Abstract
Five Escherichia coli colonies/patient were studied to evaluate the reliability of a multiplex real-time PCR assay for detection of diarrheagenic Escherichia coli groups, using a pool of five colonies rather than individual colonies. Sensitivity and specificity were 98% and 100%, respectively, at a fifth of the cost of the individual colony analysis.
Diarrheal disease remains a leading cause of mortality and morbidity in the world (1, 2), particularly in developing countries. In Peru, enteric infections represent the third most important cause of mortality in the first 5 years of life (5). Diarrheagenic Escherichia coli strains are important causes of diarrhea in children in the developing world (6) and to a lesser extent the developed world (3). E. coli associated with diarrhea has been classified into six groups based on clinical, epidemiological, and molecular criteria (Table 1). Although these E. coli strains commonly cause gastroenteritis in children living in developing countries, they are not routinely sought in clinical laboratories. A multiplex real-time PCR to simultaneously identify eight virulence genes associated with the six classes of diarrheagenic E. coli was previously developed (4). To make this assay less expensive for use in developing countries, we conducted this study to determine the sensitivity and specificity of multiplex real-time PCR analysis using a pool of five colonies rather than analyzing individual colonies.
TABLE 1.
Sensitivity and specificity of pool analysis
| Diarrheagenic E. coli (relevant gene[s]) | No. (%) of positive results
|
% Sensitivity of pool analysis | % Specificity of pool analysis | |
|---|---|---|---|---|
| Individual colony | Pool analysis | |||
| EAEC (aagR) | 155 (13.4) | 154 (13.3) | 99 | 100 |
| EPEC (eaeA) | 74 (6.4) | 73 (6.3) | 99 | 100 |
| DAEC (daaD) | 40 (3.5) | 38 (3.3) | 95 | 100 |
| ETEC (st, lt) | 25 (2.2) | 24 (2.1) | 96 | 100 |
| STEC/EHEC (eaeA, stx1, stx2) | 6 (0.5) | 6 (0.5) | 100 | 100 |
| All | 300 | 295 | 98 | 100 |
Abbreviations.
The following abbreviations are used in this article: EPEC, enteropathogenic E. coli; ETEC, enterotoxigenic E. coli; STEC, Shiga toxin-producing E. coli; EAEC, enteroaggregative E. coli; EIEC, enteroinvasive E. coli; and DAEC, diffusely adherent E. coli.
Stool specimens were collected from 1,025 children 2 to 12 months old from a diarrhea surveillance study conducted in low-socioeconomic-status communities of Lima, Peru, during 2006 and 2007 (8) and were cultured on MacConkey agar plates. After overnight incubation at 37°C, five lactose-positive E. coli colonies/patient were subcultured on a new MacConkey agar plate to assure the purity of individual colonies. Prototypical strains (DAEC-5019, STEC-91/8626, ETEC-H10407, EPEC-2348/69, EIEC-213, and EAEC-042) were used as positive controls, and E. coli C600 was used as a negative control.
DNA was extracted by boiling either a single colony in 50 μl or a pool of five colonies in 100 μl of PCR water for 5 min, followed by 15 min at room temperature and a centrifugation at 13,000 rpm for 15 min. Two microliters of this crude lysate was used as a template in a 25-μl total PCR volume.
PCR was performed using a PTC-200 thermal cycler and real-time fluorescence monitoring by a Chromo 4 optical detector (Bio-Rad). Each multiplex PCR assay contained the sets of primers previously validated (4) and 0.5 U of Phusion polymerase (Finnzyme) in high-fidelity Phusion buffer with a final concentration of 200 μM deoxynucleoside triphosphates and 4 mM MgCl2. SYBR green I (Cambrex Bio Science) was diluted as recommended by the manufacturer, and a hot start of 98°C for 30 s was used to prevent nonspecific amplification. The protocol consisted of incubation at 98°C for 20 s, 60°C for 20 s, 72°C for 30 s, and 75°C for 10 s. After 25 cycles, a melting curve was determined with a ramp speed of 2.5°C/s between 73°C and 95°C with a reading every 0.2°C. Melting points were automatically calculated by the Opticon Monitor software program, which plotted the negative derivative of fluorescence with respect to temperature.
We analyzed 1,158 pools from children (<1 year of age) with (858) or without (292) diarrhea, who were enrolled in the cohort study. Three hundred nineteen of 1,158 (27.5%) pools were positive for diarrheagenic E. coli, and 839 of 1,158 (72.5%) were negative. The most common pathogens were EAEC (12.5% for the diarrhea group versus 16.1% for controls), EPEC (5.8% for the diarrhea group versus 7.9% for controls), DAEC (3.7% for the diarrhea group versus 2.1% for controls), and ETEC (2.5% for the diarrhea group versus 1.0% for controls). EIEC strains were not detected in these samples.
We also analyzed 2,065 individual colonies (1,595 colonies from positive pools and 500 colonies from 100 randomly selected negative pools). There was a trend, although not statistically significant, for a higher number of positive colonies per sample in the diarrhea group than in the control group for some pathogens (i.e., for DAEC, 3.04 ± 1.64 in the diarrhea group versus 1.94 ± 1.47 in the control group) in the diarrhea group. For the purpose of the pool-versus-individual-colony analysis, diarrhea and control samples were grouped together.
In a pool-colony analysis, the specificity was 100% for each diarrheagenic E. coli group, and the sensitivity ranged from 96% to 100% (Table 1). There were no false positives; in no case (0/319) did a peak occur at a melting point inconsistent with data for the prototype strain genotype. There were 5 false-negative pools (5/100), as indicated by individual colony analysis, which were positive for DAEC, EPEC, ETEC, and EAEC (1 strain each). The sensitivity was 98%; when false-negative pool samples were run a second time, the sensitivity improved to 100%.
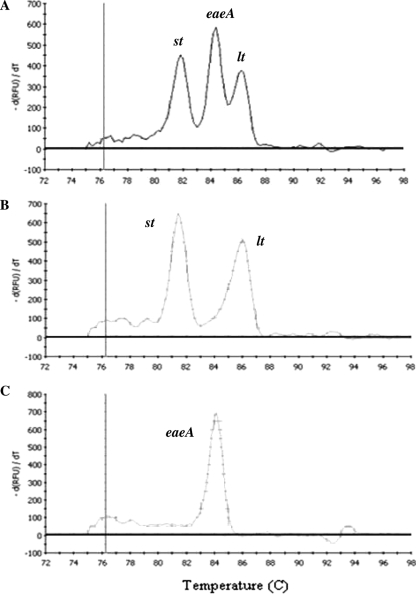
Among positive samples, mixed infections could also be detected (Fig. 1). The most common mixed infections were EAEC plus EPEC (9/20), EAEC plus ETEC (3/20), and EPEC plus ETEC (3/20). There was no competition between different set of primers used to detect the genes associated with diarrheagenic E. coli virulence.
FIG. 1.
Mixed infection detected in a pool analysis colony showing both an EPEC (eae+) and an ETEC (st+ lt+) infection (A) and confirmed by individual colony analyses corresponding to an ETEC (st+ lt+) colony (B) and an EPEC (eae+) colony (C). −d(RFU)/dt, negative derivative of fluorescence (RFU, relative fluorescence units).
Conventional PCR for diagnosis of diarrheagenic E. coli has limitations in the developing world. The cost, the time delay required to do gel analysis of PCR products, and the inability to analyze large numbers of strains are major impediments to this approach. For this reason, we developed and validated a multiplex real-time PCR for detection of diarrheagenic E. coli groups (4). The “gold standard” is the individual colony analysis, but the cost limits its use as a routine test for use in clinical laboratories.
In this study, we were able to save time and effort involved in testing for virulence factors, reducing the number of gene detection assays by use of a sensitive and specific colony pool multiplex real-time PCR. An advantage of this technique is that there is no need to run an electrophoresis gel to determine the presence of the amplicons because each has a characteristic melting temperature that is detected in the denaturation curve. Indeed, this approach also allows identification of mixed infections involving two or three diarrheagenic E. coli strains. Individual positive colonies can be defined in a subsequent PCR if required for epidemiologic or antibiotic susceptibility studies. The rapid detection of diarrheagenic E. coli has important treatment implications. Some diarrheagenic E. coli strains (e.g., ETEC) respond to antimicrobial agents, while for others (e.g., STEC), antibiotics should be avoided. Furthermore, diarrheagenic E. coli strains are commonly resistant to commonly used antibiotics (7), so that optimal treatment depends on rapid detection of the specific pathogen.
The choice of five colonies for analysis represents a compromise between the cost of analysis and the need to detect infection.
In summary, the analysis of a pool of five colonies for the detection of diarrheagenic E. coli by multiplex real-time PCR is a dramatically more cost-effective, sensitive, and specific technique than individual colony analysis.
Acknowledgments
Theresa Ochoa is supported by PHS-FIC grant 1K01TW007405, and Tom Cleary is supported by PHS-NICHD grant R01-HD051716. This study was partially funded by Claudio Lanata's Institutional Research Fund.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health and Human Development or the National Institutes of Health.
We have no conflict of interest to report.
Footnotes
Published ahead of print on 8 April 2009.
REFERENCES
- 1.Black, R. E., S. S. Morris, and J. Bryce. 2003. Where and why are 10 million children dying every year? Lancet 3612226-2234. [DOI] [PubMed] [Google Scholar]
- 2.Bryce, J., C. Boschi-Pinto, K. Shibuya, R. E. Black, and the WHO Child Health Epidemiology Reference Group. 2005. WHO estimates of the causes of death in children. Lancet 3651147-1152. [DOI] [PubMed] [Google Scholar]
- 3.Cohen, M. B., J. P. Nataro, D. I. Bernstein, J. Hawkins, N. Roberts, and M. A. Staat. 2005. Prevalence of diarrheagenic Escherichia coli in acute childhood enteritis: a prospective controlled study. J. Pediatr. 14654-61. [DOI] [PubMed] [Google Scholar]
- 4.Guion, C. E., T. J. Ochoa, C. M. Walker, F. Barletta, and T. G. Cleary. 2008. Detection of diarrheagenic Escherichia coli by use of melting-curve analysis and real-time multiplex PCR. J. Clin. Microbiol. 461752-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huicho, L., M. Trelles, and F. Gonzales. 2006. National and sub-national under-five mortality profiles in Peru: a basis for informed policy decisions. BMC Public Health 6173-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ochoa, T. J., J. Ruiz, M. Molina, L. J. Del Valle, M. Vargas, A. I. Gil, L. Ecker, F. Barletta, E. Hall, T. G. Cleary, and C. F. Lanata. High frequency of antimicrobial resistance in diarrheagenic E. coli in Peruvian children. Am. J. Trop. Med. Hyg., in press. [PMC free article] [PubMed]
- 8.Ochoa, T. J., L. Ecker, F. Barletta, M. Mispireta, A. Gil, C. Contreras, M. Molina, I. Amemiya, H. Verastegui, E. R. Hall, T. G. Cleary, and C. F. Lanata. Age-related susceptibility to infection with diarrheagenic E. coli in infants from peri-urban areas of Lima, Peru. Clin. Infect. Dis., in press. [DOI] [PMC free article] [PubMed]