Treatment outcomes for serious infections caused by methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. Clin. Infect. Dis. 38521-528. [DOI] [PubMed] [Google Scholar]
- 14.Hussain, F., S. Boyle-Vavra, P. B. Shete, and R. S. Daum. 2002. Evidence for continuum of decreased vancomycin susceptibility in unselected Staphylococcus clinical isolates. Clin. Infect. Dis. 186661-667. [DOI] [PubMed] [Google Scholar]
- 15.Jones, R. N. 2006. Microbiological features of vancomycin in the 21st century: minimum inhibitory concentration creep, bactericidal/static activity, and applied breakpoints to predict clinical outcomes or detect resistant strains. Clin. Infect. Dis. 42S13-S24. [DOI] [PubMed] [Google Scholar]
- 16.Khatib, R., L. B. Johnson, M. Sharma, M. G. Fakih, R. Ganga, and K. Riederer. 2009. Persistent Staphylococcus aureus bacteremia: incidence and outcome trends over time. Scand. J. Infect. Dis. 414-9. [DOI] [PubMed] [Google Scholar]
- 17.Khatib, R., K. Riederer, S. Saeed, L. B. Johnson, M. G. Fakih, M. Sharma, M. S. Tabriz, and A. Khosrovaneh. 2005. Time to positivity in Staphylococcus aureus bacteremia: possible correlation with the source and outcome of infection. Clin. Infect. Dis. 41594-598. [DOI] [PubMed] [Google Scholar]
- 18.Khatib, R., M. Sharma, A. H. Naqvi, K. Riederer, M. O. Almoujahed, and M. G. Fakih. 2003. Molecular analysis of Staphylococcus aureus blood isolates shows lack of polyclonal bacteremia. J. Clin. Microbiol. 411717-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khosrovaneh, A., K. Riederer, S. Saeed, M. Sharma, L. B. Johnson, M. Fakih, and R. Khatib. 2004. Frequency of reduced vancomycin susceptibility and heterogeneous subpopulation in persistent or recurrent methicillin resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 381328-1330. [DOI] [PubMed] [Google Scholar]
- 20.Kim, H. B., W. B. Park, K. D. Lee, Y. J. Choi, S. W. Park, M. D. Oh, E. C. Kim, and K. W. Choe. 2003. Nationwide surveillance for Staphylococcus aureus with reduced susceptibility to vancomycin in Korea. J. Clin. Microbiol. 412279-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim, M. N., S. H. Hwang, Y. J. Pyo, H. M. Mun, and C. H. Pai. 2002. Clonal spread of Staphylococcus aureus heterogeneously resistant to vancomycin in a university hospital in Korea. J. Clin. Microbiol. 401376-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klevens, R. M., M. A. Morrison, J. Nadle, S. Petit, K. Gersham, S. Ray, L. H. Harrison, R. Lynfield, G. Dumyati, J. M. Townes, A. S. Craig, E. R. Zell, G. E. Fosheim, L. K. McDougal, R. B. Carey, and S. K. Fridkin. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2981763-1771. [DOI] [PubMed] [Google Scholar]
- 23.Li, J. S., D. J. Sexton, N. Mick, R. Nettle, V. G. Fowler, Jr., T. Ryan, T. Bashore, and G. R. Corey. 2006. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. 30633-638. [DOI] [PubMed] [Google Scholar]
- 24.Liu, C., and H. F. Chambers. 2003. Staphylococcus aureus with heterogeneous resistance to vancomycin: epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob. Agents. Chemother. 473040-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lodise, T. P., J. Graves, A. Evans, E. Graffunder, M. Helmecke, B. M. Lomaestro, and K. Stellrecht. 2008. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob. Agents Chemother. 523315-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maor, Y., M. Hagin, N. Belausov, N. Keller, D. Ben-David, and G. Rahav. 2009. Clinical features of heteroresistant vancomycin-intermediate Staphylococcus aureus bacteremia versus those of methicillin-resistant S. aureus bacteremia. J. Infect. Dis. 199619-624. [DOI] [PubMed] [Google Scholar]
- 27.Maor, Y., G. Rahav, N. Belausov, D. Ben-David, G. Smollan, and N. Keller. 2007. Prevalence and characteristics of heteroresistant vancomycin-intermediate Staphylococcus aureus bacteremia in a tertiary care center. J. Clin. Microbiol. 451511-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchese, A., G. Balistreri, E. Tonoli, E. A. Debbia, and G. C. Schito. 2000. Heterogeneous vancomycin resistance in methicillin-resistant Staphylococcus strains isolated in a large Italian hospital. J. Clin. Microbiol. 38866-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohr, J. F., and B. E. Murray. 2007. Point: vancomycin is not obsolete for the treatment of infections caused by methicillin resistant Staphylococcus aureus. Clin. Infect. Dis. 441536-1542. [DOI] [PubMed] [Google Scholar]
- 30.Moise, P. A., A. Sakoulas, A. Forrest, and J. J. Schentag. 2007. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 512582-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plipat, N., G. Livni, H. Bertram, and R. B. Thomson, Jr. 2005. Unstable heteroresistance is common among clinical isolates of methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 432494-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prakash, V., J. S. Lewis II, and J. H. Jorgensen. 2008. Vancomycin MICs for methicillin-resistant Staphylococcus aureus isolates differ based upon the susceptibility test method used. Antimicrob. Agents Chemother. 524528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ross, T. L., W. G. Merz, M. Farkosh, and K. C. Carroll. 2005. Comparison of automated repetitive sequence-based PCR microbial typing system to pulse-field gel electrophoresis for analysis of outbreaks of methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 435642-5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rybak, M. J., S. N. Leonard, K. L. Rossi, C. M. Cheung, H. S. Sadar, and R. N. Jones. 2008. Characterization of vancomycin heteroresistant Staphylococcus aureus (hVISA) from the Detroit metropolitan area over 22-year period (1986-2007). J. Clin. Microbiol. 462950-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakoulas, G., P. A. Moise-Broder, J. Schentag, A. Forrest, R. C. Mollering, Jr., and G. M. Eliopoulos. 2004. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J. Clin. Microbiol. 422398-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwaber, M. J., S. B. Wright, Y. Carmeli, L. Venkataraman, P. C. DeGirolami, A. Gramatikova, T. M. Perl, G. Sakoulas, and H. S. Gold. 2003. Clinical implications of varying degrees of vancomycin susceptibility in methicillin-resistant Staphylococcus aureus bacteremia. Emerg. Infect. Dis. 9657-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soriano, A., F. Marco, A. J. Martinez, E. Pisos, M. Almela, V. P. Dimova, D. Alamo, M. Ortega, J. Lopez, and J. Mensa. 2008. Influence of vancomycin minimal inhibitory concentration on the treatment of methicillin resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 46193-200. [DOI] [PubMed] [Google Scholar]
- 38.Steinkraus, G., R. White, and L. Friedrich. 2007. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA) vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001-05. J. Antimicrob. Chemother. 60788-794. [DOI] [PubMed] [Google Scholar]
- 39.Tenover, F., and R. C. Moellering, Jr. 2007. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin. Infect. Dis. 441208-1215. [DOI] [PubMed] [Google Scholar]
- 40.Walsh, T. R., A. Bolmstrom, A. Qwarnstrom, P. Ho, M. Wootton, R. A. Howe, A. P. MacGowan, and D. Diekema. 2001. Evaluation of current methods for detection of staphylococci with reduced susceptibility to glycopeptides. J. Clin. Microbiol. 392439-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walsh, T. R., and R. A. Howe. 2002. The prevalence and mechanism of vancomycin resistance in Staphylococcus aureus. Annu. Rev. Microbiol. 56657-675. [DOI] [PubMed] [Google Scholar]
- 42.Wang, G., J. F. Hindler, K. W. Ward, and D. A. Bruckner. 2006. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J. Clin. Microbiol. 443883-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong, S. S., P. L. Ho, P. C. Woo, and K. Y. Yuen. 1999. Bacteremia caused by Staphylococcus aureus with inducible vancomycin heteroresistance. Clin. Infect. Dis. 29760-767. [DOI] [PubMed] [Google Scholar]
- 44.Wootton, M., A. P. MacGowan, T. R. Walsh, and R. A. Howe. 2007. A multicenter study evaluating the current strategies for isolating Staphylococcus aureus strains with reduced susceptibility to glycopeptides. J. Clin. Microbiol. 45329-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang, K., J. A. McClure, S. Elsayed, T. Louie, and J. M. Conly. 2005. Novel multiplex PCR assay for characterization and concomitant subtyping of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 435026-5033. [DOI] [PMC free article] [PubMed] [Google Scholar]


