Abstract
In recent years, the proportion of Salmonella enterica infections represented by S. enterica serovar Newport has increased markedly among humans and animals. Multilocus variable-number tandem-repeat analysis (MLVA) has proven to be useful in discriminating other highly clonal Salmonella serovars. Here, we report on the development of a highly discriminatory MLVA for Salmonella serovar Newport.
Multilocus variable-number tandem-repeat analyses (MLVA) have been developed for subtyping bacterial pathogens, including Salmonella enterica (12, 13, 16, 20). MLVA detects polymorphisms at genomic loci that are composed of tandemly repeated DNA sequences. Pulsed-field gel electrophoresis (PFGE) has been used for many years to subtype Salmonella enterica with satisfactory results (7), but MLVA has the advantages of a shorter turnaround time, additional discriminatory power, and the possibility of providing phylogenetic information to investigators. We report here the development and application of an MLVA protocol for Salmonella enterica serovar Newport, a clinically and epidemiologically important serovar characterized by multidrug resistance (5, 6, 9, 11, 14, 19).
Bovine isolates from the northwestern United States were obtained from previous field studies conducted at Washington State University (Pullman) and from clinical submissions to the Washington Animal Disease Diagnostic Laboratory (Pullman). Isolates from the northeastern United States originated from several independent cattle herd outbreaks and from epidemiologically independent sources (humans and wild birds) (see Table S1 in the supplemental material). Veterinary isolates were serotyped by the National Veterinary Services Laboratory (Ames, IA), and human isolates were serotyped by the state public health reference laboratories in Washington and New York State by using a standard protocol (15).
Fourteen S. Newport isolates from diverse sources and with diverse PFGE profiles were initially assayed using a previously published protocol for S. Typhimurium (13). Two of the published loci, the STTR5 and STTR6 loci, were polymorphic (see Table S2 in the supplemental material). Tandem-repeat finder software (3) was used to identify potential variable-number tandem-repeat (VNTR) loci in the S. Newport genome (GenBank accession number NC_011080). Sixty-seven repeat loci were identified, including the STTR3, STTR5, STTR6, and STTR7 loci, previously reported by Lindstedt et al. for serovar Typhimurium (12, 13). Published primers for STTR7 (12) did not amplify a product, and STTR3 products were identical between S. Newport isolates. Among the remaining repeat loci, those with the highest number of repeat copies and the fewest mismatches in the repeated sequences were investigated further. Fourteen of the repeat loci met our criteria, and primers for these loci were designed using Primer3 (18), targeting an amplicon size between 150 and 600 bp (see Table S3 in the supplemental material). PCRs that produced products with visible fragment size differences between several strains of S. Newport (assessed using gel electrophoresis) were repeated with dye-labeled primers, and those products were submitted for fragment sizing by capillary electrophoresis (Laboratory for Biotechnology and Bioanalysis, Washington State University, Pullman). The Newport-A, Newport-B, Newport-L, and Newport-M loci were polymorphic in our set of test isolates, generated the expected product sizes, and could be assayed simultaneously (Table 1).
TABLE 1.
Primer sequences used for six VNTR target loci from Salmonella enterica serovar Newport
| Target | Forward primera | Reverse primer | Repeat size (bp)b | Amplicon size (bp) |
|---|---|---|---|---|
| PCR 1 | ||||
| STTR6 | 5′-FAM-TCGGGCATGCGTTGAAA | 5′-CTGGTGGGGAGAATGACTGG | 6 | 397 |
| Newport-A | 5′-PET-ACTGAAAGGAAGGGGAGAGC | 5′-GTCAGGGTGGAATAGAATGC | 9 | 429 |
| Newport-L | 5′-VIC-GAAGTACCGAAGTGGGTGAT | 5′-CGTCCGTTAGAGGAACGTAT | 51 | 529 |
| PCR 2 | ||||
| STTR5 | 5′-PET-ATGGCGAGGCGAGCAGCAGT | 5′-GGTCAGGCCGAATAGCAGGAT | 6 | 564 |
| Newport-B | 5′-VIC-GGCCGATATAGCTCAGTTGG | 5′-GAACCTCGCTTAGGGTTGTG | 12 | 350 |
| Newport-M | 5′-FAM-GGTCATAGAGGGTCTGCAT | 5′-ATGGAGCACAGACCACTAAC | 36 | 378 |
6-Carboxyfluorescein (FAM), PET, and VIC are 5′-conjugated fluorescent dyes (Applied Biosystems).
Expected repeat size for Salmonella enterica serovar Newport strain SL254 (GenBank accession number NC_011080).
To avoid overlap in combinations of fragment sizes and dye colors, two separate triplex reactions were conducted using an iCycler thermal cycler (Bio-Rad, Hercules, CA) in 25-μl volumes (Table 1). All primers were purchased from Applied Biosystems (Foster City, CA). A colony boiled-lysate suspension was used as the PCR template, and cycling conditions for both reactions included an initial denaturation at 94°C for 15 min followed by 25 cycles of 94°C for 30 s, 55°C for 1 min, and 72°C for 1.5 min, with a final extension step at 72°C for 10 min. A size standard (LIZ600; Applied Biosystems) (0.125 μl) and Hi-Di formamide (19.375 μl) were added to the PCR products (0.5 μl) (total volume, 20 μl for capillary electrophoresis). Capillary electrophoresis was conducted using a 3730 DNA analyzer with a POP-7 polymer (Applied Biosystems). The resulting electropherograms were analyzed using GeneMarker software (Softgenetics LLC, State College, PA). Fragment sizes for each of the VNTR loci were analyzed as character data by BioNumerics software. Cluster analysis of the categorical coefficient was performed using the unweighted-pair group method using arithmetic averages (UPGMA) algorithm. The Simpson diversity index (SDI) and 95% confidence intervals (CI) around the SDI were calculated as previously described (8, 10).
Isolates were also assayed using PFGE according to a previously published protocol (17). Bacterial DNA was digested in agarose plugs with the restriction enzyme XbaI, and the resulting DNA fragments were gel separated using a CHEF-DRII (Bio-Rad, Hercules, CA) apparatus. PFGE profiles were analyzed with BioNumerics software (Applied Maths, Sint-Martens-Latem, Belgium) by clustering Dice similarity coefficients, using the UPGMA algorithm with a 1% position tolerance and 0% optimization. Pearson's correlation coefficient for the similarity matrices generated by MLVA and PFGE was 59.8%, as calculated using the “congruence of experiments” feature in BioNumerics.
To determine the repeatability of MLVA results, 20 systematically chosen isolates were assayed twice. Among these, one replicate reaction produced an additional repeat length (STTR5 locus) compared with the initial PCR amplification result. Thus, for 120 individual allele determinations (20 isolates × 6 loci), 119 (99.2%) were stably repeated.
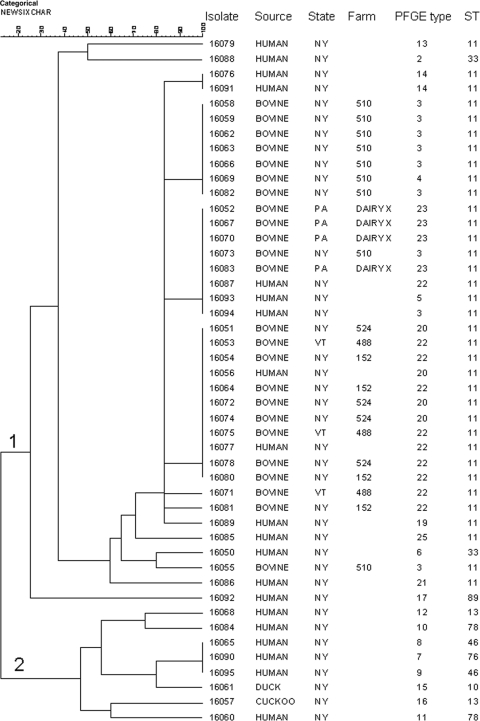
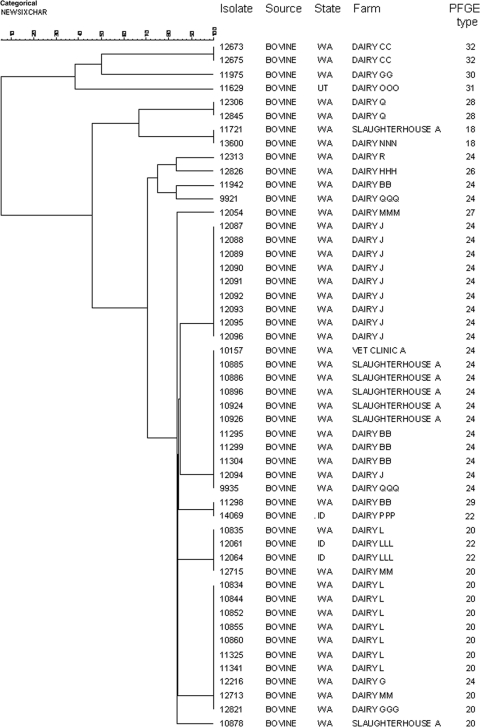
MLVA divided 132 S. Newport isolates into 40 different types, where a type is defined as a set of alleles that is different from other isolates at one or more loci and where a null amplification product is considered a distinct allele after confirmation by a repeat assay (see Table S1 in the supplemental material). The number of isolates within each type ranged from 1 to 26, with an SDI of 0.91 (95% CI, 0.88 to 0.93). The SDI was 0.90 (95% CI, 0.86 to 0.95) for XbaI PFGE of the same group of isolates. The discriminatory power of MLVA relative to that of PFGE varied by region: for the northeastern isolates (Fig. 1), the SDIs (95% CI) for MLVA and PFGE were 0.90 (0.85 to 0.95) and 0.92 (0.87 to 0.97), respectively, while those for the northwestern isolates (Fig. 2) were 0.88 (0.83 to 0.93) for MLVA and 0.71 (0.61 to 0.82) for PFGE.
FIG. 1.
Dendrogram from cluster analysis of northeastern U.S. S. Newport isolates. The UPGMA algorithm was used to cluster categorical coefficients of MLVA allele data. STs were assigned by Alcaine et al. (1).
FIG. 2.
Dendrogram from cluster analysis of northwestern U.S. bovine S. Newport isolates. The UPGMA algorithm was used to cluster categorical coefficients of MLVA allele data.
In the MLVA-generated cluster analysis of northeastern U.S. isolates, most (20 of 24) isolates from herd outbreaks of salmonellosis among cattle clustered with others from the same outbreak. The few exceptions, including isolates 16073 and 16055 from farm 510 and isolates 16071 and 16081 from farms 488 and 152, respectively, may have been misclassified by MLVA, but it is equally possible that they represent true dissemination of strains. MLVA clustering was consistent with multilocus sequence type (ST) data available from previous investigations (1, 2). Cluster 1 includes primarily ST 11 isolates, and cluster 2 includes human and avian isolates with diverse multiple MLVA types, PFGE types, and STs (Fig. 1). The cluster analysis of northwestern isolates from bovine sources provides additional support for the close correlation between our MLVA protocol and known epidemiologic data. As for the northeastern isolates, most isolates from the same farm or operation clustered together with few exceptions, and PFGE types corresponded well with MLVA types (Fig. 2).
The MLVA protocol for S. Newport presented here demonstrated excellent repeatability and close congruence with PFGE and multilocus sequence typing, as well as having strong correspondence with epidemiological data. Our finding of occasional allele instability with this method is consistent with the instability of VNTR loci reported for Salmonella enterica serovar Typhimurium (4), and discrepancies between PFGE and MLVA may in part be explained by such instability (for example, isolates 10834 and 10835 in Fig. 2 differed at a single locus and by one repeat length). However, given its relative stability and concordance with PFGE, the higher processing capacity and more-rapid turnaround of MLVA make it a strong complementary or alternative technique for regional surveillance and outbreak investigation.
Supplementary Material
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract numbers N01-AI-30054 and N01-AI-30055.
This work could not have been completed without the help and guidance of Derek Pouchnik and the staff at the WSU Genomics Core Laboratory, School of Molecular Biosciences.
Footnotes
Published ahead of print on 22 April 2009.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Alcaine, S. D., Y. Soyer, L. D. Warnick, W. L. Su, S. Sukhnanand, J. Richards, E. D. Fortes, P. McDonough, T. P. Root, N. B. Dumas, Y. Grohn, and M. Wiedmann. 2006. Multilocus sequence typing supports the hypothesis that cow- and human-associated Salmonella isolates represent distinct and overlapping populations. Appl. Environ. Microbiol. 727575-7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcaine, S. D., S. S. Sukhnanand, L. D. Warnick, W. L. Su, P. McGann, P. McDonough, and M. Wiedmann. 2005. Ceftiofur-resistant Salmonella strains isolated from dairy farms represent multiple widely distributed subtypes that evolved by independent horizontal gene transfer. Antimicrob. Agents Chemother. 494061-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson, G. 1999. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 27573-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Call, D. R., L. Orfe, M. A. Davis, S. Lafrentz, and M. S. Kang. 2008. Impact of compounding error on strategies for subtyping pathogenic bacteria. Foodborne Pathog. Dis. 5505-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2008. Outbreak of multidrug-resistant Salmonella enterica serotype Newport infections associated with consumption of unpasteurized Mexican-style aged cheese—Illinois, March 2006-April 2007. MMWR Morb. Mortal. Wkly. Rep. 57432-435. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2002. Outbreak of multidrug-resistant Salmonella Newport—United States, January-April 2002. MMWR Morb. Mortal. Wkly. Rep. 51545-548. [PubMed] [Google Scholar]
- 7.Gerner-Smidt, P., K. Hise, J. Kincaid, S. Hunter, S. Rolando, E. Hyytia-Trees, E. M. Ribot, and B. Swaminathan. 2006. PulseNet USA: a five-year update. Foodborne Pathog. Dis. 39-19. [DOI] [PubMed] [Google Scholar]
- 8.Grundmann, H., S. Hori, and G. Tanner. 2001. Determining confidence intervals when measuring genetic diversity and the discriminatory abilities of typing methods for microorganisms. J. Clin. Microbiol. 394190-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta, A., J. Fontana, C. Crowe, B. Bolstorff, A. Stout, S. Van Duyne, M. P. Hoekstra, J. M. Whichard, T. J. Barrett, and F. J. Angulo. 2003. Emergence of multidrug-resistant Salmonella enterica serotype Newport infections resistant to expanded-spectrum cephalosporins in the United States. J. Infect. Dis. 1881707-1716. [DOI] [PubMed] [Google Scholar]
- 10.Hunter, P. R., and M. A. Gaston. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 262465-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karon, A. E., J. R. Archer, M. J. Sotir, T. A. Monson, and J. J. Kazmierczak. 2007. Human multidrug-resistant Salmonella Newport infections, Wisconsin, 2003-2005. Emerg. Infect. Dis. 131777-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindstedt, B. A., E. Heir, E. Gjernes, and G. Kapperud. 2003. DNA fingerprinting of Salmonella enterica subsp. enterica serovar Typhimurium with emphasis on phage type DT104 based on variable number of tandem repeat loci. J. Clin. Microbiol. 411469-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindstedt, B. A., T. Vardund, L. Aas, and G. Kapperud. 2004. Multiple-locus variable-number tandem-repeats analysis of Salmonella enterica subsp. enterica serovar Typhimurium using PCR multiplexing and multicolor capillary electrophoresis. J. Microbiol. Methods 59163-172. [DOI] [PubMed] [Google Scholar]
- 14.Lopes, V. C., S. D. Wedel, J. B. Bender, K. E. Smith, F. T. Leano, D. J. Boxrud, D. C. Lauer, B. T. Velayudhan, and K. V. Nagaraja. 2006. Emergence of multidrug-resistant Salmonella enterica serotype Newport in Minnesota. Clin. Infect. Dis. 43210-213. [DOI] [PubMed] [Google Scholar]
- 15.Popoff, M. Y., J. Bockemuhl, and L. L. Gheesling. 2004. Supplement 2002 (no. 46) to the Kauffmann-White scheme. Res. Microbiol. 155568-570. [DOI] [PubMed] [Google Scholar]
- 16.Ramisse, V., P. Houssu, E. Hernandez, F. Denoeud, V. Hilaire, O. Lisanti, F. Ramisse, J. D. Cavallo, and G. Vergnaud. 2004. Variable number of tandem repeats in Salmonella enterica subsp. enterica for typing purposes. J. Clin. Microbiol. 425722-5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribot, E. M., M. A. Fair, R. Gautom, D. N. Cameron, S. B. Hunter, B. Swaminathan, and T. J. Barrett. 2006. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog. Dis. 359-67. [DOI] [PubMed] [Google Scholar]
- 18.Rozen, S., and H. Skaletsy. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132365-386. [DOI] [PubMed] [Google Scholar]
- 19.Varma, J. K., R. Marcus, S. A. Stenzel, S. S. Hanna, S. Gettner, B. J. Anderson, T. Hayes, B. Shiferaw, T. L. Crume, K. Joyce, K. E. Fullerton, A. C. Voetsch, and F. J. Angulo. 2006. Highly resistant Salmonella Newport-MDRAmpC transmitted through the domestic US food supply: a FoodNet case-control study of sporadic Salmonella Newport infections, 2002-2003. J. Infect. Dis. 194222-230. [DOI] [PubMed] [Google Scholar]
- 20.Witonski, D., R. Stefanova, A. Ranganathan, G. E. Schutze, K. D. Eisenach, and M. D. Cave. 2006. Variable-number tandem repeats that are useful in genotyping isolates of Salmonella enterica subsp. enterica serovars Typhimurium and Newport. J. Clin. Microbiol. 443849-3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.