Abstract
Mycobacterium arosiense is a newly described species. After noticing it was misidentified as Mycobacterium intracellulare by the commercial identification system GenoType CM (Hein, Nehren, Germany), we detected 4 such strains among 33 that were previously misidentified as M. intracellulare. Three more strains were found among unidentified mycobacteria not tested previously with GenoType. The first case of pulmonary disease due to M. arosiense is reported here, and the novel species, of which so far only one strain had been investigated, is further characterized.
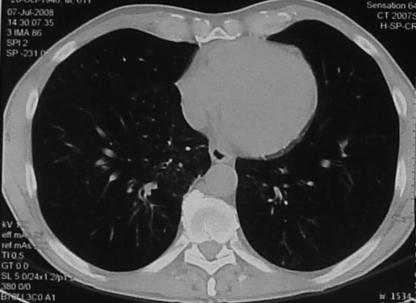
A 62-year-old male, previously a smoker, was hospitalized because of a cough and slight fever (37.6°C). His history included Hodgkin's lymphoma, treated with radiotherapy and a splenectomy at the age of 39, and gastric carcinoma, treated with a gastrectomy at the age of 53. In the last 5 months, the patient had been occasionally treated with amoxicillin-clavulanic acid; following each cycle, the fever and cough appeared to improve but invariably relapsed 15 to 20 days later. A chest X ray revealed bilateral fibronodular infiltration and calcified hilar nodules. A computed tomography scan showed, in the right apex of the lung, alveolar pseudonodular opacity and, in the upper left lobe, bronchiectasis and pleural thickening; typical tree-in-bud pictures (4), considered suggestive of mycobacterial infection, were present in the upper left lobe, both lower lobes, the middle lobe, and the lingula (Fig. 1). Both a skin test and a gamma interferon-releasing assay (8) for tuberculosis were negative. More-remarkable blood parameters included elevated erythrocyte sedimentation rate and C-reactive protein (104 mm/h and 1,590 μg/liter, respectively) and leukocytosis (16.4 × 109 cells/liter) with neutrophilia (79%). When microbiological investigations of samples of sputum, urine, stool, blood, and pharyngeal swabs as well as serology for more-frequent viral and bacterial infections scored negative, the patient, who although untreated had turned afebrile, was discharged. Two weeks later, however, the growth on liquid MGIT medium (Becton Dickinson, Sparks, MD) of mycobacteria from four (out of four) sputum samples led to the start of an antituberculosis treatment with isoniazid, rifampin, pyrazinamide, and ethambutol. A new sample of sputum, collected at that time, grew mycobacteria 2 weeks later. Once the isolates were identified with GenoType CM (Hein, Nehren, Germany) (10) as belonging to the species Mycobacterium intracellulare, clarithromycin was added to the therapy, and isoniazid and pyrazinamide were removed. Since then, apart from episodes of epigastralgia and esophageal candidosis, the cough disappeared, and the conditions of the man clearly improved; the only sample of sputum he was able to produce remained negative in culture. At present, the patient has completed his fourth month of therapy, which is planned to be continued for at least 1 year (3), and is well. In the meantime, a thorough revision of the identification of the strain led to its being identified as belonging to the new species Mycobacterium arosiense.
FIG. 1.
Computed tomography scan showing a typical tree-in-bud picture.
M. arosiense is a recently described, slow-growing, yellow-pigmented, and scotochromogenic species (2). The only strain reported so far was grown repeatedly from osteomyelitic bone lesions from a young boy with a hereditary partial gamma interferon receptor alpha-1 deficiency (2).
We recently noticed that one strain identified by the reverse hybridization system GenoType as Mycobacterium intracellulare presented genetic sequences identical to the ones reported for M. arosiense. A thorough revision of the identification of the 33 strains assigned to M. intracellulare by GenoType revealed that 4 of them belonged to the new species M. arosiense. Such a finding allows us to hypothesize that M. arosiense is a mycobacterium that is less rare than it has been considered so far and adds clinical and microbiological information to the characterization of the new species, based thus far on a single strain.
GenoType was performed on mycobacterial colonies subcultured on Middlebrook 7H11 agar, according to the recommendations of the producer (10).
DNAs extracted from 33 strains isolated from independent clinical specimens, mostly respiratory materials, and identified as M. intracellulare by GenoType CM were initially submitted to genetic sequencing, following a previously reported protocol, of the internal transcribed spacer (ITS) region interposed between the 16S rRNA and the 23S rRNA genes (9). Four strains presenting ITS sequences identical to the one reported for M. arosiense were further sequenced by following the standard procedures and, in particular, were investigated for the almost complete 16S rRNA genes (5), a trait of about 400 bp of the gene coding for the 65-kDa heat shock protein (hsp65) (6) and a trait of about 700 bp of the gene coding for the beta subunit of RNA polymerase (rpoB) (1). A similar characterization was performed on three more strains isolated previously, when GenoType was not yet in use in our laboratory, and were considered, on the basis of partial sequencing of 16S rRNA genes, as not belonging to any known species (genetic sequences of M. arosiense were, at the time of their isolation, not yet available in GenBank).
The susceptibility testing was performed, according to NCCLS recommendations (7), by determining the MICs for amikacin, ciprofloxacin, clarithromycin, ethambutol, gatifloxacin, linezolid, minocycline, moxifloxacin, rifabutin, rifampin, streptomycin, and trimethoprim-sulfamethoxazole using the commercially available microplates Sensititre MAISLOW (Trek Diagnostic Systems, Cleveland, OH).
Clinical information was obtained from the patients' records.
The seven strains characterized here presented identical sequences, 100% overlapping the ones reported for M. arosiense, in the ITS region (GenBank/EMBL/DDBJ accession number EU370533) and in the 16S rRNA (GenBank/EMBL/DDBJ accession number EF054881), hsp65 (GenBank/EMBL/DDBJ accession number EU370531), and rpoB (GenBank/EMBL/DDBJ accession number EU350732) genes.
They were all slow growers and presented scotochromogenic yellow pigmentation. Most of them had grown, in primary cultures, only in liquid media, although in subcultures, they were also able to grow on solid media (both egg and agar based).
The antimicrobial pattern (Table 1) was very homogeneous: all the strains were resistant to quinolones, ethambutol, and streptomycin and susceptible to rifamycins and clarithromycin. They were moderately susceptible to amikacin and linezolid and moderately resistant to minocycline.
TABLE 1.
MICs for M. arosiense strainsa
| Drug(s) | MIC for indicated strain (μg/ml)
|
Interpretationb | |||||
|---|---|---|---|---|---|---|---|
| FI-06102 | FI-07062 | FI-07154 | FI-08110 | FI-08139 | FI-08141 | ||
| Amikacin | 16 | 32 | 16 | 32 | 32 | 32 | S/I |
| Ciprofloxacin | >16 | >16 | >16 | >16 | >16 | >16 | R |
| Clarithromycin | 1 | 0.5 | 0.5 | 1 | 1 | 1 | S |
| Ethambutol | 16 | >32 | 16 | 32 | 16 | 32 | R |
| Gatifloxacin | 8 | >8 | >8 | >8 | >8 | >8 | R |
| Linezolid | 8 | 8 | 8 | 16 | 16 | 16 | S/I |
| Minocycline | 8 | 16 | 8 | 16 | 16 | 16 | I/R |
| Moxifloxacin | 2 | 4 | 4 | 8 | 8 | 4 | R |
| Rifabutin | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | S |
| Rifampin | 0.5 | 1 | 0.5 | 1 | 1 | 1 | S |
| Streptomycin | 32 | 32 | 32 | 32 | 64 | 32 | R |
| Trimethoprim-sulfamethoxazole | >152 | >152 | 152 | 152 | >152 | >152 | R |
Only one strain was tested of the two strains suspected to result from a cross-contamination.
S, susceptible; R, resistant; I, intermediate.
The strains had been grown in hospitals in four different cities in Italy. For two of the strains, isolated almost simultaneously in the same hospital center from urine samples of different patients, a laboratory cross-contamination cannot be excluded; all the others were, on the contrary, unquestionably independent.
The clinical significance of the M. arosiense strain could be confirmed, on the basis of the criteria of the American Thoracic Society (3), in one patient only, whose case is reported here. Two strains were grown from outpatients whose clinical information was not available; the other patients, except for one, had pulmonary conditions compatible with mycobacteriosis, but in most cases, either other possible causes of disease could not be excluded or the isolation had been obtained from a single sample of sputum (Table 2).
TABLE 2.
Microbiological and clinical features of the strains of M. arosiense
| Strain | Microscopyc | No. of positive cultures/no. of cultures performed (type of specimen) | Patient information
|
Isolating center/year | |||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Sexd | Underlying disease | Clinical presentation | Treatment | Follow-up | ||||
| FI-07062 | Neg | 1/1 (sputum) | 74 | M | Adenocarcinoma | Pneumopathy | No | Improved | A/2006 |
| FI-06102 | Neg | 1/1 (bronchial aspirate) | 77 | M | Diabetes | Bronchiectasis, chronic obstructive pulmonary disease | No | Unchanged | B/2006 |
| FI-07154 | Neg | 1/3 (sputum) | 73 | F | Diabetes | Bronchiectasis, interstitial pneumonia | No | Unchanged | C/2007 |
| FI-08110a | Neg | 1/1 (sputum) | 75 | F | D/2008 | ||||
| FI-08139b | Neg | 1/1 (urine) | 32 | F | AIDS | Chronic cystitis | No | Unchanged | D/2008 |
| FI-08141 | Neg | 5/6 (sputum) | 62 | M | History of Hodgkin's lymphoma and gastric carcinoma | Disseminated pneumopathy | Clarithromycin, ethambutol, rifampin | Improved | B/2008 |
| FI-08192a,b | Neg | 1/1 (urine) | 69 | M | D/2008 | ||||
Strain from an outpatient—clinical information not available.
Strain possibly resulting from laboratory cross-contamination.
Neg, negative.
M, male; F, female.
Many new species have been described in the last years, in the wake of the increased diffusion of genetic investigation methods (11). Unlike most of them, M. arosiense seems, on the basis of our findings, to play a major role. Its identification as M. intracellulare by a worldwide diffused commercial system makes it unrecognizable for many diagnostic laboratories; however, it is likely it will be more frequently detected in the future as a consequence of the increasing use of genetic sequencing.
Its potential pathogenicity, at least in immunocompromised patients, is supported, as an adjunct to the present case in which the patient had a history of lymphoma, gastrectomy, and splenectomy and was bronchiectasic, by the previous one in which it was responsible for disseminated osteomyelitis (2). Unlike other species, the antimicrobial susceptibility in vitro of M. arosiense appears to correlate well with its clinical response in vivo; in fact, a treatment with clarithromycin and a rifamycin turned out to be effective in both cases in which it was undertaken.
Footnotes
Published ahead of print on 22 April 2009.
REFERENCES
- 1.Adékambi, T., P. Colson, and M. Drancourt. 2003. rpoB-based identification of nonpigmented and late-pigmenting rapidly growing mycobacteria. J. Clin. Microbiol. 415699-5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bang, D., T. Herlin, M. Stegger, Å. B. Andersen, P. Torkko, E. Tortoli, and B. V. Thomsen. 2008. Mycobacterium arosiense sp. nov., a slowly growing, scotochromogenic species causing osteomyelitis in an immunocompromised child. Int. J. Syst. Evol. Microbiol. 582398-2402. [DOI] [PubMed] [Google Scholar]
- 3.Griffith, D. E., T. Aksamit, B. A. Brown-Elliott, A. Catanzarro, C. Daley, F. Gordin, S. M. Holland, R. Horsburg, G. Huitt, M. F. Iademarco, M. Iseman, K. Olivier, S. Rouss, C. F. von Reyn, R. J. Wallace, Jr., K. Winthrop, ATS Mycobacterial Disease Subcommittee, American Thoracic Society, and Infectious Diseases Society of America. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 165367-416. [DOI] [PubMed] [Google Scholar]
- 4.Jeong, Y. J., K. S. Lee, W. J. Koh, J. Han, T. S. Kim, and O. J. Kwon. 2004. Nontuberculous mycobacterial pulmonary infection in immunocompetent patients: comparison of thin-section CT and histopathologic findings. Radiology 231880-886. [DOI] [PubMed] [Google Scholar]
- 5.Kirschner, P., B. Springer, U. Vogel, A. Meier, A. Wrede, M. Kiekenbeck, F. C. Bange, and E. C. Böttger. 1993. Genotypic identification of mycobacteria by nucleic acid sequence determination: report of a 2-year experience in a clinical laboratory. J. Clin. Microbiol. 312882-2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McNabb, A., D. Eisler, K. Adie, M. Amos, M. Rodrigues, G. Stephens, W. A. Black, and J. Isaac-Renton. 2004. Assessment of partial sequencing of the 65-kilodalton heat shock protein gene (hsp65) for routine identification of mycobacterium species isolated from clinical sources. J. Clin. Microbiol. 423000-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NCCLS. 2003. Susceptibility testing for mycobacteria, nocardiae and other aerobic actinomycetes. Approved standard M24-A. National Committee for Clinical Laboratory Standards, Wayne, PA. [PubMed]
- 8.Pai, M., L. W. Riley, and J. M. Colford, Jr. 2004. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect. Dis. 4761-766. [DOI] [PubMed] [Google Scholar]
- 9.Roth, A., M. Fisher, M. E. Hamid, S. Michalke, W. Ludwig, and H. Mauch. 1998. Differentiation of phylogenetically related slowly growing mycobacteria based on 16S-23S rRNA gene internal transcribed spacer sequences. J. Clin. Microbiol. 36139-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russo, C., E. Tortoli, and D. Menichella. 2006. Evaluation of the new GenoType Mycobacterium assay for identification of mycobacterial species. J. Clin. Microbiol. 44334-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tortoli, E. 2003. Impact of genotypic studies on mycobacterial taxonomy: the new mycobacteria of the 1990s. Clin. Microbiol. Rev. 16319-354. [DOI] [PMC free article] [PubMed] [Google Scholar]