Abstract
Low-colony-number counts on solid media are considered characteristic of cross-contamination, although they are normally observed in true-positive cultures from some groups of patients. The aim of this study was to evaluate low-yield growth cultures as a microbiological marker for cross-contamination. We evaluated 106 cultures with <15 colonies from 94 patients, and the proportions of false-positive cultures were 0.9% per sample and 1.1% per patient, which indicates that low-yield growth is not a reliable marker of cross-contamination.
Isolation and identification of Mycobacterium tuberculosis from clinical specimens remain the definitive methods for diagnosing tuberculosis (TB). Occasionally, false-positive cultures occur due to contaminated clinical equipment, clerical errors, or cross-contamination of specimens (1, 3, 11, 14, 16). Indications of cross-contamination include culture results inconsistent with the patient's clinical course, unexpected drug resistance, single culture-positive specimens, and solid-medium cultures with low-colony-number counts (4). A single positive culture is the most commonly reported indicator of false positivity. When molecular-strain-typing methods were used to investigate the source of single positive cultures, outbreaks of laboratory cross-contamination that had been unrecognized by clinicians or laboratory personnel were identified (7, 11). Drug treatment trials usually consider cultures yielding ≤10 colonies negative to avoid false-positive culture results. However, in cultures of specimens from some patients having active TB but minimal radiographic disease, having human immunodeficiency virus coinfection, or already receiving anti-TB treatment, growth of few colonies is normally observed (5, 8, 12). No studies have systematically evaluated the predictive value of low-colony-number counts of M. tuberculosis on solid media as a microbiological indicator (4).
This study was conducted in the mycobacteriology laboratory of a large urban teaching hospital in Brazil, where about 30 specimens are processed daily and the M. tuberculosis positivity rate is 8%. From January 2003 to January 2005, 12,984 respiratory samples from 8,656 patients suspected of having TB were received. M. tuberculosis was isolated from 2,399 (18.5%) specimens obtained from 2,141 patients. Isolates from smear-negative, low-colony-number (<15 colonies) cultures with corresponding clinical information were included in the study; 106 isolates from 94 patients met these criteria. Quantification of growth and identification of isolates were done according to standard procedures (6, 9). To investigate cross-contamination episodes, the DNA fingerprint patterns of the 106 isolates were compared to those of positive specimens processed on the same day, resulting in the analysis of 279 isolates processed on 92 days.
The rapid PCR-based epidemiological typing (RAPET) method was used to screen 279 isolates. The PCR mixture contained 20 mM Tris-HCl (pH 8,4), 50 mM KCl, 2.5 mM MgCl2, 0.2 mM of each deoxynucleoside triphosphate, 40 pmol of the primer, and 1 U of Taq polymerase; the PCR primers and cycling conditions were as described by Yates et al. (17). PCR mixtures showing similar patterns were digested with HaeIII. Isolates that had similar RAPET patterns were subjected to restriction fragment length polymorphism (RFLP) analysis with IS6110, using a standardized method (15). Isolates were considered identical when they had matching RAPET fingerprint patterns which were confirmed by RFLP analysis. When an isolate from a low-growth-rate culture had the same fingerprint pattern as an isolate from a culture processed on the same day, the low-growth-rate culture was considered to be cross-contaminated, especially when the patient had no clinical indication of active TB and no epidemiological link with the presumed source of M. tuberculosis. When there were no isolates with matching fingerprint patterns and the patient had a clinical course consistent with active TB, the culture was considered to be a true positive.
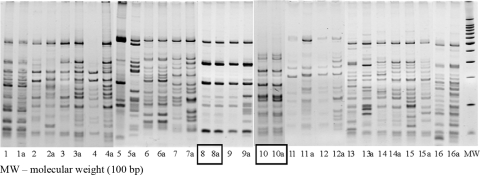
To investigate cross-contamination episodes, the RAPET PCR patterns from each of the isolates included in the study were compared to the RAPET PCR patterns of all culture-positive specimens processed concurrently. After RAPET, 16 isolates showed a molecular pattern similar to that of a true-positive specimen processed in the same batch. These isolates were subjected to the RAPET restriction enzyme analysis and RFLP analysis. After RAPET restriction analysis, only two of the isolates had patterns identical to that of a true-positive specimen (Fig. 1). Subsequently, RFLP analysis showed that the fingerprint patterns of each of the pairs were indistinguishable (Fig. 2). Thus, molecular results suggested the occurrence of two cross-contamination episodes.
FIG. 1.
Comparison of RAPET patterns of M. tuberculosis isolates from suspected false-positive cultures and isolates from cultures processed on the same day. Patterns generated by HaeIII digestion of PCR products are shown.
FIG. 2.
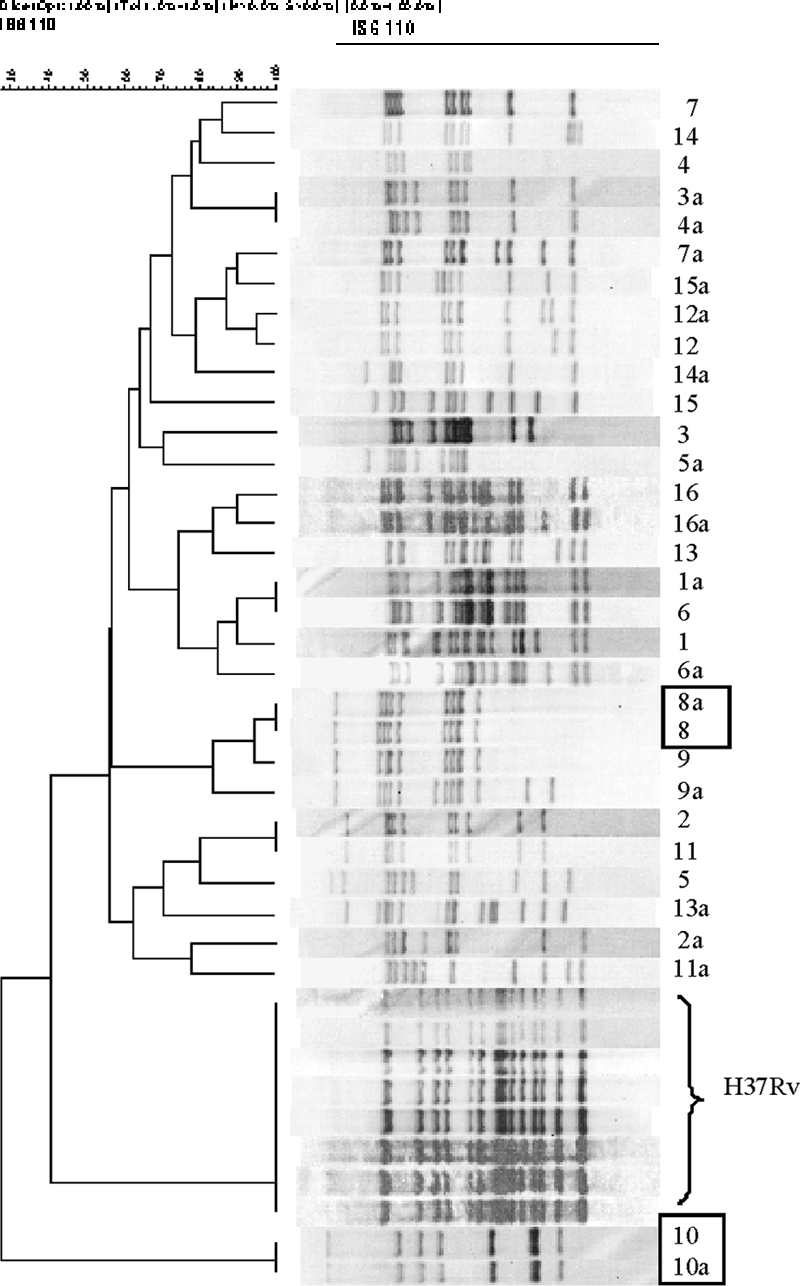
IS6110 RFLP patterns from M. tuberculosis isolates that showed similar patterns of PCR products after the first step of RAPET analysis. The rectangles represent the two possible cross-contaminated cultures.
Next, demographic and clinical data for the paired patients were reviewed. The patients were not epidemiologically linked. Medical records showed that patients 1 and 2 had clinical courses consistent with active TB (Table 1). The second pair was assessed to involve a cross-contamination episode between patients 3 and 4, as patient 3 had been treated for TB for 2 years at the time the specimen was collected, whereas patient 4 had a clinical course inconsistent with active TB, had no other positive smears or cultures, and was not reported as a TB case by the responsible clinician.
TABLE 1.
Clinical and laboratory characteristics of patients whose M. tuberculosis cultures were suspected to be false positive and patients with isolates matching those of another patient whose specimen was processed on the same day
| Patient | Isolate no. | Status of specimen | Smear result | No. of M. tuberculosis colonies on Ogawa | Clinical signs and symptoms | Panel decision | Final diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | 8a | Follow-up (mo 1) | Negative | 0a | 40-Yr-old man with chest pain, wt loss, adynamia, and fever; chest radiograph showed two right lung zones involved | TB | TB |
| 2 | 8 | Initial | Negative | 2 | 27-Yr-old woman with productive cough for 30 days, household contact; chest radiograph was normal | TB | TB |
| 3 | 10 | Follow-up (yr 4) | 3+ positive | 20 | 39-Yr-old woman with multi-resistant TB, nonproductive cough; chest radiograph suggested active disease | TB | TB |
| 4 | 10a | Initial | Negative | 10 | 79-Yr-old woman, household contact, no clinical signs of active TB disease | Cross-contamination | Not TB |
Positive in Bactec 12B.
Thus, out of the 106 M. tuberculosis isolates from cultures growing <15 colonies, only 1 (0.9%) was considered a false positive, which corresponds to 1.1% (1/94) of the patients. Considering the time intervals when specimens were collected, the cross-contamination rates were 1.2% for diagnostic and 0% for follow-up cultures. The lack of false-positive results for patients receiving anti-TB drugs confirms the importance of low-growth-rate culture results in the evaluation and classification of a patient's response to TB treatment.
Our results differ from those of MacGregor et al., who evaluated 36 cultures, yielding <5 colonies from 31 patients, and observed a cross-contamination rate of 33% (10). However, in this study, only clinical and radiological data were used for final assessment of true- and false-positive cases of TB, as molecular-typing techniques were unavailable. The association between low-colony-number counts and cross-contamination events, first mentioned by MacGregor et al., has been accepted for decades. Others have reported that low-growth-rate cultures were associated with false positivity; however, these studies did not prospectively investigate the validity of this association (1, 5, 13).
Although false-positive cultures are more likely to have few colonies on solid media, the opposite was not true in our study. Braden et al. reached a similar conclusion in their evaluation of the number of colonies observed in cultures from 15 TB patients (3). Five or more colonies were discriminatory for those cultures considered unlikely cross-contaminated, compared with those considered cross-contaminated; however, a lower colony number was not discriminatory for the two groups. Bhattacharya et al. reported that, although the two episodes of cross-contamination detected in their laboratory showed growth with five or fewer colonies, the same was observed for 16 (30%) specimens from 54 patients with culture-confirmed TB (2).
Although the growth of few colonies of M. tuberculosis on solid media is commonly attributed to cross-contamination, our findings suggest that, even for a single specimen, this result should not be considered indicative of a false-positive culture. Such results must be carefully considered in the context of the clinical and epidemiologic information and strain genotyping performed when these data are unclear.
Acknowledgments
This work was supported by the Tuberculosis Research Unit at Case Western Reserve University, established with funds from the U.S. National Institutes of Allergy and Infectious Diseases, National Institutes of Health and Human Services, under contract no. NO1-AI95383 and HHSN266200700022C/NO1-AI-70022, and in part by grant no. 37396285/2007 from Fundação de Apoio à Ciência e Tecnologia do Espírito Santo.
Footnotes
Published ahead of print on 22 April 2009.
REFERENCES
- 1.Bauer, J., V. O. Thomsen, S. Polsen, and A. B. Andersen. 1997. False-positive results from cultures of Mycobacterium tuberculosis due to laboratory cross-contamination confirmed by restriction fragment length polymorphism. J. Clin. Microbiol. 35988-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharya, M., S. Dietrich, L. Mosher, F. Siddiqui, B. E. Reisberg, W. S. Paul, and J. R. Warren. 1998. Cross-contamination of specimens with Mycobacterium tuberculosis: clinical significance, causes, and prevention. Am. J. Clin. Pathol. 109324-330. [DOI] [PubMed] [Google Scholar]
- 3.Braden, C., G. Templeton, W. Stead, J. Bates, D. Cave, and S. Valway. 1997. Retrospective detection of laboratory cross-contamination of Mycobacterium tuberculosis cultures with use of DNA fingerprinting analysis. Clin. Infect. Dis. 2435-40. [DOI] [PubMed] [Google Scholar]
- 4.Burman, W. J., and R. R. Reves. 2000. Review of false-positive cultures for Mycobacterium tuberculosis and recommendations for avoiding unnecessary treatment. Clin. Infect. Dis. 311390-1395. [DOI] [PubMed] [Google Scholar]
- 5.Burman, W. J., B. L. Stone, R. R. Reves, M. L. Wilson, Z. Yang, H. El-Hajj, J. H. Bates, and M. D. Cave. 1997. The incidence of false-positive cultures for Mycobacterium tuberculosis. Am. J. Respir. Crit. Care Med. 155321-326. [DOI] [PubMed] [Google Scholar]
- 6.Collins, C. H., J. M. Grange, and M. D. Yates. 1997. Tuberculosis bacteriology: organization and practice, 2nd ed. Butterworth-Heinemann, Oxford, United Kingdom.
- 7.Fine, A., B. Nivin, C. Driver, K. Kaye, R. Jovell, and M. Salfinger. 1997. A pseudo-outbreak of tuberculosis: laboratory cross-contamination discovered through routine surveillance. Clin. Infect. Dis. 45402. [Google Scholar]
- 8.Frieden, T. R., C. L. Woodley, J. T. Crawford, D. Lew, and S. M. Dooley. 1996. The molecular epidemiology of tuberculosis in New York City: the importance of nosocomial transmission and laboratory error. Tuber. Lung Dis. 77407-413. [DOI] [PubMed] [Google Scholar]
- 9.Kent, P. T., and G. P. Kubica. 1985. Public health mycobacteriology: a guide for the level III laboratory. Centers for Disease Control and Prevention, Atlanta, GA.
- 10.MacGregor, R. R., L. W. Clark, and F. Bass. 1975. The significance of isolating low numbers of Mycobacterium tuberculosis in culture of sputum specimens. Chest 68518-523. [DOI] [PubMed] [Google Scholar]
- 11.Nivin, B., P. I. Fujiwara, J. Hannifan, and B. N. Kreisworth. 1998. Cross-contamination with Mycobacterium tuberculosis: an epidemiological and laboratory investigation. Infect. Control Hosp. Epidemiol. 19500-503. [DOI] [PubMed] [Google Scholar]
- 12.Nunn, P., B. Williams, K. Floyd, C. Dye, G. Elzinga, and M. Raviglione. 2005. Tuberculosis control in the era of HIV. Nat. Rev. Immunol. 5819-826. [DOI] [PubMed] [Google Scholar]
- 13.Schoch, O. D., G. E. Pfyffer, D. Buhl, and A. Paky. 2003. False-positive Mycobacterium tuberculosis culture revealed by restriction fragment length polymorphism analysis. Infection 31189-191. [DOI] [PubMed] [Google Scholar]
- 14.Small, P. M., N. B. McClenny, S. P. Singh, G. K. Schoolnik, L. S. Tompkins, and P. A. Mickelsen. 1993. Molecular strain typing of Mycobacterium tuberculosis to confirm cross-contamination in the mycobacteriology laboratory and modifications of procedures to minimize occurrence of false-positive cultures. J. Clin. Microbiol. 311677-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Embden, J. D., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, and T. M. Shinnick. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wurtz, R., P. Demarais, W. Trainor, J. McAuley, F. Kocka, L. Mosher, and S. Dietrich. 1996. Specimen contamination in mycobacteriology laboratory detected by pseudo-outbreak of multidrug-resistant tuberculosis: analysis by routine epidemiology and confirmation by molecular technique. J. Clin. Microbiol. 341017-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yates, M. D., F. A. Drobniewski, and S. M. Wilson. 2002. Evaluation of a rapid PCR-based epidemiological typing method for routine studies of Mycobacterium tuberculosis. J. Clin. Microbiol. 40712-714. [DOI] [PMC free article] [PubMed] [Google Scholar]