Abstract
In a point-prevalence study performed in 145 Spanish hospitals in 2006, we collected 463 isolates of Staphylococcus aureus in a single day. Of these, 135 (29.2%) were methicillin (meticillin)-resistant S. aureus (MRSA) isolates. Susceptibility testing was performed by a microdilution method, and mecA was detected by PCR. The isolates were analyzed by pulsed-field gel electrophoresis (PFGE) after SmaI digestion, staphylococcal chromosomal cassette mec (SCCmec) typing, agr typing, spa typing with BURP (based-upon-repeat-pattern) analysis, and multilocus sequence typing (MLST). The 135 MRSA isolates showed resistance to ciprofloxacin (93.3%), tobramycin (72.6%), gentamicin (20.0%), erythromycin (66.7%), and clindamycin (39.3%). Among the isolates resistant to erythromycin, 27.4% showed the M phenotype. All of the isolates were susceptible to glycopeptides. Twelve resistance patterns were found, of which four accounted for 65% of the isolates. PFGE revealed 36 different patterns, with 13 major clones (including 2 predominant clones with various antibiotypes that accounted for 52.5% of the MRSA isolates) and 23 sporadic profiles. Two genotypes were observed for the first time in Spain. SCCmec type IV accounted for 6.7% of the isolates (70.1% were type IVa, 23.9% were type IVc, 0.9% were type IVd, and 5.1% were type IVh), and SCCmec type I and SCCmec type II accounted for 7.4% and 5.2% of the isolates, respectively. One isolate was nontypeable. Only one of the isolates produced the Panton-Valentine leukocidin. The isolates presented agr type 2 (82.2%), type 1 (14.8%), and type 3 (3.0%). spa typing revealed 32 different types, the predominant ones being t067 (48.9%) and t002 (14.8%), as well as clonal complex 067 (78%) by BURP analysis. The MRSA clone of sequence type 125 and SCCmec type IV was the most prevalent throughout Spain. In our experience, PFGE, spa typing, SCCmec typing, and MLST presented good correlations for the majority of the MRSA strains; we suggest the use of spa typing and PFGE typing for epidemiological surveillance, since this combination is useful for both long-term and short-term studies.
Methicillin (meticillin)-resistant Staphylococcus aureus (MRSA) is a major cause of hospital-acquired infections worldwide (5, 25). The appearance of MRSA in the community and the potential risk of it entering hospitals are also matters of concern (29, 44). Moreover, the increasing prevalence of multidrug resistance and the emergence of isolates with intermediate or high-level vancomycin resistance emphasize the importance of the use of infection control measures (2, 49, 50). Although the rates of isolation of MRSA have been increasing throughout the world for the last few decades and in some areas the rates reach >50%, there are considerable variations in the prevalence of MRSA according to geographic area (3, 18, 21, 39, 44). In Spain, the prevalence of MRSA increased from 1.5% in 1986 to 29.2% in 2006, although it seems to have stabilized (13). Despite the worldwide increase in isolation rates, only a limited number of clones of MRSA have spread in most countries (20).
Historically, the dissemination of epidemic clones such as EMRSA type 15 (EMRSA-15), EMRSA-16, the Iberian clone, and the Brazilian clone, as well as the high incidence of the community-acquired MRSA USA300 clone, has led to the increased use of molecular typing methods (11, 38, 42, 47, 53).
In recent years, a variety of molecular techniques have been used for the typing of MRSA isolates. Of these, SmaI macrorestriction analysis is the “gold standard” for the analysis of the local epidemiology in the short term, spa typing in combination with BURP (based-upon-repeat-pattern) analysis has become a frontline tool for routine epidemiological typing, and multilocus sequence typing (MLST)-staphylococcal chromosomal cassette mec (SCCmec) typing is the reference method for the definition of MRSA clones (10, 34, 37, 46).
The aim of the present study was to determine which clones are circulating in Spain and whether the strains have spread between hospitals by analyzing a representative sample of isolates collected in a point-prevalence study. Isolates were grouped by using pulsed-field gel electrophoresis (PFGE) and spa typing and were assigned to MRSA clones on the basis of MLST and SCCmec typing. The congruence between the different grouping methods was assessed.
(This study was presented in part at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, 2007 [O. Cuevas, C. Marcos, P. Trincados, T. Boquete, E. Cercenado, E. Bouza, and A. Vindel; abstr. C2-148].)
MATERIALS AND METHODS
Bacterial isolates.
A point-prevalence study involving 145 Spanish hospitals on a single day in 2006 yielded a total of 463 clinical isolates of S. aureus. Full details of the study design and identification of the isolates have been published previously (13, 14). Of the total number of isolates tested, 135 were MRSA.
Susceptibility testing.
Antimicrobial susceptibility testing was performed by an automated broth microdilution method with the Pos Combo 23S panel (MicroScan; Siemens, Sacramento, CA), according to the manufacturer's guidelines. MIC breakpoints were determined according to the recommendations of the Clinical and Laboratory Standards Institute (CLSI) (9). Full details of the antimicrobial susceptibility tests have been published elsewhere (13, 14).
Methicillin resistance detection.
The mecA gene was detected by PCR, as described by Geha et al. (24).
PFGE.
All 135 MRSA isolates were genotyped by PFGE after SmaI digestion of chromosomal DNA, prepared by using a modification of the protocol described by Cookson et al. (10). This technique has been fully described previously (12). Analysis of the gels was performed according to the criteria of Tenover et al. (48), and a dendrogram was constructed with Molecular Analyst software (Bio-Rad) by using the Dice correlation coefficient (28) and the unweighted pair-group method with averages with a tolerance position of 0.8%. According to the findings of our previous studies (12, 53), each PFGE type was assigned the letter E, followed by a number that correlated with the date of isolation, while each subtype was assigned the letter of the main genotype to which it was the closest. Sporadic strains were indicated in each case. In our previous studies (12, 53), PFGE type E1 corresponded to a MRSA isolate of sequence type 247 (ST247) and SCCmec type I (ST247-MRSA-I); types E3 and E10 corresponded to ST146-MRSA-IV; types E6, E9, E15, and E17 corresponded to ST228-MRSA-I; types E7, E8, and E11 corresponded to ST125-MRSA-IV; type E12 corresponded to ST36-MRSA-II; type E16 corresponded to ST228-MRSA-IV; and type E13 corresponded to ST22-MRSSA-IV (53). PFGE type A was a community-acquired genotype that corresponded to ST8-MRSA-IV (6).
Multiplex PCR for SCCmec typing.
SCCmec types were determined by use of a multiplex PCR strategy that generated a specific amplification pattern for each SCCmec structural type, according to the method described by Oliveira and de Lencastre (40). Additional typing of the isolates was performed by two different PCR methods in order to detect SCCmec IV subtypes IVa, IVb, IVc, IVd, and IVh (37) and SCCmec type V (56).
Detection of PVL genes.
The Panton-Valentine leukocidin (PVL) genes (lukS-PV and lukF-PV) were detected by PCR by the method described by Lina et al. (32). S. aureus ATCC 49775 (a PVL-positive strain) was used as a positive amplification control.
Determination of accessory gene regulator (agr) types.
A scheme of two PCRs based on the method described by Shopsin et al. (45) was used for the determination of the specific agr groups.
spa typing and BURP analysis.
The polymorphic X region of the protein A gene (spa) was amplified from all MRSA isolates, as described previously (27, 46). By application of the BURP algorithm implemented by the software, spa types with more than five repeats were clustered into different groups, with the calculated cost between the members of a group being less than or equal to 6. The spa type was assigned by using Ridom StaphType software (36).
MLST.
Several representative strains from each type and subtype of PFGE were selected for determination of the ST. None of the sporadic PFGE genotypes were typed by this method. MLST typing was performed by the method described by Enright et al. (16). Allelic profiles and ST types were assigned by using the MLST database (http://www.mlst.net).
RESULTS
Resistance patterns.
Of the 135 MRSA isolates studied, 93.3% were resistant to ciprofloxacin, 72.6% were resistant to tobramycin, 20% were resistant to gentamicin, 66.7% were resistant to erythromycin, and 39.3% were resistant to clindamycin. Of the isolates resistant to erythromycin, 27.4% showed the M phenotype. All the isolates were susceptible to vancomycin (MICs ≤ 2 mg/liter) and teicoplanin (MICs ≤ 8 mg/liter). Full details of the susceptibilities of the isolates have been published elsewhere (13). Table 1 shows the different resistance patterns of the MRSA strains. Twelve different patterns were found, of which four accounted for 65% of the isolates. Seven isolates (5.2%) were resistant only to oxacillin. Multiresistance to one, two, three, four, and five additional antibiotics was observed in 6.7%, 25.1%, 29.6%, 20.8%, and 12.6% of the MRSA isolates, respectively.
TABLE 1.
Resistance phenotypes of MRSA
| Resistance profilea | No. (%) of isolates |
|---|---|
| Oxacillin only | 7 (5.2) |
| ERY | 1 (0.8) |
| CIP | 8 (5.9) |
| TOB + CIP | 26 (19.2) |
| ERY + CIP | 7 (5.2) |
| ERY + CLI | 1 (0.7) |
| ERY + TOB + CIP | 23 (17.0) |
| ERY + CIP + CLI | 13 (9.6) |
| GEN + TOB + CIP | 4 (3.0) |
| ERY + CIP + CLI + TOB | 22 (16.3) |
| ERY + CIP + TOB + GEN | 6 (4.5) |
| ERY + CIP + TOB + GEN + CLI | 17 (12.6) |
Abbreviations: CIP, ciprofloxacin; CLI, clindamycin; ERY, erythromycin; GEN, gentamicin; TOB, tobramycin.
PFGE.
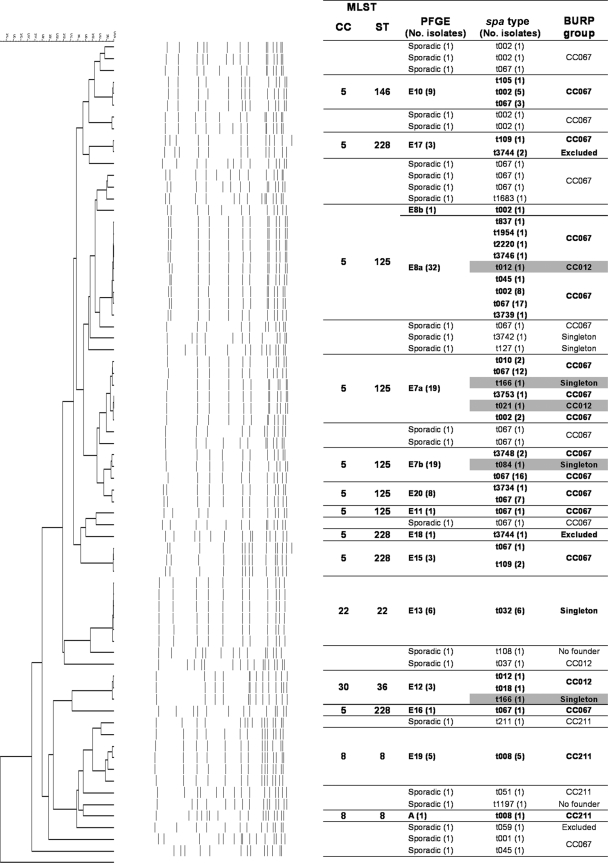
Genotyping by PFGE of the 135 MRSA isolates grouped 112 into 13 genotypes (E7, E8, E10, E11, E12, E13, E15, E16, E17, E18, E19, E20, and A). Two genotypes (E19 and E20) were observed for the first time in Spain. Genotypes E7 (with subtypes E7a and E7b; 28.1%) and E8 (with subtypes E8a and E8b; 24.4%) predominated and together accounted for 52.5% of the isolates. The remaining 23 isolates belonged to 23 sporadic profiles that were each represented by a single isolate. Figure 1 shows the genetic relationships between the 36 PFGE patterns identified.
FIG. 1.
Dendrogram showing the genetic relationships between the 135 MRSA isolates and correlations between the different typing methods. Group violations are marked in gray.
SCCmec types.
The distribution of the different SCCmec types among the different genotypes is shown in Table 2. SCCmec type IV accounted for 86.7% of the isolates (117), with 70.1% of these carrying SCCmec type IVa, 23.9% carrying SCCmec type IVc, 0.9% carrying SCCmec type IVd, and 5.1% carrying SCCmec type IVh. SCCmec type I was identified in 10 isolates (7.4%), and SCCmec type II was found in 7 isolates (5.2%). One isolate could not be typed by any of the methods of SCCmec typing used in this study.
TABLE 2.
Correlation between the different molecular typing methods
| agr type (no. of isolates) | PFGE (no. of isolates) | CC by BURST analysis | spa CC by use of BURP algorithm (no. of isolates) | No. (%) of isolates of SCCmec type:
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | IVa | IVc | IVd | IVh | ||||
| 1 (20) | E13 (6) | CC22 | Singletons (6) | 6 | |||||
| E19 (5) | CC8 | CC211 (5) | 4 | 1 | |||||
| A (1) | CC8 | CC211 (1) | 1 | ||||||
| Sporadic (8) | CC067 (3) | 3 | |||||||
| CC211 (2) | 1 | 1 | |||||||
| Nonfounder (1) | 1 | ||||||||
| Singleton (1) | 1 | ||||||||
| Excluded (1) | 1 | ||||||||
| 2 (111) | E7 (38) | CC5 | CC067 (35) | 30 | 5 | ||||
| CC012 (1) | 1 | ||||||||
| Singletons (2) | 2 | ||||||||
| E8 (33) | CC5 | CC067 (32) | 21 | 11 | |||||
| CC012 (1) | 1 | ||||||||
| E10 (9) | CC5 | CC067 (9) | 6 | 3 | |||||
| E11 (1) | CC5 | CC067 (1) | 1 | ||||||
| E15 (3) | CC5 | CC067 (3) | 3 | ||||||
| E16 (1) | CC5 | CC067 (1) | 1 | ||||||
| E17 (3) | CC5 | CC067 (1) | 1 | ||||||
| Excluded (2) | 2 | ||||||||
| E18 (1) | CC5 | Excluded (1) | 1 | ||||||
| E20 (8) | CC5 | CC067 (8) | 8 | ||||||
| Sporadic (14) | CC067 (12) | 1 | 4 | 3 | 4 | ||||
| CC012 (1) | 1 | ||||||||
| Nonfounder (1) | 1 | ||||||||
| 3 (4) | E12 (3) | CC30 | CC012 (2) | 2 | |||||
| Singleton (1) | 1 | ||||||||
| Sporadic (1) | Singleton (1) | NDa | |||||||
| Total | 10 (7.4) | 7 (5.2) | 82 (60.8) | 28 (20.7) | 1 (0.8) | 6 (4.4) | |||
ND, not determined.
SCCmec type IVa (present in 60.8% of all MRSA isolates) was included in seven major genotypes (E7, E8, E10, E16, E19, E20, and A) and eight sporadic isolates. SCCmec type IVc was present in four major genotypes (E7, E8, E10, and E11) and seven sporadic isolates. SCCmec type IVh was present in genotype E13 (ST22-MRSA-IV). SCCmec type I was present in genotypes E15, E17, and E18 (ST228-MRSA-I), and SCCmec type II was present in genotype E12 (ST36-MRSA-II).
agr types.
Table 2 shows the distribution of the agr types and their correlation with the genotypes observed by PFGE and SCCmec typing. The most frequent agr type was type 2 (82.2%), which grouped strains belonging to nine major clones (E7, E8, E10, E11, E15, E16, E17, E18, and E20) and 14 sporadic MRSA strains. agr type 1 was present in 14.8% of the strains belonging to three major clones (E13, E19, and A) and to eight sporadic MRSA strains. Only 3.0% of the MRSA strains harbored agr type 3; these strains belonged to the E12 clone (three strains), and agr type 3 was found in one sporadic MRSA isolate. None of the MRSA strains presented agr type 4.
PVL genes.
Only one isolate presented PVL. The MRSA strain showed PFGE genotype A (community acquired) and was also resistant to erythromycin (Table 2). The origin of the isolate was a wound infection from a child.
spa types.
The different spa types observed are shown in Table 3. Among the 135 MRSA strains, 32 different spa types were identified: 21 were represented by a single strain, and 7 were new spa types not described previously. spa type t067 was the most frequent (48.9% of the isolates), followed by spa type t002 (14.8%). By application of the BURP algorithm, the MRSA strains were clustered into three groups: clonal complex 067 (CC067; 77.7%), CC211 (6.0%), and CC012 (3.7%). The only PVL-positive isolate belonged to spa type t008 (spa CC211). Two spa types were considered nonfounders, five were singletons (one of which, t032, grouped six MRSA strains), and two were excluded (3%).
TABLE 3.
Distribution of spa types and BURP groups (spa CC)
| spa CC (by use of BURP algorithm) | No. (%) of isolates | spa type | No. of isolates |
|---|---|---|---|
| CC067 | 105 (77.7) | t067a | 66 |
| t002 | 20 | ||
| t001 | 1 | ||
| t010 | 2 | ||
| t045 | 2 | ||
| t105 | 1 | ||
| t109 | 3 | ||
| t837 | 1 | ||
| t1683 | 1 | ||
| t1954 | 1 | ||
| t2220 | 1 | ||
| t3734 | 1 | ||
| t3739 | 1 | ||
| t3746 | 1 | ||
| t3748 | 2 | ||
| t3753 | 1 | ||
| CC211 | 8 (6.0) | t008 | 6 |
| t051 | 1 | ||
| t211 | 1 | ||
| CC012 | 5 (3.7) | t012 | 2 |
| t018 | 1 | ||
| t021 | 1 | ||
| t037 | 1 | ||
| Singletons | 11 (8.1) | t032 | 6 |
| t084 | 1 | ||
| t127 | 1 | ||
| t166 | 2 | ||
| t3742 | 1 | ||
| Nonfounders | 2 (1.5) | t108 | 1 |
| t1197 | 1 | ||
| Excluded | 4 (3.0) | t059 | 1 |
| t3744 | 3 |
The most frequent spa types are marked in boldface.
Correlation between the different molecular typing methods.
The correlations between the BURP group (spa CC), spa type, PFGE genotype, agr type, and BURST group MLST type and the SCCmec type are shown in Table 2 and Fig. 1. Both PFGE and spa typing showed 100% typeability and excellent reproducibility, although PFGE showed more discriminatory power than spa typing. MRSA strains belonging to different epidemic PFGE genotypes (E7, E8, E10, E11, E15, E16, and E20) presented either spa type t067 (43.0%) or spa type t002 (11.9%), and both were grouped in CC067 and MLST CC5 (ST125 and ST228). The six isolates designated genotype E13 by PFGE belonged to spa type t032 and were grouped as singletons, corresponding to clone ST22-MRSA-IVh. On the other hand, 12 MRSA strains considered sporadic isolates on the basis of their PFGE patterns belonged to spa types t067 (8 strains; 5.9%) and t002 (4 strains; 2.9%). In addition, we observed discrepancies between the two methods in the classification of five MRSA isolates. Three isolates belonging to genotype E7, one isolate belonging to genotype E8, and another isolate belonging to genotype E12 presented different spa types, namely, types t012 and t021 (CC012, ST30-MRSA-IV) (Fig. 1).
In general, we observed a high degree of concordance between the MLST clonal complexes and the BURP groups (Fig. 1). With the exception of the five isolates described above, isolates belonging to MLST CC5 (ST125 with PFGE genotypes E7, E8, E11, and E20; ST146 with PFGE genotype E10; and ST228 with PFGE genotypes E15, E16, E17, and E18) were grouped in BURP CC067. MLST CC8 (ST8 with PFGE genotypes E19 and A) presented spa type t008, which belonged to BURP group CC211. CC22 (ST22 with genotype E13, which is related to clone EMRSA-15) presented spa type t032, which appeared in this analysis as a singleton. Finally, MLST CC30 (ST36, genotype E12, which is related to clone EMRSA-16) presented spa types t012 and t018 and grouped in BURP CC012 and spa type t166 (singleton).
DISCUSSION
MRSA is among the most frequently identified antimicrobial drug-resistant pathogens worldwide and has evolved in a relatively few lineages. It has been demonstrated that some lineages are ecologically highly successful and that most isolates belong to pandemic clones (17). The present study revealed that >90% of the isolates were multiresistant and that >30% were resistant to at least four antimicrobial agents. In 2002, the predominant (23.9%) pattern among MRSA isolates in Spain involved resistance to ciprofloxacin, erythromycin, clindamycin, gentamicin, and tobramycin (12). In the present study, performed 4 years later by use of the same methodology, we observed a significant decrease in this multiresistance pattern (P = 0.018), which was represented by only 12.6% of the isolates. In contrast, the rate of multiresistance to ciprofloxacin, erythromycin, and tobramycin increased significantly, from 8.2% in 2002 (12) to 17.0% in 2006 (P = 0.042). These results indicate that strains are becoming more susceptible and that the M phenotype of resistance to macrolides and the presence of the ant4′ gene, which confers resistance to tobramycin but not to gentamicin, are becoming more prevalent. Of the 135 MRSA isolates, only one was community acquired and presented the M phenotype of resistance to macrolides.
We characterized the MRSA isolates by using different molecular typing tools. After analysis of the data, we found interesting clinical and epidemiological findings. First, spa type t067 (ST5-MRSA-IV) was dominant among the Spanish MRSA isolates, a situation not described in other countries. Second, we found a high degree of clonality of the MRSA isolates obtained in this nationwide prevalence study, which demonstrates that most isolates belong to pandemic clones; and third, we found that PFGE, spa typing, SCCmec typing, and MLST presented a good correlation for most MRSA strains.
The PFGE analysis revealed 36 different genotypes that included two predominant clones (E7 and E8) and one community-acquired clone (profile A). PFGE is known to be a highly discriminatory and valuable technique for the typing of S. aureus (10) and has been used by the Spanish Reference Laboratory for staphylococci for local investigations and national surveillance of MRSA since 1996 (53). It has been argued that the stability of PFGE may be insufficient for its application to long-term epidemiological studies due to the high degrees of genetic variation that have been observed among pandemic clones with a long evolutionary history (4). However, we have already reported that the predominant clones in Spain did not undergo significant changes from 1996 (53) to 2002 (12). Moreover, in the present study (conducted with strains collected in 2006), we identified the same predominant clones as well as two new clones, E19 (ST8) and E20 (ST125), with the latter clone being closely related to the predominant E7 and E8 clones. These genotypes belong to ST125, which continues to be responsible for more than half of the nosocomial MRSA infections in Spain (59.3%), although it is unusual in the rest of Europe (23, 42). Our results validate the use of PFGE for long-term nationwide epidemiological studies, although we consider that this technique presents difficulties in interlaboratory reliability. Nevertheless, multicenter studies by PFGE are now possible due to the standardization of the electrophoresis conditions (8, 10) and the availability of normalization and analysis software (15).
The most frequent SCCmec type found was SCCmec type IV, which was present in 86.7% of the isolates. Its presence in the predominant clones, the majority clones, sporadic isolates, and the community-acquired clone suggests a great degree of promiscuity and successful persistence (43). Since SCCmec type IV is currently one of the most frequent nosocomial SCCmec types found in several countries (1, 22, 33, 44, 47), the antimicrobial resistance patterns of isolates presenting this type varied considerably. In our study, 97.5% and 78.6% of MRSA isolates with multiresistance to three or four antimicrobials, respectively, showed this type. The permanence of this type of SCCmec in hospitals over long periods of time has probably favored its multiresistance due to antibiotic pressure. In our study, most type IV strains belonged to subtype IVa (70.1%), followed by subtype IVc (23.9%). In one study performed in the United States (1), subtype IVa was identified in 87.1% of MRSA isolates and subtype IVd was identified in 5.7% of isolates. In a Japanese study, type IV SCCmec strains were also the most frequent, comprising 53.6% of all strains, and the frequencies of type IVc and type IVd were 38.1% and 10.3%, respectively (33).
SCCmec types I and II, which have historically been associated with multiresistance (resistance to more than three antimicrobials), were very uncommon in this study and in certain cases were associated with sporadic isolates.
The characterization of SCCmec did not allow 100% typeability in our study and showed weak discriminatory power. Even when we used three different typing schemes, one isolate (a sporadic isolate) was nontypeable. In addition, the elevated number of isolates harboring SCCmec type IV limited the discriminatory power of this technique. Although this type can be differentiated into many subtypes, a second multiplex PCR is necessary, increasing the cost of type determinations (37). Since new alleles are frequently described, an ever increasing number of primers will be necessary in order to discriminate between different subtypes (7, 30).
Only 1 of the 135 MRSA isolates was PVL positive and was from a community-acquired wound infection in a child. This isolate presented the characteristics most frequently described among PVL-positive MRSA isolates in Spain, including PFGE profile A, ST8, and spa type t008 (CC008) (6). The finding of only one PVL-positive isolate could be due to the characteristics of our study: a point-prevalence study performed on a single day in 145 Spanish hospitals (13). However, we have previously described a higher prevalence of PVL-positive MRSA isolates (6), which is consistent with the increased prevalence of PVL-positive MRSA isolates in Europe (52, 54).
Concerning the agr types, one previous study indicated that the genotypes determined by PFGE, MLST, and spa are so strongly correlated with the agr types that the former can be used to predict the latter indistinctly and that no MLST, spa, or PFGE pattern occurs in more than one agr group (55). In our study, most isolates presented agr type 2 (82.2%), and strains belonging to BURP group CC012 presented agr group 2 or 3 indistinctly. The same study cited above (55) also indicates that in certain cases, strains belonging to the same MLST type can present different agr groups. These observations need to be confirmed by additional data, although interstrain recombination and intrastrain rearrangements would be important sources of variation that could explain these observations (43). In our study, in all cases there were unequivocal correlations between the MLST and the agr types. We have not found in the literature any studies analyzing the agr types of a large series of MRSA isolates.
Recently, a method based on the sequence of the protein A gene (spa typing) represents a marked improvement in the typing of MRSA. It is reproducible and easy to use, and the availability of a central database (http://spa.ridom.de) enables comparisons to be made with data obtained in different laboratories and countries. Several studies have demonstrated that it is applicable to both local and global epidemiological studies (31, 35). The application of this method in Spain revealed that two spa types (t067 and t002) were dominant (63.7% of all MRSA strains).
The high frequency of t067 (48.9%) in Spain contrasted with the relatively low frequency of t067 (0.97%) found in other European countries (Austria, Denmark, Finland, Germany, The Netherlands, Norway, Sweden, and Switzerland) (http://spa.ridom.de).
In the case of spa type t002, the global frequency was to be found 5.79% (Austria, Belgium, Canada, China, Croatia, Cyprus, Denmark, Estonia, Finland, France, Germany, Hungary, Iceland, Israel, Italy, Japan, Lebanon, The Netherlands, Norway, Romania, Sweden, Switzerland, Taiwan, the United Kingdom, and the United States), whereas the frequency of this type was higher in Spain (14.8%). The high frequency of t067 and t002 in Spanish hospitals limits the usefulness of spa typing for local investigations and makes it necessary to differentiate these frequent strains by PFGE.
Concerning the correlation between the different typing methods used in our study, SCCmec types encompassed multiple MLST types, spa types, MLST CCs, and spa CCs, a fact that has been observed elsewhere (41, 51).
When we compared the PFGE genotypes using spa typing, the predominant clones (E7 and E8) presented a variety of spa types, although most belonged to the same BURP group (CC067). However, we observed discordant results (group violations) between the spa type assignment and the profile obtained by PFGE, as described in other studies (26, 43). In addition, we encountered MRSA isolates that had different profiles—E7, E8 and E11 (ST125), and E10 (ST146)—but that shared the same spa type (t002 and t067). These discrepancies have also been described by Hallin et al. (26), suggesting that they could be due to intergenomic recombination. An elevated number of sporadic isolates, as defined by their PFGE profiles, harbored the same spa type as the predominant clones. This could be due to the high discriminatory power of PFGE. These different profiles could reflect the occurrence of genetic events (19). A recent report suggests that the combination of PFGE and spa typing for epidemiological surveillance studies makes it possible to maintain the discriminatory power and typeability needed in short-term and long-term studies (19).
The application of MLST is especially useful for long-term epidemiological studies due to the low mutation rate of the seven housekeeping genes analyzed by the method (16). However, we consider spa typing and PFGE typing for epidemiological surveillance to be the most useful techniques for both long-term and short-term studies. In our study, this combination of typing techniques predicted the MLST CCs, except for the five isolates included as group violations. Other studies have also suggested that this combination reasonably predicts the MLST CCs (19).
In summary, this study demonstrates that strains of MRSA in Spain have become significantly more susceptible to gentamicin and clindamycin than they were in previous years and that there is persistence of the ST125-MRSA-IV clone, which includes the previously described predominant clones E7 and E8 and the new closely related clone, E20. The use of spa typing in this study allowed us to detect two predominant types (t067 and t002) in Spain that are very uncommon in other countries. In our experience, PFGE, spa typing, SCCmec typing, and MLST presented a good correlation for most MRSA strains. Due to the high percentage of spa types t067 and t002 (CC067 BURP), we consider that spa typing should be combined with PFGE to provide the necessary discriminatory power and typeability for local and long-term epidemiological studies, as well as the possibility of interlaboratory comparisons. The combination of PFGE and the assignment of BURP CC could predict the eBURST CC without the need to perform MLST for a larger number of MRSA strains.
Supplementary Material
Acknowledgments
This study was supported by the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III—FEDER, Spanish Network for the Research in Infectious Diseases (REIPI RD06/0008). We are grateful to Wyeth-Farma for financing the transport of samples and the laboratory material used in this study.
We thank T. Ito for the Staphylococcus aureus control strains used for the subtyping of SCCmec IV. We thank Thomas O'Boyle for his help with the translation of the manuscript.
The members of the Spanish Group for the Study of Staphylococcus and the staff of the microbiology services of all participating hospitals are presented in the supplemental material.
Footnotes
Published ahead of print on 1 April 2009.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Abdel-Haq, N., H. Al-Tatari, P. Chearskul, H. Salimnia, B. I. Asmar, M. R. Fairfax, and M. Amjad. 20 November 2008. Methicillin-resistant Staphylococcus aureus (MRSA) in hospitalized children: correlation of molecular analysis with clinical presentation and antibiotic susceptibility testing (ABST) results. Eur. J. Clin. Microbiol. Infect. Dis. [Epub ahead of print.]. [DOI] [PubMed]
- 2.Appelbaum, P. C. 2006. The emergence of vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 12(Suppl. 1)16-23. [DOI] [PubMed] [Google Scholar]
- 3.Bell, J. M., and J. D. Turnidge. 2002. High prevalence of oxacillin-resistant Staphylococcus aureus isolates from hospitalized patients in Asia-Pacific and South Africa: results from SENTRY Antimicrobial Surveillance Program, 1998-1999. Antimicrob. Agents Chemother. 46879-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanc, D. S., P. Francioli, and P. M. Hauser. 2002. Poor value of pulsed-field gel electrophoresis to investigate long-term scale epidemiology of methicillin-resistant Staphylococcus aureus. Infect. Genet. Evol. 2145-148. [DOI] [PubMed] [Google Scholar]
- 5.Boyce, J. M., B. Cookson, K. Christiansen, S. Hori, J. Vuopio-Varkila, S. Kocagoz, A. Y. Oztop, C. M. Vandenbroucke-Grauls, S. Harbarth, and D. Pittet. 2005. Meticillin-resistant Staphylococcus aureus. Lancet Infect. Dis. 5653-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cercenado, E., O. Cuevas, M. Marin, E. Bouza, P. Trincado, T. Boquete, B. Padilla, and A. Vindel. 2008. Community-acquired methicillin-resistant Staphylococcus aureus in Madrid, Spain: transcontinental importation and polyclonal emergence of Panton-Valentine leukocidin-positive isolates. Diagn. Microbiol. Infect. Dis. 61143-149. [DOI] [PubMed] [Google Scholar]
- 7.Chongtrakool, P., T. Ito, X. X. Ma, Y. Kondo, S. Trakulsomboon, C. Tiensasitorn, M. Jamklang, T. Chavalit, J. H. Song, and K. Hiramatsu. 2006. Staphylococcal cassette chromosome mec (SCCmec) typing of methicillin-resistant Staphylococcus aureus strains isolated in 11 Asian countries: a proposal for a new nomenclature for SCCmec elements. Antimicrob. Agents Chemother. 501001-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung, M., H. de Lencastre, P. Matthews, A. Tomasz, I. Adamsson, M. Aires de Sousa, T. Camou, C. Cocuzza, A. Corso, I. Couto, A. Dominguez, M. Gniadkowski, R. Goering, A. Gomes, K. Kikuchi, A. Marchese, R. Mato, O. Melter, D. Oliveira, R. Palacio, R. Sa-Leao, I. Santos Sanches, J. H. Song, P. T. Tassios, and P. Villari. 2000. Molecular typing of methicillin-resistant Staphylococcus aureus by pulsed-field gel electrophoresis: comparison of results obtained in a multilaboratory effort using identical protocols and MRSA strains. Microb. Drug Resist. 6189-198. [DOI] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2007. Performance standards for antimicrobial susceptibility testing, 17th informational supplement. M100-S17, vol. 27, no. 1. Clinical and Laboratory Standards Institute, Wayne, PA.
- 10.Cookson, B. D., D. A. Robinson, A. B. Monk, S. Murchan, A. Deplano, R. de Ryck, M. J. Struelens, C. Scheel, V. Fussing, S. Salmenlinna, J. Vuopio-Varkila, C. Cuny, W. Witte, P. T. Tassios, N. J. Legakis, W. van Leeuwen, A. van Belkum, A. Vindel, J. Garaizar, S. Haeggman, B. Olsson-Liljequist, U. Ransjo, M. Muller-Premru, W. Hryniewicz, A. Rossney, B. O'Connell, B. D. Short, J. Thomas, S. O'Hanlon, and M. C. Enright. 2007. Evaluation of molecular typing methods in characterizing a European collection of epidemic methicillin-resistant Staphylococcus aureus strains: the HARMONY collection. J. Clin. Microbiol. 451830-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox, R. A., C. Conquest, C. Mallaghan, and R. R. Marples. 1995. A major outbreak of methicillin-resistant Staphylococcus aureus caused by a new phage-type (EMRSA-16). J. Hosp. Infect. 2987-106. [DOI] [PubMed] [Google Scholar]
- 12.Cuevas, O., E. Cercenado, E. Bouza, C. Castellares, P. Trincado, R. Cabrera, and A. Vindel. 2007. Molecular epidemiology of methicillin-resistant Staphylococcus aureus in Spain: a multicentre prevalence study (2002). Clin. Microbiol. Infect. 13250-256. [DOI] [PubMed] [Google Scholar]
- 13.Cuevas, O., E. Cercenado, M. Goyanes, A. Vindel, P. Trincado, T. Boquete, A. Marin, and E. Bouza. 2008. Staphylococcus spp. in Spain: present situation and evolution of the resistance to antimicrobials (1986-2006). Enferm. Infecc. Microbiol. Clin. 26269-277. (In Spanish.) [DOI] [PubMed] [Google Scholar]
- 14.Cuevas, O., E. Cercenado, A. Vindel, J. Guinea, M. Sanchez-Conde, M. Sanchez-Somolinos, and E. Bouza. 2004. Evolution of the antimicrobial resistance of Staphylococcus spp. in Spain: five nationwide prevalence studies, 1986 to 2002. Antimicrob. Agents Chemother. 484240-4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duck, W. M., C. D. Steward, S. N. Banerjee, J. E. McGowan, Jr., and F. C. Tenover. 2003. Optimization of computer software settings improves accuracy of pulsed-field gel electrophoresis macrorestriction fragment pattern analysis. J. Clin. Microbiol. 413035-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enright, M. C., N. P. Day, C. E. Davies, S. J. Peacock, and B. G. Spratt. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 381008-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enright, M. C., D. A. Robinson, G. Randle, E. J. Feil, H. Grundmann, and B. G. Spratt. 2002. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc. Natl. Acad. Sci. USA 997687-7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Antimicrobial Resistance Surveillance System. 2006. EARSS annual report 2006. European Antimicrobial Resistance Surveillance System, Bilthoven, The Netherlands.
- 19.Faria, N. A., J. A. Carrico, D. C. Oliveira, M. Ramirez, and H. de Lencastre. 2008. Analysis of typing methods for epidemiological surveillance of both methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains. J. Clin. Microbiol. 46136-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feil, E. J., and M. C. Enright. 2004. Analyses of clonality and the evolution of bacterial pathogens. Curr. Opin. Microbiol. 7308-313. [DOI] [PubMed] [Google Scholar]
- 21.Feil, E. J., E. K. Nickerson, N. Chantratita, V. Wuthiekanun, P. Srisomang, R. Cousins, W. Pan, G. Zhang, B. Xu, N. P. Day, and S. J. Peacock. 2008. Rapid detection of the pandemic methicillin-resistant Staphylococcus aureus clone ST 239, a dominant strain in Asian hospitals. J. Clin. Microbiol. 461520-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fey, P. D., B. Said-Salim, M. E. Rupp, S. H. Hinrichs, D. J. Boxrud, C. C. Davis, B. N. Kreiswirth, and P. M. Schlievert. 2003. Comparative molecular analysis of community- or hospital-acquired methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47196-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fossum, A. E., and G. Bukholm. 2006. Increased incidence of methicillin-resistant Staphylococcus aureus ST80, novel ST125 and SCCmecIV in the south-eastern part of Norway during a 12-year period. Clin. Microbiol. Infect. 12627-633. [DOI] [PubMed] [Google Scholar]
- 24.Geha, D. J., J. R. Uhl, C. A. Gustaferro, and D. H. Persing. 1994. Multiplex PCR for identification of methicillin-resistant staphylococci in the clinical laboratory. J. Clin. Microbiol. 321768-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grundmann, H., M. Aires-de-Sousa, J. Boyce, and E. Tiemersma. 2006. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet 368874-885. [DOI] [PubMed] [Google Scholar]
- 26.Hallin, M., A. Deplano, O. Denis, R. De Mendonca, R. De Ryck, and M. J. Struelens. 2007. Validation of pulsed-field gel electrophoresis and spa typing for long-term, nationwide epidemiological surveillance studies of Staphylococcus aureus infections. J. Clin. Microbiol. 45127-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harmsen, D., H. Claus, W. Witte, J. Rothganger, D. Turnwald, and U. Vogel. 2003. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 415442-5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunter, P. R. 1990. Reproducibility and indices of discriminatory power of microbial typing methods. J. Clin. Microbiol. 281903-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kluytmans-Vandenbergh, M. F., and J. A. Kluytmans. 2006. Community-acquired methicillin-resistant Staphylococcus aureus: current perspectives. Clin. Microbiol. Infect. 12(Suppl. 1)9-15. [DOI] [PubMed] [Google Scholar]
- 30.Kondo, Y., T. Ito, X. X. Ma, S. Watanabe, B. N. Kreiswirth, J. Etienne, and K. Hiramatsu. 2007. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob. Agents Chemother. 51264-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koreen, L., S. V. Ramaswamy, E. A. Graviss, S. Naidich, J. M. Musser, and B. N. Kreiswirth. 2004. spa typing method for discriminating among Staphylococcus aureus isolates: implications for use of a single marker to detect genetic micro- and macrovariation. J. Clin. Microbiol. 42792-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lina, G., Y. Piemont, F. Godail-Gamot, M. Bes, M. O. Peter, V. Gauduchon, F. Vandenesch, and J. Etienne. 1999. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 291128-1132. [DOI] [PubMed] [Google Scholar]
- 33.Ma, X. X., T. Ito, P. Chongtrakool, and K. Hiramatsu. 2006. Predominance of clones carrying Panton-Valentine leukocidin genes among methicillin-resistant Staphylococcus aureus strains isolated in Japanese hospitals from 1979 to 1985. J. Clin. Microbiol. 444515-4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maiden, M. C., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 953140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mellmann, A., T. Weniger, C. Berssenbrugge, U. Keckevoet, A. W. Friedrich, D. Harmsen, and H. Grundmann. 2008. Characterization of clonal relatedness among the natural population of Staphylococcus aureus strains by using spa sequence typing and the BURP (based upon repeat patterns) algorithm. J. Clin. Microbiol. 462805-2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mellmann, A., T. Weniger, C. Berssenbrugge, J. Rothganger, M. Sammeth, J. Stoye, and D. Harmsen. 2007. Based upon repeat pattern (BURP): an algorithm to characterize the long-term evolution of Staphylococcus aureus populations based on spa polymorphisms. BMC Microbiol. 798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milheirico, C., D. C. Oliveira, and H. de Lencastre. 2007. Multiplex PCR strategy for subtyping the staphylococcal cassette chromosome mec type IV in methicillin-resistant Staphylococcus aureus: ‘SCCmec IV multiplex.’ J. Antimicrob. Chemother. 6042-48. [DOI] [PubMed] [Google Scholar]
- 38.Moore, P. C., and J. A. Lindsay. 2002. Molecular characterization of the dominant UK methicillin-resistant Staphylococcus aureus strains, EMRSA-15 and EMRSA-16. J. Med. Microbiol. 51516-521. [DOI] [PubMed] [Google Scholar]
- 39.National Nosocomial Infections Surveillance (NNIS) System Report. 2002. Data summary from January 1992 to June 2002, issued August 2002. Am. J. Infect. Control 30458-475. [DOI] [PubMed] [Google Scholar]
- 40.Oliveira, D. C., and H. de Lencastre. 2002. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 462155-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliveira, D. C., A. Tomasz, and H. de Lencastre. 2001. The evolution of pandemic clones of methicillin-resistant Staphylococcus aureus: identification of two ancestral genetic backgrounds and the associated mec elements. Microb. Drug Resist. 7349-361. [DOI] [PubMed] [Google Scholar]
- 42.Perez-Roth, E., F. Lorenzo-Diaz, N. Batista, A. Moreno, and S. Mendez-Alvarez. 2004. Tracking methicillin-resistant Staphylococcus aureus clones during a 5-year period (1998 to 2002) in a Spanish hospital. J. Clin. Microbiol. 424649-4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robinson, D. A., and M. C. Enright. 2004. Evolution of Staphylococcus aureus by large chromosomal replacements. J. Bacteriol. 1861060-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seybold, U., E. V. Kourbatova, J. G. Johnson, S. J. Halvosa, Y. F. Wang, M. D. King, S. M. Ray, and H. M. Blumberg. 2006. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin. Infect. Dis. 42647-656. [DOI] [PubMed] [Google Scholar]
- 45.Shopsin, B., B. Mathema, P. Alcabes, B. Said-Salim, G. Lina, A. Matsuka, J. Martinez, and B. N. Kreiswirth. 2003. Prevalence of agr specificity groups among Staphylococcus aureus strains colonizing children and their guardians. J. Clin. Microbiol. 41456-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strommenger, B., C. Braulke, D. Heuck, C. Schmidt, B. Pasemann, U. Nubel, and W. Witte. 2008. spa typing of Staphylococcus aureus as a frontline tool in epidemiological typing. J. Clin. Microbiol. 46574-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tenover, F. C. 2006. Community-associated methicillin-resistant Staphylococcus aureus: it's not just in communities anymore. Clin. Microbiol. Newsl. 2833-36. [Google Scholar]
- 48.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 332233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tenover, F. C., L. M. Weigel, P. C. Appelbaum, L. K. McDougal, J. Chaitram, S. McAllister, N. Clark, G. Killgore, C. M. O'Hara, L. Jevitt, J. B. Patel, and B. Bozdogan. 2004. Vancomycin-resistant Staphylococcus aureus isolate from a patient in Pennsylvania. Antimicrob. Agents Chemother. 48275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsiodras, S., H. S. Gold, G. Sakoulas, G. M. Eliopoulos, C. Wennersten, L. Venkataraman, R. C. Moellering, and M. J. Ferraro. 2001. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 358207-208. [DOI] [PubMed] [Google Scholar]
- 51.Vainio, A., M. Karden-Lilja, S. Ibrahem, A. M. Kerttula, S. Salmenlinna, A. Virolainen, and J. Vuopio-Varkila. 2008. Clonality of epidemic methicillin-resistant Staphylococcus aureus strains in Finland as defined by several molecular methods. Eur. J. Clin. Microbiol. Infect. Dis. 27545-555. [DOI] [PubMed] [Google Scholar]
- 52.Vandenesch, F., T. Naimi, M. C. Enright, G. Lina, G. R. Nimmo, H. Haffernan, N. Liassine, M. Bes, T. Greenland, M. E. Reverdy, and J. Etienne. 2003. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 9978-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vindel, A., P. Trincado, E. Gomez, R. Cabrera, T. Boquete, C. Sola, S. Valdezate, and J. A. Saez-Nieto. 2006. Prevalence and evolution of methicillin-resistant Staphylococcus aureus in Spanish hospitals between 1996 and 2002. J. Clin. Microbiol. 44266-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Witte, W., C. Braulke, C. Cuny, B. Strommenger, G. Werner, D. Heuck, U. Jappe, C. Wendt, H. J. Linde, and D. Harmsen. 2005. Emergence of methicillin-resistant Staphylococcus aureus with Panton-Valentine leukocidin genes in central Europe. Eur. J. Clin. Microbiol. Infect. Dis. 241-5. [DOI] [PubMed] [Google Scholar]
- 55.Wright, J. S., III, K. E. Traber, R. Corrigan, S. A. Benson, J. M. Musser, and R. P. Novick. 2005. The agr radiation: an early event in the evolution of staphylococci. J. Bacteriol. 1875585-5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang, K., J. A. McClure, S. Elsayed, T. Louie, and J. M. Conly. 2005. Novel multiplex PCR assay for characterization and concomitant subtyping of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 435026-5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.