Abstract
Diagnosis of human toxocariasis currently relies on serologic tests that use Toxocara excretory-secretory (TES) antigen to detect immunoglobulin G (IgG) antibodies to the larvae. In general, however, these assays do not have adequate specificity for use in countries in which other soil-transmitted helminths are endemic. The use of recombinant antigens in these assays, however, is promising for improving the specificity of the diagnosis of toxocariasis. Toward this goal, we developed an IgG4 enzyme-linked immunosorbent assay (ELISA) involving three recombinant antigens: rTES-30USM (previously produced), rTES-26, and rTES-120. The latter two antigens were produced by reverse transcription-PCR cloning; subcloned into glutathione S-transferase (GST)-tagged and His-tagged prokaryotic expression vectors, respectively; and expressed in Escherichia coli. The recombinant proteins were subsequently purified by affinity chromatography using GST and His-Trap resins. The diagnostic potential of each purified recombinant antigen was tested with various immunoglobulin classes (IgG, IgM, and IgE) and IgG subclasses. The IgG4 ELISA was determined to have the highest specificity and was further evaluated using a panel of serum samples. The rTES-26 IgG4 ELISA showed 80.0% (24/30 samples positive) sensitivity, and both the rTES-30USM IgG4 ELISA and rTES-120 IgG4 ELISA had 93.0% (28/30) sensitivity. Combined use of rTES-120 and rTES-30 IgG4 ELISA for the diagnosis of toxocariasis provided 100% sensitivity. The specificities of rTES-26, rTES-30USM, and rTES-120 antigens were 96.2%, 93.9%, and 92.0%, respectively. These results indicate that the development of a diagnostic test using the three recombinant antigens will allow for more-accurate detection of toxocariasis.
Human toxocariasis is a worldwide parasitic zoonosis, caused most commonly by the intestinal parasites the dog roundworm (Toxocara canis) and also the cat roundworm (Toxocara cati) (2). It commonly manifests as visceral larva migrans, ocular larva migrans, and covert toxocariasis. Toxocariasis is probably one of the most common zoonotic helminthiases in temperate climates and developed countries (20). In Malaysia, the seroprevalence rate is approximately 20% (6).
Definitive diagnosis of toxocariasis is based on the detection of Toxocara larvae from biopsy tissues, but this test is time-consuming and difficult to perform. Therefore, diagnosis is commonly based on clinical and serologic diagnosis. Currently, common routine serology tests are applied for detection, such as commercial immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA) kits in which Toxocara excretory-secretory (TES) antigens obtained from culture of T. canis second-stage (L2) larvae are used (22). The use of the native TES antigen for serodiagnosis of human toxocariasis is a laborious and time-consuming technique, and the production capacity is limited by the culture volume (22). Further, the specificity is often low, especially in developing countries, where infections with helminths that cause cross-reactions, particularly soil-transmitted helminths, are prevalent (10, 15).
The use of recombinant antigens offers significant benefits for detection because their production is basically limitless, and assays using recombinant antigens have increased sensitivity and specificity compared to those of assays using native TES antigens. Several investigators have reported recombinant antigens that are potentially useful for the serodiagnosis of toxocariasis, namely, TES-30 (16, 27, 28) and TES-120 (3, 4), but more validation studies are needed to establish their specificity and sensitivity for use as diagnostic reagents.
Accurate diagnosis is important for patient management, understanding the epidemiology of toxocariasis, and establishing preventive measures. Thus, the aim of this study was to develop a robust diagnostic test for human toxocariasis based on three recombinant antigens, rTES-30USM (previously produced), rTES-26, and rTES-120.
MATERIALS AND METHODS
Isolation and cultivation of T. canis eggs.
Live adult female T. canis worms were obtained postmortem from the small intestinal tracts of naturally infected puppies and dogs. The adult worms were washed in normal saline solution, the uteri of gravid female worms were dissected, and fertile eggs were collected.
The in vitro cultivation of the L2 larvae was performed according to the method described previously (1, 13) with some modifications. Briefly, eggs were digested in acid-pepsin solution for 30 min and then incubated in 2% neutral formalin in a sterile 250-ml flask for 14 days at 30°C with occasional inspection. To the 10% egg suspension, an equal volume of ∼4% sodium hypochlorite solution (Sigma-Aldrich; ready solution) was added for 30 min until the eggs lost their outer pitted shells. A hand tissue homogenizer (B. Braun) was used to disrupt the decoated eggs. The egg suspension was then placed in RPMI 1640 medium containing 100 IU/ml penicillin, 100 μg/ml streptomycin, and 2.5 μg/ml amphotericin B (Fungizone). The suspension was then incubated in a 37°C CO2 incubator with a mixture of 5% CO2 and 95% N2 bubbled through the suspension. After 1 h, the mixture was transferred onto two layers of gauze in a modified Baermann apparatus. The live L2 larvae were collected from the bottom of the apparatus after approximately 8 h. The larvae were then homogenized in lysis buffer. The mRNA was extracted from T. canis L2 larvae using the Straight A's mRNA isolation system in accordance with the manufacturer's instructions (Novagen, Germany). The yield/concentration and purity of the mRNA sample were then quantified by spectrophotometry (Eppendorf) at wavelengths of 260 and 280 nm.
Human serum samples.
A total of 242 serum samples, collected after obtaining informed consent from the subjects and approval from the institutional ethics review board, were used in this study. Thirty serum samples were obtained from patients with clinical and hematologic evidence of toxocariasis and were seropositive by a commercial IgG ELISA kit (Cypress Toxocara IgG; Cypress, Belgium); 28 samples came from patients with soil-transmitted helminth infections, namely, Ascaris worms, Trichuris worms, and/or hookworm (STH); 30 samples came from patients with serologic diagnosis of extraintestinal amoebiasis; 20 samples came from patients with a serologic diagnosis of toxoplasmosis; 28 samples came from patients with Brugia malayi microfilaremia; five samples came from patients with Strongyloides stercoralis in their stool; one sample came from a patient with Gnathostoma spinigerum in the eye; and 100 serum samples came from healthy individuals.
Oligonucleotide design.
The complete coding sequences (open reading frames) of the TES-26 and TES-120 genes were obtained from GenBank (accession numbers U29761 and U39815, respectively). Primers were designed and analyzed for high-efficiency amplification with OligoPerfect Primer Designer and Vector NTi version 6.0 software (Informac Inc., Invitrogen).
RT-PCR.
Reverse transcription-PCR (RT-PCR) was performed by using the commercial StrataScript One-Tube RT-PCR System with the Easy-A high-fidelity PCR cloning enzyme kit (Stratagene), following the manufacturer's instructions. The specific primers for each DNA sequence were as follows: TES26F, 5′-CACCATGTCAGTTGTACACAAAGCTTGC-3′; TES26R, 5′-TTAGGCCTGCGATCGATAGA-3′; TES120F, 5′-ATGCACGTCCTTACCGTCGCT-3′; TES120R, 5′-ACAGAAGCCGCACGTCAGTGG-3′. The PCR mixture comprised 39.5 μl RNase-free water, 5 μl of 10× RT-PCR buffer, 1 μl of forward and reverse primers (20 pmol/μl each), 1 μl of 40 mM deoxynucleoside triphosphate mix, 1 μl of mRNA sample, 1 μl of diluted StrataScript reverse transcriptase (2.5 U/μl), and 0.5 μl of Easy-A HiFi PCR cloning enzyme. These were added sequentially to an 0.2-ml PCR tube in a total reaction volume of 50 μl. The amplification process was then performed as follows: first-strand synthesis at 42°C for 15 min; StrataScript reverse transcriptase inactivation at 95°C for 1 min; 40 cycles of denaturation at 95°C for 30 s, template-primer annealing at 60°C for 30 s, and extension at 68°C for 2 min; and final extension at 68°C for 5 min.
Cloning of genes encoding TES-26 and TES-120.
The A-tailed freshly purified RT-PCR products were cloned into a TOPO TA cloning vector (PCR2.1 TOPO TA; Invitrogen), followed by transformation into the TOP10 Escherichia coli host (Invitrogen). The orientation of the recombinant plasmids was confirmed by PCR screening using both gene-specific primers (TES26F and TES26R or TES120F and TES120R) in tandem with a vector-specific primer (M13R) and a gene-specific primer (TES26R or TES120R), followed by DNA sequencing. Sequences of the engineered genes were then compared with the appropriate published sequences using Vector NTi software version 6.0.
Repair of base mutations.
Four DNA base errors were detected in TES-26/TOPO, and no base errors were detected in TES-120/TOPO. Thus, in vitro PCR-based site-directed mutagenesis was performed to correct the four base errors (124, 502, 613, and 768 bp) in TES-26/TOPO by using a commercially available kit (QuikChange XL; Stratagene). Four specific forward and reverse primers (Table 1) at the particular regions were designed according to the kit manufacturer's instructions. Plasmid DNA template (4 μl) was added to a PCR mixture consisting of 5 μl of 10× mutagenesis buffer, 2 μl each of 1 μM forward and reverse mutagenic primers, 1 μl of 10 mM deoxynucleoside triphosphates, 1 μl of Pfu DNA polymerase (2.5 U/μl), and water (DNase and RNase free; Sigma) to a final volume of 50 μl. PCR was performed using the following parameters: one cycle at 95°C (5 min); 12 cycles at 95°C (30 s), 60°C (1 min), and 72°C (5 min); and finally one cycle at 4°C (5 min). The PCR product was then digested with 1 μl of 10 U/μl DpnI enzyme (Fermentas) at 37°C for 1 h, followed by transformation into the TOP10 E. coli host (Invitrogen). The corrected DNA sequences of the recombinant plasmids were then verified by sequencing.
TABLE 1.
Mutagenic primers used to repair base errors in TES-26 recombinant plasmids
| Mutagenic primer | Sequence (5′ to 3′) (25-mer) | Purpose |
|---|---|---|
| TES26MF1 | TTCACACGACCAGTCAGCCAAGTTC | G to replace A |
| TES26MR1 | GAACTTGGCTGACTGGTCGTGTGAA | G to replace A |
| TES26MF2 | AGCGCCGCGAACGGCCAGCAGGGTC | G to replace C |
| TES26MR2 | GACCCTGCTGGCCGTTCGCGGCGCT | G to replace C |
| TES26MF3 | CCGCCGCTAATACTGGTGTGCACCG | C to replace G |
| TES26MR3 | CGGTGCACACCAGTATTAGCGGCGG | C to replace G |
| TES26MF4 | TATGCTGGCAATTTCTATCGATCGC | T to replace C |
| TES26MR4 | GCGATCGATAGAAATTGCCAGCATA | T to replace C |
Subcloning into bacterial expression vectors.
All recombinant plasmids and expression vectors were digested with EcoRI enzyme (Fermentas). After digestion, TES-26 and TES-120 recombinant plasmids were subcloned into pET42 version b (Novagen, Germany) and pPROExHT version a (Life Technologies), respectively, using a T4 rapid DNA ligation kit (Roche Diagnostics). After the construct was verified by sequencing, the recombinant plasmids were transformed into an expression host, BL21(DE3) (Novagen, Germany).
Expression and purification of TES-26 and TES-120 recombinant proteins.
Each of the recombinant bacteria was cultured in Terrific broth containing 30 μg/ml kanamycin (for rTES-26) or 50 μg/ml ampicillin (for rTES-120) and incubated at 37°C until the optical density (OD) at 600 nm reached mid-log phase (OD at 600 nm of 0.5). The expression was then induced with isopropyl-β-d-thiogalactopyranoside to a final concentration of 1 mM at 30°C in an incubator shaker. The culture was harvested after 3 h (for rTES-26) or 5 h (for rTES-120). The affinity purification of TES-26 glutathione S-transferase (GST)-tagged and TES-120 histidine-tagged recombinant proteins was performed under native conditions, as described by the manufacturer, because both recombinant proteins were present in sufficient amounts in soluble form. The cells of rTES-26 were lysed in buffer (pH 7.3) containing 43 mM NaH2PO4, 14.7 mM KH2PO4, 1.37 M NaCl, and 27 mM KCl by using a French press (Thermo Spectronic) and purified using GST resin (Novagen, Germany). The cells of rTES-120 were lysed in buffer (pH 7.0) containing 50 mM NaH2PO4, 500 mM NaCl, and 10 mM imidazole using the same method as that described above. rTES-120 was then purified with a His-Trap column (GE Healthcare) using an AKTAprime machine (GE Healthcare). A restriction-grade site-specific protease, factor Xa enzyme, was used for specific cleavage/removal of the GST tag in TES-26 GST-tagged recombinant protein by using a commercial kit (factor Xa cleavage capture kit; Novagen, Germany). After cleavage of the target protein, factor Xa was removed by affinity chromatography using Xarrest agarose (Novagen, Germany). The sizes of the expressed target proteins were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis, and the immunoreactivities of these recombinant proteins were analyzed by Western blotting using serum samples and IgG4 antibody detection.
Western blotting.
The rTES-26 and rTES-120 antigens (20 μg/ml) were separated by 10% SDS-PAGE and transferred onto a nitrocellulose membrane (Osmonic) by using a semidry transblot apparatus (Bio-Rad). The membrane was cut into strips and blocked with 1% casein blocking solution (Roche Diagnostics) for 1 h. The strips were then incubated with serum samples (diluted 1:100 in 0.5% blocking solution) at 4°C overnight, followed by monoclonal anti-human IgG4-horseradish peroxidase (Zymed) at 1:2,000 (in 0.5% blocking solution) for 30 min. BM chemiluminescence blotting reagent (Roche Diagnostics) and X-ray films (Kodak) were used to develop the blots.
ELISA.
ELISAs based on rTES-26 and rTES-120 and the previously produced rTES-30USM recombinant antigens were developed, and the diagnostic value of the assays was evaluated using monoclonal anti-human IgG subclasses (IgG1, IgG2, IgG3, and IgG4), IgE, and IgM. The final assay was then evaluated using a panel of sera from patients with positive Toxocara serology results or other helminth-related infections and from healthy individuals to determine its sensitivity and specificity. Each well of the 96-well flat-bottomed microtiter plate (Nunc Immuno Maxisorp) was coated with 100 μl of each recombinant antigen at the optimum concentration for each antigen in 0.02 M bicarbonate buffer, pH 9.6 (Table 2). The plate was then covered and incubated in a humid chamber at 4°C overnight followed by 2 h at 37°C. The plate was washed in phosphate-buffered saline (PBS), pH 7.2, containing 0.05% (vol/vol) Tween 20, pH 7.2, to remove unadsorbed antigen. After a washing step of five washes for 5 min each with PBS with Tween 20, each well was blocked with 1.0% blocking reagent (Roche Diagnostics) for 1 h at 37°C. The plate was again washed as previously described, followed by the addition of serum samples (100 μl, 1:50 in PBS, duplicate wells), and incubated at 37°C for 2 h. After the excess serum samples were washed off, mouse monoclonal anti-human IgG1- to IgG4-, IgE-, and IgM-horseradish peroxidase (Zymed) were added at an optimized dilution in PBS (Table 2) and incubated at 37°C for 30 min. Following a final washing step, 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid-diammonium salt) substrate (Roche Diagnostics) was added and the ODs were measured after 30 min as absorbance at 405 nm (reference, 490 nm) using an ELISA spectrophotometer (Tecan, Germany). The OD readings were blanked with the PBS, and an OD reading of 0.200 was used as the cutoff value to discriminate between the positive and negative results. This cutoff value was based on the mean OD reading plus three standard deviations of 30 serum samples from healthy individuals.
TABLE 2.
Optimum conditions for each rTES ELISA
| rTES | Antigen concn (μg/ml) | Serum dilution | Conjugate dilution |
|---|---|---|---|
| rTES-26 | 1:50 | ||
| IgG1-HRPa | 5 | 1:15,000 | |
| IgG2-HRP | 10 | 1:1,000 | |
| IgG3-HRP | 10 | 1:1,000 | |
| IgG4-HRP | 10 | 1:1,000 | |
| IgE-HRP | 5 | 1:2,000 | |
| IgM-HRP | 10 | 1:1,000 | |
| rTES-32 | 1:50 | ||
| IgG1-HRP | 5 | 1:15,000 | |
| IgG2-HRP | 10 | 1:1,000 | |
| IgG3-HRP | 10 | 1:1,000 | |
| IgG4-HRP | 20 | 1:1,000 | |
| IgE-HRP | 2.5 | 1:1,000 | |
| IgM-HRP | 10 | 1:1,000 | |
| rTES-120 | 1:50 | ||
| IgG1-HRP | 5 | 1:15,000 | |
| IgG2-HRP | 10 | 1:1,000 | |
| IgG3-HRP | 10 | 1:1,000 | |
| IgG4-HRP | 10 | 1:1,000 | |
| IgE-HRP | 2.5 | 1:1,000 | |
| IgM-HRP | 10 | 1:1,000 |
HRP, horseradish peroxidase.
Statistical analysis.
Analyses of the differences in sensitivity and specificity between ELISAs with the different recombinant antigens were performed using Pearson's chi-square test. One-way analysis of variance was used to compare the ODs obtained among the IgG4 ELISAs using rTES-26, rTES-30USM, and rTES-120. Unless otherwise indicated, a P value of 0.05 or less was considered to be significant.
RESULTS
Gel electrophoresis of the amplified RT-PCR products of the DNA sequence encoding TES-26 and TES-120 proteins produced bands at the expected sizes of 793 and 528 bp, respectively (Fig. 1). All four mutations that occurred at 124, 502, 613, and 789 bp in TES-26/TOPO were successfully corrected (data not shown).
FIG. 1.
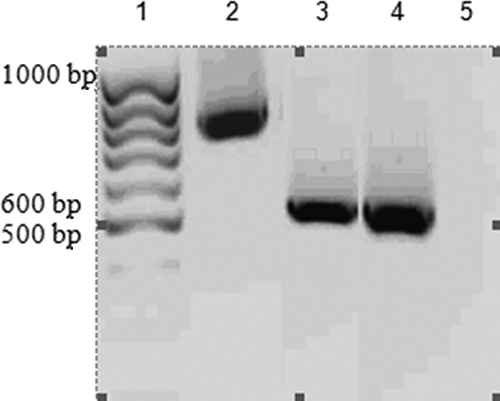
Agarose gel electrophoresis of the amplified RT-PCR products of genes encoding TES-26 and TES-120. Lane 1, 100-bp DNA ladder; lane 2, amplified product of genes encoding TES-26; lane 3, amplified product of genes encoding TES-120; lane 4, positive control; lane 5, negative control.
The SDS-PAGE profiles of the purified TES-26 GST fusion and TES-120 His-tagged proteins showed distinctive and thick bands at molecular masses of approximately 72 kDa and 26 kDa, respectively (Fig. 2). The histidine tag is a very small peptide (approximately 0.8 kDa), is not immunogenic, and is rarely disruptive to the properties of the proteins on which it is attached. Therefore, the His tag was not removed. The TES-120 His-tagged protein was thus used directly for developing the immunoassay to detect Toxocara infection. The GST tag, on the other hand, is a large molecule (approximately 26 kDa) that affects the immunogenicity of the protein; thus, the GST tag was removed prior to developing the immunoassay. After removal of the GST tag, the molecular mass of the TES-26 recombinant protein was approximately 31 kDa.
FIG. 2.
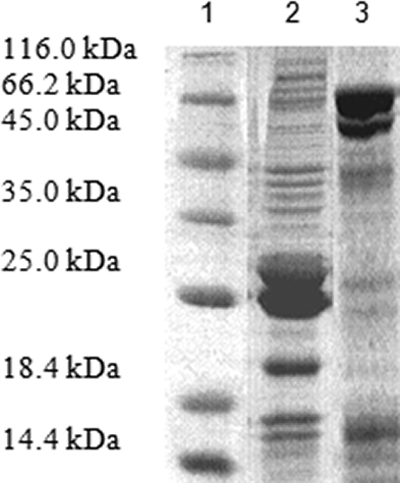
SDS-PAGE of the purified TES-26 GST fusion and TES-120 His-tagged proteins. Lane 1, protein marker; lane 2, rTES-120 antigen (26 kDa); lane 3, rTES-26 antigen (72 kDa).
The antigenicity study by Western blot analysis of the cleaved TES-26 and TES-120 recombinant proteins showed reactivity with serum samples from only toxocariasis patients, as indicated by the presence of bands with molecular masses of approximately 31 and 26 kDa, respectively. Sera from healthy individuals and patients with other helminthic infections showed no reactivity (Fig. 3a and b).
FIG. 3.
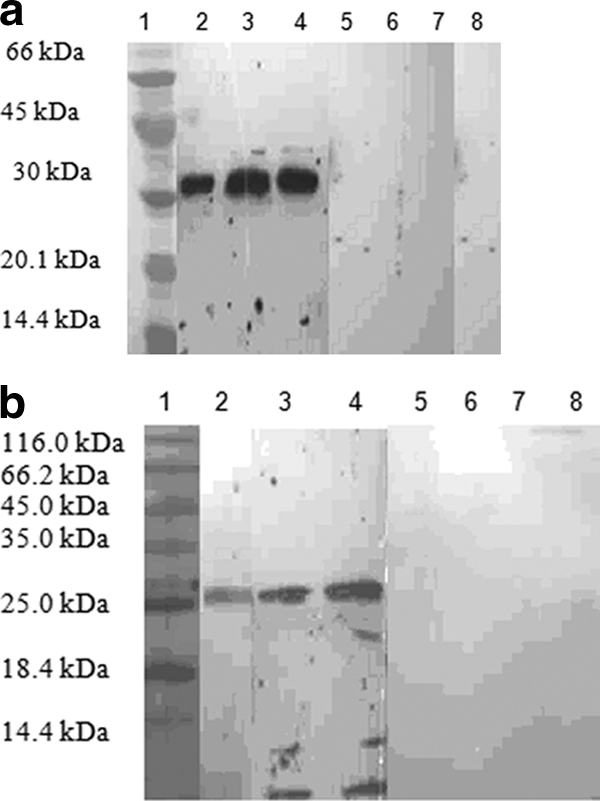
(a) Western blot analysis of rTES-26 antigen probed with various categories of infected and noninfected serum. Lane 1, protein marker; lanes 2, 3, and 4, sera from three different Toxocara-infected patients; lane 5, serum from trichuriasis patient; lane 6, serum from toxoplasmosis patient; lanes 7 and 8, sera from apparently healthy people. (b) Western blot analysis of rTES-120 antigen probed with sera from variously infected and healthy individuals. Lane 1, protein marker; lanes 2, 3, and 4, sera from three different Toxocara-infected patients; lane 5, serum from trichuriasis patient; lane 6, serum from toxoplasmosis patient; lanes 7 and 8, sera from apparently healthy people.
The sensitivity and cross-reactions of sera from patients with other helminthic infections in rTES-ELISA with IgG subclasses (IgG1 to IgG4), IgE, and IgM are shown in Table 3. The IgG4 ELISA demonstrated the least cross-reactivity, and hence the highest specificity, whereas the IgG1 ELISA demonstrated the lowest specificity. The decreasing order of the assay specificities was as follows: IgG4 > IgG3 > IgG2 > IgG1. The greatest assay sensitivity was also demonstrated by IgG4, and the decreasing order of the assay sensitivities was as follows: IgG4 > IgG2 > IgG1 ≥ IgG3.
TABLE 3.
Sensitivities and cross-reactions of sera from patients with other helminthic infections in rTES ELISAs using IgG subclasses, IgE, and IgM
| ELISA and antigen | No. of positive samples/total no. of samples (%)
|
||||||
|---|---|---|---|---|---|---|---|
| Sensitivity to toxocariasis | Cross-reactivity with serum from source
|
Total cross-reactivity | |||||
| Patients with:
|
Healthy individuals | ||||||
| STH infection | B. malayi microfilaremia | Amoebiasis | Toxoplasmosis | ||||
| IgG1 | |||||||
| rTES-26 | 8/20 (40) | 8/15 | 3/10 | 4/10 | 6/10 | 1/20 | 22/65 (33.8) |
| rTES-30USM | 8/20 (40) | 8/15 | 3/10 | 5/10 | 6/10 | 1/20 | 23/65 (35.4) |
| rTES-120 | 10/20 (50) | 8/15 | 3/10 | 5/10 | 6/10 | 2/20 | 24/65 (36.9) |
| IgG2 | |||||||
| rTES-26 | 11/20 (55) | 5/15 | 2/10 | 3/10 | 3/10 | 3/20 | 16/65 (24.6) |
| rTES-30USM | 11/20 (55) | 8/15 | 2/10 | 5/10 | 3/10 | 3/20 | 21/65 (32.3) |
| rTES-120 | 15/20 (75) | 8/15 | 2/10 | 5/10 | 4/10 | 3/20 | 22/65 (33.8) |
| IgG3 | |||||||
| rTES-26 | 8/20 (40) | 4/15 | 2/10 | 2/10 | 3/10 | 3/20 | 14/65 (21.5) |
| rTES-30USM | 8/20 (40) | 5/15 | 2/10 | 4/10 | 5/10 | 2/20 | 18/65 (27.7) |
| rTES-120 | 9/20 (45) | 5/15 | 2/10 | 4/10 | 4/10 | 3/20 | 18/65 (27.7) |
| IgE | |||||||
| rTES-26 | 10/20 (50) | 4/15 | 3/10 | 3/10 | 2/10 | 3/20 | 15/65 (23) |
| rTES-30USM | 11/20 (55) | 5/15 | 3/10 | 3/10 | 3/10 | 3/20 | 17/65 (26.2) |
| rTES-120 | 11/20 (55) | 5/15 | 3/10 | 3/10 | 3/10 | 3/20 | 17/65 (26.2) |
| IgM | |||||||
| rTES-26 | 1/12 (8.33) | 2/11 | 0/5 | 0/5 | 1/4 | 2/5 | 5/30 (16.7) |
| rTES-30USM | 1/12 (8.33) | 2/11 | 1/5 | 0/5 | 1/4 | 2/5 | 6/30 (20) |
| rTES-120 | 4/12 (33.3) | 3/11 | 3/5 | 1/5 | 0/4 | 3/5 | 10/30 (33.3) |
The sensitivity evaluation results of the final developed IgG4 ELISA are shown in Table 4. Of 30 positive human serum samples examined, 28 (93.3%) were reactive for anti-Toxocara IgG4 antibodies with rTES-30USM and rTES-120; the IgG4 ELISA using rTES-26 antigen gave 80.0% (24/30) sensitivity. The increased level of sensitivity of rTES-30USM and rTES-120 IgG4 ELISA was significant in comparison with the rTES-26 IgG4 ELISA (P < 0.001, as determined by Pearson chi-square test). However, there was no significant difference in mean ODs obtained with 30 Toxocara-infected serum samples among the three tests (P = 0.76; determined by one-way analysis of variance).
TABLE 4.
Sensitivity evaluations of rTES-26, rTES-30USM, and rTES-120 IgG4 ELISAs
| IgG4 ELISA type | No. of samples (n = 30)
|
Sensitivity (%) | |
|---|---|---|---|
| Positive | Negative | ||
| rTES-26 | 24 | 6 | 80.0 |
| rTES-30USM | 28 | 2a | 93.3 |
| rTES-120 | 28 | 2a | 93.3 |
Two serum samples that were negative by rTES-30USM were positive by rTES-120 and vice versa. Thus, the combination of rTES-30USM and rTES-120 provided 100% sensitivity.
The specificity evaluation of each of the assays is shown in Table 5. The specificity values obtained were as follows: 96.2% by rTES-26, 93.9% by rTES-30USM, and 92.0% by rTES-120. At an alpha level of 0.05, there was no significant difference between the specificities of rTES-26 and rTES-120 (P = 0.059), rTES-26 and rTES-30USM, or rTES-30USM and rTES-120. Minimal cross-reactivities were observed with serum samples from patients with ascariasis, trichuriasis, amoebiasis, filariasis, toxoplasmosis, and strongyloidiasis; none of the sera from healthy individuals were reactive. Meanwhile, when 30 individuals were used as healthy controls, the specificities (number of positive samples/total number of samples) were as follows: 94.4% (134/142), 88.0% (125/142), and 90.8% (129/142) for rTES-26, rTES-120 and rTES-30USM, respectively. These data show high values for all parameters and show also that the specificity values of the tests were still high even when a much-reduced number of healthy individuals was considered.
TABLE 5.
Specificity evaluations of rTES-26, rTES-30USM, and rTES-120 IgG4 ELISAs
| Serum source | No. of negative samples/total no. of samples (no. of false-positive samples) for IgG4 ELISA
|
||
|---|---|---|---|
| rTES-26 (96.2% specificity) | rTES-30USM (93.9% specificity) | rTES-120 (92.0% specificity) | |
| Patients infected with | |||
| Ascaris lumbricoides, Trichuris trichiura, or hookworm | 27/28 (1) | 26/28 (2) | 24/28 (4) |
| Strongyloides stercoralis | 4/5 (1) | 4/5 (1) | 4/5 (1) |
| Gnathostoma spinigerum | 1/1 (0) | 1/1 (0) | 1/1 (0) |
| Entamoeba histolytica | 28/30 (2) | 25/30 (5) | 23/30 (7) |
| Brugia malayi (microfilaremia) | 26/28 (2) | 25/28 (3) | 25/28 (3) |
| Toxoplasma gondii | 18/20 (2) | 18/20 (2) | 18/20 (2) |
| Healthy individuals | 100/100 (0) | 100/100 (0) | 100/100 (0) |
| Total | 204/212 (8) | 199/212 (13) | 195/212 (17) |
DISCUSSION
The development of a highly specific, sensitive, and reliable assay to detect the presence of anti-Toxocara antibodies is an important goal toward improving the diagnosis of human toxocariasis. TES antigen derived from T. canis L2 maintained in defined medium in vitro has been extensively used for the immunodiagnosis of human toxocariasis. Serum samples from patients with filariasis and with STH infections such as ascariasis and strongyloidiasis, however, cross-react with the native TES antigen in immunoassays (10, 12, 15, 16, 27, 28). This may not be a major problem in developed countries where STH infections are not prevalent, but it is a significant problem in tropical countries where STH infections are endemic (7, 10). Thus, native TES antigen is useful only for differential diagnosis, and test interpretation is problematic when the result is positive (15).
The diagnosis of human toxocariasis is currently performed by detecting IgG in an ELISA format. The IgG assay, however, provides false-positive reactions with other parasitic helminths, whereas the IgG4 antibody greatly increased the specificity of the assay for toxocariasis (15, 16, 26). Similarly, in the diagnosis of lymphatic filariasis, antifilarial IgG4 is often used as a marker of active infection (8, 9, 19, 25), but to date, there is no commercially available IgG4 test for the detection of Toxocara infection.
Previously rTES-30USM has been successfully cloned by assembly PCR and expressed in the prokaryotic expression vector by our group (16). rTES-120 has been cloned and expressed as insoluble protein in the pTrcHis2 prokaryotic vector and yeast expression vectors (3, 4). Both of these recombinant antigens have potential in the diagnosis of toxocariasis in an IgG ELISA (3, 4, 28). Evaluation of the diagnostic values of both recombinant antigens, however, requires further validation. In addition, previous studies indicate that TES-30 recombinant antigens demonstrated high sensitivity and specificity for the detection of anti-Toxocara IgG4 subclass antibodies (16).
A 26-kDa antigen is being used as one of six serodiagnostic markers for confirmation of toxocariasis in a commercial Western blot IgG kit that uses native TES antigen (Testline, France). Furthermore previous studies have shown that low-molecular-mass bands (24 to 35 kDa) of native TES antigen were more specific for toxocariasis, while the high-molecular-weight TES antigen bands showed reactivity with sera from various helminth infections (11, 17). Therefore, we hypothesized that rTES-26 might be useful as a serodiagnostic marker for toxocariasis. However, it is interesting that previously rTES-26 was reported to be of poor diagnostic value when tested with serum from toxocariasis patients (18/118; 11.5%) (5). The investigators themselves thought the low reactivity was unexpected since the gene selection was based on reactivity to antisera from TES antigen-immunized mice. Their results are in apparent contradiction to the results of the present study; the reasons could be due to differences in expression vectors, purification methods, and the secondary antibodies employed.
In this study, the genes encoding TES-26 and TES-120 were successfully cloned via RT-PCR and expressed in the appropriate prokaryotic expression vectors for the expression of the recombinant proteins. The target proteins proved to be immunologically reactive. Because both recombinant proteins were well expressed in soluble form, the proteins were purified under native conditions. The predicted molecular masses of rTES-26 and rTES-120 recombinant proteins were 70 and 26 kDa, respectively. These values corresponded with the sizes of the proteins observed using SDS-PAGE/Western blotting. The purified recombinant proteins were then used to develop an ELISA for the detection of specific antibody in sera from patients infected with T. canis by using various antibody classes and subclasses.
Increased levels of total IgE in many cases of toxocariasis indicated that IgE specific for TES antigen was present. High levels of IgE specific for TES antigen are also observed in the serum of patients with clinical signs suggestive of Toxocara infection (22, 23). Not all patients with elevated total IgE levels, however, demonstrated specific anti-Toxocara IgE. Pawlowski (18) reported that Toxocara-specific IgE was present in half of the patients in that study, a finding consistent with the results in the present study, where only half of the patients with toxocariasis had increased IgE levels.
The demonstration of increased Toxocara-specific IgM has been considered indicative of acute infection (21). In our study, however, IgM was not a good marker for Toxocara infection because only 1 of 30 samples was positive by the assay. This result is not surprising, because toxocariasis is often not an acute disease.
When the three recombinant antigens were tested in ELISAs using all the IgG subclasses (IgG1 to IgG4), the results clearly indicated that only the IgG4 assay displayed good specificity. Thus, IgG4 ELISA was employed in the final development of the diagnostic assay for toxocariasis. The use of rTES-26 IgG4 ELISA alone gave a sensitivity of 80.0%, and rTES-30USM and rTES-120 IgG4 ELISA gave similar sensitivities of 93.3%. When both rTES-30USM and rTES-120 were used in separate wells, 100% sensitivity was obtained. The three recombinant antigens were found not to be different in terms of the mean ODs of 30 samples from patients with toxocariasis.
The results obtained differed from those obtained in a study by Watthanakulpanich et al. (24). They reported that with native TES antigen, anti-Toxocara IgG2 gave the greatest sensitivity (98%) (IgG2 > IgG3 > IgG4 > IgG1 > IgG) whereas anti-Toxocara IgG3 gave the greatest specificity (81%) (IgG3 > IgG1 > IgG > IgG2 and IgG4). These differences may be due to the fact that native antigen was employed in their study whereas recombinant antigens were used in our study. The result of the present study, however, is in agreement with other previous studies that reported a significant increase in the specificity for detection of toxocariasis when an IgG4 assay (instead of an IgG assay) was used (15, 16, 26).
In terms of specificity, all three recombinant antigens displayed high specificities. Some of the false-positive cases recorded in the present study may have been due to coinfections with Toxocara. The high specificities obtained can be explained by the fact that the recombinant antigens (unlike native TES antigen) are single or homogenous molecules. In addition, the recombinant antigens are not glycosylated because they are expressed by bacteria, which reduces the cross-reactivities with antibodies that recognize sugar moieties (14).
Previous reports showed that the low-molecular-mass bands (24 to 35 kDa) were more specific for toxocariasis, while higher-molecular-mass bands showed reactivity with sera from patients with various helminth infections (11, 17). These findings are thus consistent with the pattern of specificities obtained in the present study, namely, rTES-26 > rTES-30USM > rTES-120.
Although the sensitivity of rTES-26 was lower than that of rTES-30USM/rTES-120, it is still important to include it in the panel of recombinant antigens in the final assay because TES-26 displayed a very high specificity of 96.2%. Furthermore, the additional infection marker will increase the robustness of the assay, as the final assay remains to be tested with a larger number of samples and in different settings where the organism is endemic.
When a patient's sample is tested using the final assay developed in this study, the results can be interpreted as follows. When all three recombinant antigen IgG4 ELISAs are positive and the clinical symptoms are consistent with Toxocara infection, there is high likelihood that it is a true-positive case. The same applies for when rTES-26 and rTES-120 or rTES-26 and rTES-30USM ELISAs are positive. When only the rTES-30USM or rTES-120 IgG4 ELISA is positive, there is good evidence that the patient is infected.
Conclusion.
A sensitive and specific IgG4 ELISA for detecting toxocariasis was successfully developed using three recombinant antigens: rTES-26, rTES-30USM, and rTES-120 IgG4 ELISA antigens. The assay enabled serodiagnosis of human toxocariasis with high sensitivity and specificity. The use of three recombinant antigens instead of a single recombinant antigen will provide a more robust assay for use in different parts of the world.
Acknowledgments
This study was funded by a research grant from the Malaysian Ministry of Science and Innovation (06-02-05-4261 EA019).
We thank the USM Health Center, Penang; Department of Microbiology and Parasitology, School of Medical Sciences, USM; and R. M. Rohela from the Faculty of Medicine, University of Malaya, Kuala Lumpur, for providing serum samples. We also thank Seberang Prai City Council, Penang, and the Veterinary Laboratories at Bukit Tengah, Penang; Kota Bharu, Kelantan; and Kangar, Perlis, for assistance in the collection of adult T. canis worms.
Footnotes
Published ahead of print on 15 April 2009.
REFERENCES
- 1.Chung, L. Y., B. H. Fang, J. H. Chang, S. M. Chye, and C. M. Yen. 2004. The infectivity and antigenicity of Toxocara canis eggs can be retained after long-term preservation. Ann. Trop. Med. Parasitol. 98251-260. [DOI] [PubMed] [Google Scholar]
- 2.Despommier, D. 2003. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin. Microbiol. Rev. 16265-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fong, M. Y., and Y. L. Lau. 2004. Recombinant expression of the larval excretory-secretory antigen TES-120 of Toxocara canis in the methylotropic yeast Pichia pastoris. Parasitol. Res. 92173-176. [DOI] [PubMed] [Google Scholar]
- 4.Fong, M. Y., Y. L. Lau, I. Init, I. Jamaiah, A. K. Anuar, and N. Rahmah. 2003. Recombinant expression of Toxocara canis excretory-secretory antigens TES-120 in Escherichia coli. Southeast Asian J. Trop. Med. Public Health 34723-726. [PubMed] [Google Scholar]
- 5.Gems, D., C. J. Ferguson, B. D. Robertson, R. Nieves, A. P. Page, M. L. Blaxter, and R. M. Maizels. 1995. An abundant, trans-spliced mRNA from Toxocara canis infective larvae encodes a 26-kDa protein with homology to phosphatidylethanolamine-binding proteins. J. Biol. Chem. 27018517-18522. [DOI] [PubMed] [Google Scholar]
- 6.Hakim, S. L., J. W. Mak, and P. L. W. Lam. 1993. ELISA seropositivity for Toxocara canis antibodies in Malaysia, 1989-1991. Med. J. Malaysia 48303-307. [PubMed] [Google Scholar]
- 7.Jacquier, P., B. Gottstein, Y. Sringelin, and J. Eckert. 1991. Immunodiagnosis of toxocariasis: evaluation of a new enzyme-linked immunosorbent assay. J. Clin. Microbiol. 291831-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klion, A. D., A. Vijaykumar, T. Oei, B. Martin, and T. B. Nutman. 2003. Serum immunoglobulin G4 antibodies to the recombinant antigen, LI-SXP-1, are highly specific for Loa loa infection. J. Infect. Dis. 187128-133. [DOI] [PubMed] [Google Scholar]
- 9.Kwan-Lim, G. E., K. P. Forsyth, and R. M. Maizels. 1990. Filarial-specific IgG4 response correlates with active Wuchereria bancrofti infection. J. Immunol. 1454298-4305. [PubMed] [Google Scholar]
- 10.Lynch, N. R., L. K. Wilkes, A. N. Hodgen, and K. J. Turner. 1988. Specificity of Toxocara ELISA in tropical population. Parasite Immunol. 10323-337. [DOI] [PubMed] [Google Scholar]
- 11.Magnaval, J. F., L. T. Glickman, P. Dorchies, and B. Morassin. 2001. Highlights of human toxocariasis. Korean J. Parasitol. 391-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magnaval, J. F., R. Fabre, P. Maurieres, J. P. Charlet, and B. De Larrard. 1991. Application of the western blotting procedure for immunodiagnosis of human toxocariasis. Parasitol. Res. 77697-702. [DOI] [PubMed] [Google Scholar]
- 13.Maizels, R. M., D. H. de Savigny, and B. M. Oglivie. 1984. Characterization of surface and excretory-secretory antigens of Toxocara canis infective larvae. Parasite Immunol. 623-27. [DOI] [PubMed] [Google Scholar]
- 14.Maizels, R. M., M. W. Kennedy, M. Meghji, B. D. Robertson, and H. V. Smith. 1987. Shared carbohydrate epitopes on the secreted and surface antigens of Toxocara canis. J. Immunol. 139207-214. [PubMed] [Google Scholar]
- 15.Noordin, R., H. V. Smith, S. Mohamad, R. M. Maizels, and M. Y. Fong. 2005. Comparison of IgG-ELISA and IgG4-ELISA for Toxocara serodiagnosis. Acta Trop. 9357-62. [DOI] [PubMed] [Google Scholar]
- 16.Norhaida, A., M. Suharni, A. T. Liza Sharmini, J. Tuda, and N. Rahmah. 2008. rTES-30USM: cloning via assembly PCR, expression and evaluation of usefulness in the detection of toxocariasis. Ann. Trop. Med. Parasitol. 102151-160. [DOI] [PubMed] [Google Scholar]
- 17.Park, S. P., I. Park, H. Y. Park, S. U. Lee, S. Huh, and J. F. Magnaval. 2000. Five cases of ocular toxocariasis confirmed by serology. Korean J. Parasitol. 38267-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pawlowski, Z. 2001. Toxocariasis in humans: clinical experiment and treatment dilemma. J. Helminthol. 75299-305. [DOI] [PubMed] [Google Scholar]
- 19.Rahmah, N., B. H. Lim, A. Khairul Anuar, R. K. Shenoy, V. Kumaraswami, S. Lokman Hakim, P. Chotechuang, K. Kanjanopas, and C. P. Ramachandran. 2001. A recombinant antigen-based IgG4-ELISA for the specific and sensitive detection of Brugia malayi infection. Trans. R. Soc. Trop. Med. Hyg. 95280-284. [DOI] [PubMed] [Google Scholar]
- 20.Schantz, P. M. 1989. Toxocara larva migrans now. Am. J. Trop. Med. Hyg. 4121-34. [DOI] [PubMed] [Google Scholar]
- 21.Shetty, A. K., and D. H. Aviles. 1999. Nephrotic syndrome associated with Toxocara canis infection. Ann. Trop. Paediatr. 19297-300. [DOI] [PubMed] [Google Scholar]
- 22.Smith, H., and N. Rahmah. 2006. Diagnostic limitations and future trends in the serodiagnosis of human toxocariasis, p. 93-102. In C. V. Holland and H. V. Smith (ed.), Toxocara: the enigmatic parasite. CABI Publishing, Wallingford, United Kingdom.
- 23.Smith, H. V., and M. W. Kennedy. 1993. Significance and quantification of antigen-specific IgE in helminthic infections of humans. J. Clin. Immunol. 16131-143. [Google Scholar]
- 24.Watthanakulpanich, D., H. V. Smith, G. Hobbs, A. J. Whalley, and D. Billington. 2008. Application of Toxocara canis excretory-secretory antigens and IgG subclass antibodies (IgG1-4) in serodiagnostic assays of human toxocariasis. Acta Trop. 10690-95. [DOI] [PubMed] [Google Scholar]
- 25.Weil, G. J., C. Stell, F. Liftis, B. W. Li, G. Mearns, E. Lobos, and T. B. Nutman. 2000. A rapid-format antibody card test for diagnosis of onchocerciasis. J. Infect. Dis. 1821796-1799. [DOI] [PubMed] [Google Scholar]
- 26.Wiechinger, W. 1998. Diagnosticher Wert der Spezifischen IgG4 antikörperbestimmung bei der toxocariasis. Ph.D. thesis. Medizinischen Fakultät der Ludwig-Maximilians-Universität zu München, Munich, Germany. http://edoc.ub.unimuenchen.de/archive/00000178/.
- 27.Yamasaki, H., K. Araki, P. K. C. Lim, N. Zasmy, J. W. Mak, R. Taib, and T. Aoki. 2000. Development of a highly specific recombinant Toxocara canis second-stage larva excretory-secretory antigen for immunodiagnosis of human toxocariasis. J. Clin. Microbiol. 381409-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamasaki, H., R. Taib, Y. I. Watanabe, J. W. Mak, N. Zasmy, K. Araki, P. K. C. Lim, K. Kita, and T. Aoki. 1998. Molecular characterization of a cDNA encoding an excretory-secretory antigen from Toxocara canis second stage larvae and its application to the immunodiagnosis of human toxocariasis. Parasitol. Int. 47171-181. [Google Scholar]


